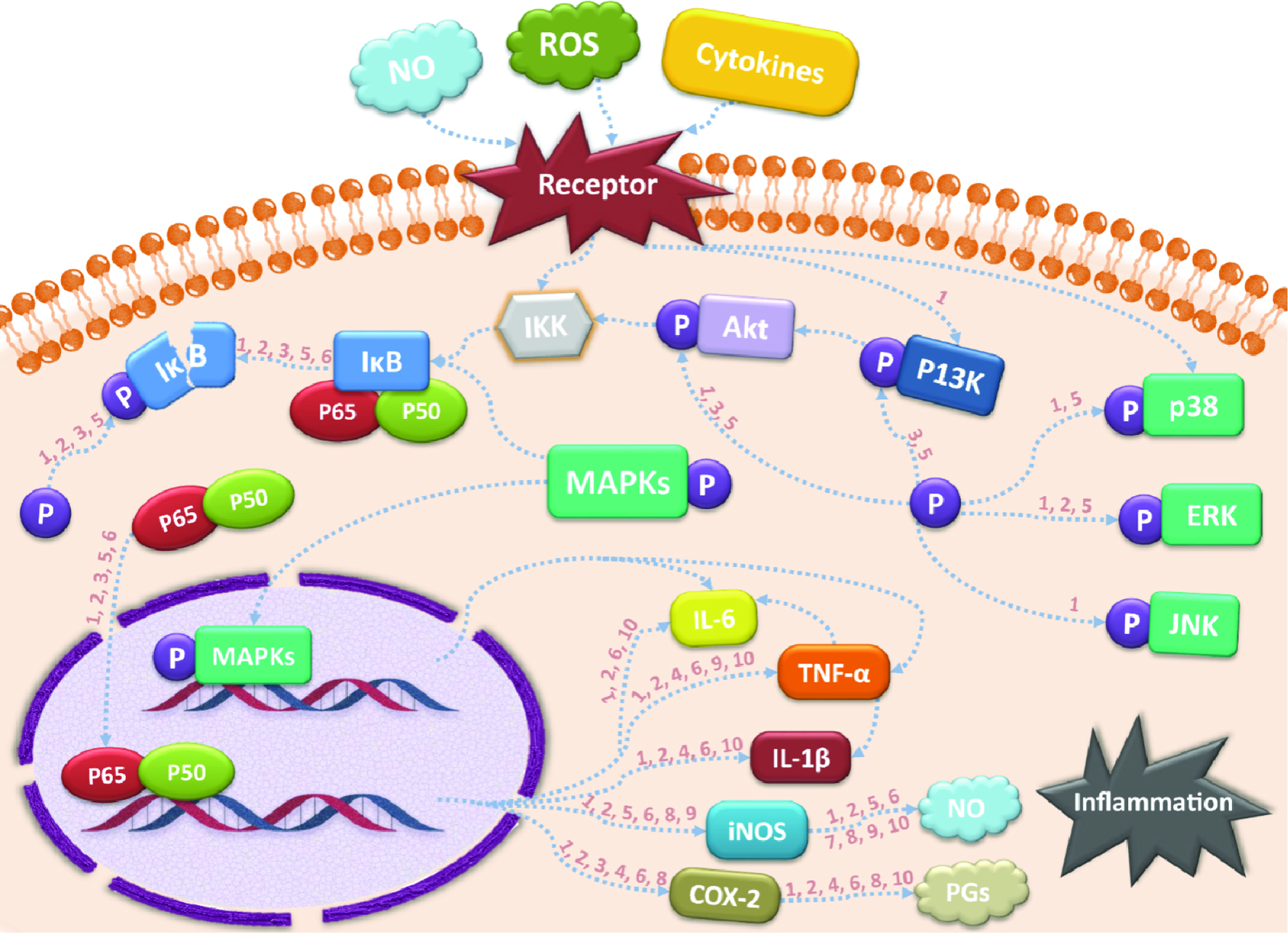
| Nobiletin | Inflammatory bowel disease | Caco-2 cell | 20, 40, 80 μM | 36 hours | MLCK expression, Akt phosphorylation ↓ NF-κB level ↓ | Xiong et al., 2015 |
| | Sprague-Dawley male rats | 20, 40 mg/kg | 7 days | iNOS, COX-2 expression ↓ TNF-α, IL-1, IL-6 level ↓
MLCK, NF-κB activation ↓
PI3K expressions, Akt phosphorylation ↓ | Xiong et al., 2015 |
| | Human intestinal mast cells | 45, 100 μM | 1/4/18 hours | TNF, CXCL8, CCL3, CCL4 expression ↓
ERK1/2 phosphorylation ↓ | Hagenlocher et al., 2017 |
| | IL-10−/− and wild-type mice | 50 mg/kg | 11 weeks | Colitis symptom, mast cells number ↓ | Hagenlocher et al.,2018 |
| | Human intestinal fibroblasts | 5–100 µM | - | IL-6, TNF, CCL2 expression ↓ | Hagenlocher et al.,2018 |
| | RAW 264.7 macrophages | 8–40 μM | 24/48 hours | NO production ↓ | Wu et al., 2017 |
| | AOM-induced male F344 rats | 0.1% in diet | 40 weeks | IL-6, IL-1β, TNF-α levels ↓ COX-2 expression ↓ | Wu et al., 2017 |
| Acute kidney injury | Cisplatin-treated adult male albino Wistar rats | 5 mg/kg | 10 days | Normalize morphological change of tubules
TNF-α expression ↓ | Malik et al., 2015 |
| Acute liver injury | LPS/GalN-treated male C57BL/6 mice | 50, 100, 200 mg/kg | - | IL-1β, IL-6, TNF-α level ↓ iNOS, COX-2 expression ↓
NF-κB activation ↓ Nrf2 and HO-1 expression↑ | He et al., 2016 |
| Acute lung injury | A549 cells | 10−4, 10−3, 10−2 mg/mL | 24 hours | TNF-α, IL-6 production ↓ NF-κB p65, IκBα phosphorylation ↓ | Li et al., 2018 |
