Figures
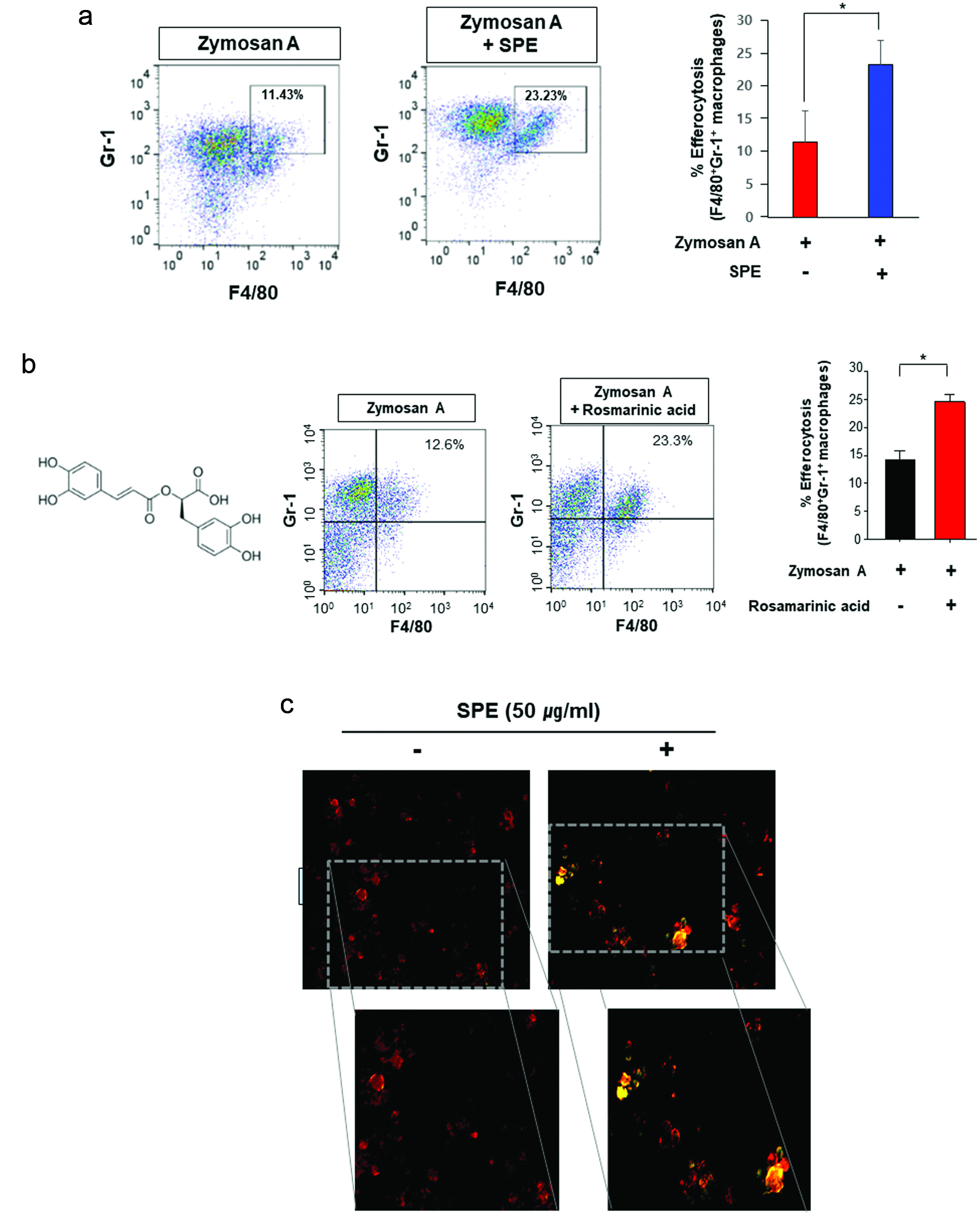
Figure 1. Potentiation of efferocytosis by the perilla extract and its pharmacologically active ingredient, rosmarinic acid in a zymosan A-induced murine peritonitis model. (a) Twelve h after intraperitoneal injection of zymosan A (30 mg/kg), SPE (100 mg/kg) was given intraperitonally to C57Bl/6 mice. Six hours later, peritoneal exudates were collected, and the proportion of macrophages engulfing neutrophils (F4/80+Gr-1+) was measured by flow cytometry as described in Materials and methods. (b) Rosmarinic acid (20 mg/kg) was administered by intraperitoneal injection 12 h after intraperitoneal injection of zymosan A. The experimental conditions for analysis of efferocytosis are same as those described for (a). (c) The thioglycolate-elicited PMs treated with SPE (50 μg/ml) for 4 h were co-incubated with zymosan A-FITC (green) for an additional 1 h. The phagocytic activity of PMs was analyzed by immunostaining using F4/80 antibody (red). The macrophages engulfing zymosan A was visualized under a fluorescence microscope.