| | LPS-treated Kunming mice | 10, 20 mg/kg | 12 hours | TNF-α, IL-6, NO production ↓ iNOS expression ↓
NF-κB p65, IκBα phosphorylation ↓ | Li et al., 2018 |
| Acute gastric lesion | Male Kunming mice | 10, 20 mg/kg, | 3 days | TNF-α, IL-6 level ↓ PEG2 level ↑
p-ERK1/2, p-JNK, p-p38 expression ↓ | Li et al., 2017 |
| Neuroinflammation | BV2 microglial cells | 25–100 µM | 20 hours | NO production ↓ iNOS expression ↓
TNF-α, IL-1β, and IL-6 level ↓ | Ho and Kuo, 2014 |
| | Male Sprague-Dawley rats | 10, 25 mg/kg | 3 days | TNF-α, IL-1β, IL-6, NF-κB, p-Akt level ↓
MMP9 expression ↓ Nrf2, HO-1 expression ↑ | Bi et al., 2016; Zhang et al., 2016b |
| Diabetic Cardiomyopathy | Male C57BL/6 mice | 50 mg/kg | 11 weeks | JNK, p38, NF-κB activation ↓ | Zhang et al., 2016a |
| Obesity-associated chronic inflammation | RAW 264.7 macrophages | 10, 50, 100 µM | 24 hours | TNF-α, NO, MCP-1 production ↓ iNOS, HO-1 expression ↓ | Namkoong et al., 2017 |
| Tangeretin | Inflammatory bowel disease | Male C57BL/6 mice | 10, 20 mg/kg | 3 days | NF-κB, MAPK activation ↓ iNOS, COX-2 expression ↓
TNF-α, IL-12, IL-17, IFN-γ expression ↓ IL-10 expression ↑ | Eun et al., 2017 |
| | Human intestinal mast cells | 45, 100 μM | 1/4/18 hours | IL-1β, TNF-α, CXCL8, CCL3, CCL4 expression ↓
ERK1/2 phosphorylation ↓ | Hagenlocher et al., 2017 |
| Acute liver injury | Cisplatin-treated Wistar rats | 100 mg/kg | 7 days | TNF-α level ↓, IL-10 expression ↑ MAPK activation ↓ | Omar et al., 2016 |
| Chronic kidney disease | Sprague-Dawley male rats | 50, 100, 200 mg/kg | 35 days | p-iNOS, p-IKKα/β, p-IκBα level ↓ p65 expression ↓
NO, IL-1β, IL-6, TNF-α production ↓ | Wu et al., 2018 |
| Acute kidney injury | Cisplatin-treated Wistar rats | 100 mg/kg | 1 week | iNOS, TNF-α level ↓, IL-10, expression ↑ NF-κB activation ↓ | Arab et al., 2016 |
| RSV-induced lung inflammation | Male BALB/c mice | 25, 50, 100 mg/kg | 3 days | IL-1β, IL-6 level ↓ NF-κB activation ↓ | Xu et al., 2015 |
| Neuroinflammation | BV2 microglial cells | 30, 50, 100 µM or 25–100 µM | 1 or 20 hours | AMPK-SIRT1-NF-κB signaling ↓
NO, TNF-α, IL-6, IL-1β level ↓
iNOS, MMP-3, MMP-8 expression ↓ | Ho and Kuo, 2014; Lee et al., 2016 |
| LPS-induced inflammation | RAW 264.7 macrophages | 6–30 μM | 24 hours | NO, PGE2, IL-1β, IL-6 level ↓ iNOS, COX-2 expression ↓ | Funaro et al., 2016 |
| 5-Demethyltangeretin | Skin inflammation | DMBA/TPA-treated female mice | 1, 5 μmol | 0.5 hour | COX-2 expression ↓ NF-κB activation ↓
PI3K, Akt phosphorylation ↓ | Ma et al., 2014 |
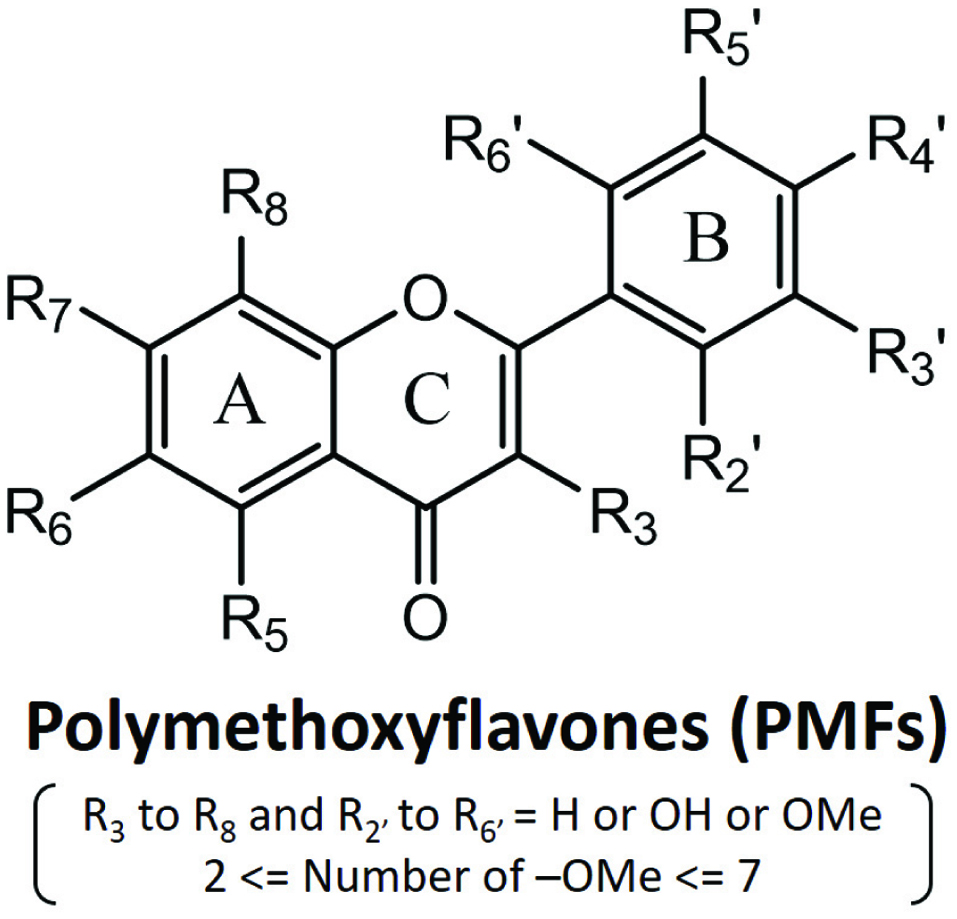
| 3,5,6,7,8,3′,4′-Heptamethoxyflavone | Neuroinflammation | C57BL/6 strain mice | 25, 50 or 100 mg/kg | 5 or 10 days | Reduce hypertrophied form of microglia
IL-1β expression ↓ | Okuyama et al., 2014; Okuyama et al., 2015 |
| LPS-induced inflammation | Human Peripheral blood monocytes | 3.7–100 μM | 0.5 hour | TNF-α (IC50 = 5 μM), MIP-1α (IC50 = 7.3 μM), IL-10 (IC50 = 12.3 μM) production ↓ | Manthey et al., 1999 |
| 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone | Neuroinflammation | BV2 microglial cells | 10, 20, 30 μM | 2 hours | iNOS expression ↓ NO level ↓ NF-κB activation ↓
Nrf2-dependent HO-1 expression ↑ | Kang et al., 2013 |
| Skin inflammation | TPA-treated female ICR mice | 1, 3 μmol | 0.5 hour | iNOS, COX-2 expression ↓ NF-κB activation ↓
STAT3, p38, ERK MAPK, PI3K, Akt phosphorylation ↓ | Lai et al., 2007 |
| Sinensetin | LPS-induced inflammation | RAW 264.7 macrophages
Murine J774 macrophages | 50 μM | 1 hour | TNF-α, PGE2, COX-2, iNOS, NO, IL-Iβ, IL-6 expression ↓ | Laavola et al., 2012; Shin et al., 2012; |
| Pentamethylquercetin | LPS-induced inflammation | Mouse peritoneal macrophages | IC50∼26 μM | 20 hours | NO production ↓ | Matsuda et al., 2003 |
| 5-hydroxy-3,7,3′,4′-tetramethoxyflavone | LPS-induced inflammation | RAW 264.7 macrophages | 100 μM | 5–20 minutes | iNOS, COX-2 expression ↓ | Sae-wong et al., 2009 |
| | RAW 264.7 macrophages | IC50∼16μM | 48 hours | NO, PGE2 production ↓ | Tewtrakul et al., 2009; Tewtrakul and Subhadhirasakul, 2008 |
| Sudachitin | LPS-induced inflammation | RAW 264.7 macrophages | 10, 30 μM | 24 hours | TNF-α, NO production ↓ iNOS expression ↓ | Yuasa et al., 2012 |
| Tetramethyl-O-scutellarin | LPS-induced inflammation | RAW 264.7 macrophages | 25, 50, 100 μM | 24 hours | PGE2, TNF-α, IL-1β, IL-6 level ↓ NO production ↓ | Hyun et al., 2017 |