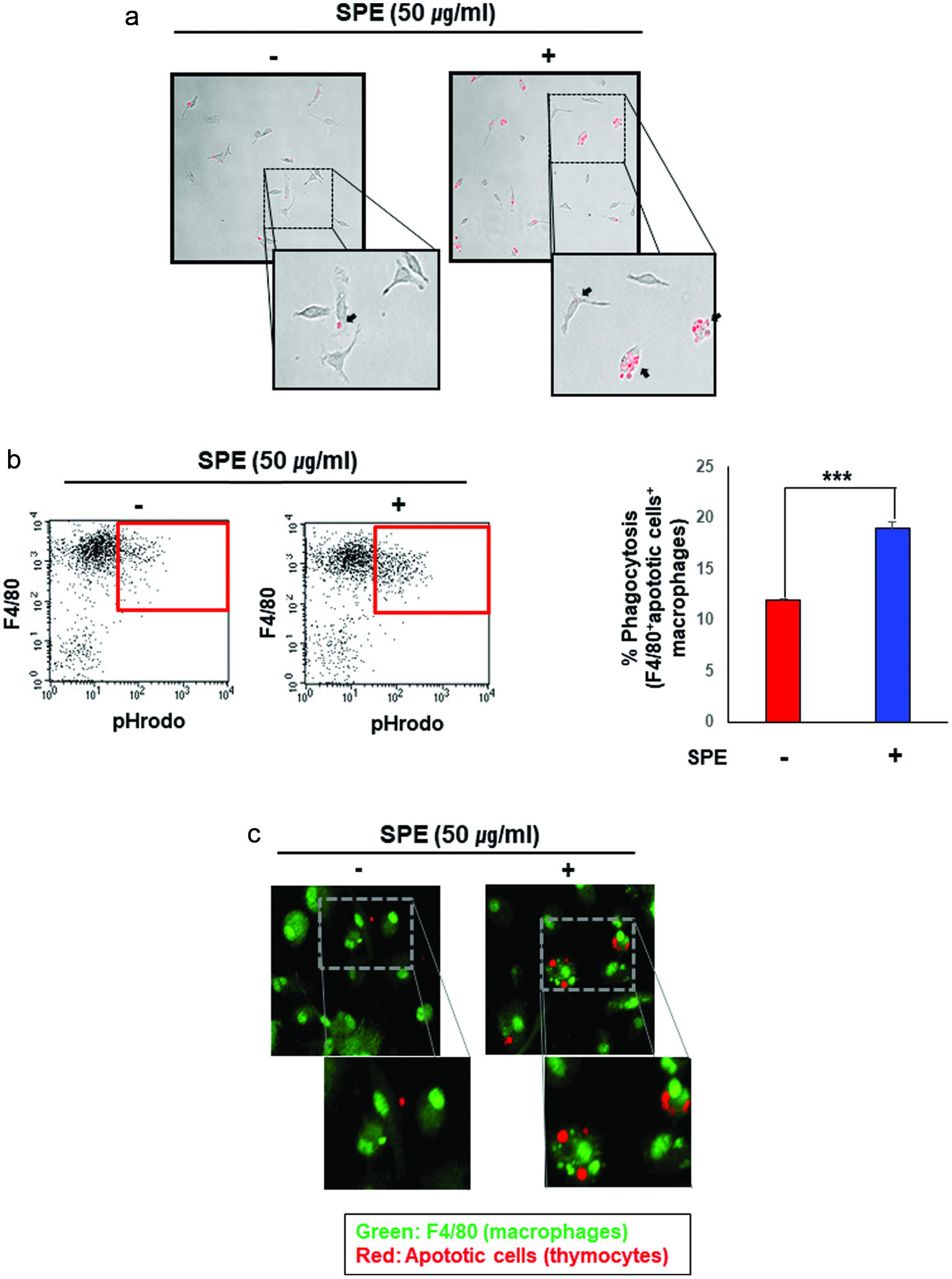
Figure 2. Enhancement of efferocytic activity of macrophages by the perilla extract. (a) The apoptotic thymocytes were prepared as described in Materials and methods. The apoptotic thymocytes labeled with a pH sensitive dye, pHrodo-SE were co-cultured with PMs pretreated for 4 h with SPE or vehicle. After incubation for 1 h, the PMs were washed to remove free apoptotic cells, and subsequently subjected to microscopy. SPE pretreated PMs engulfed apoptotic thymocytes (red puncta) more actively. (b) Macrophages were differentiated from bone marrow cells. BMDMs treated with SPE (50 μg/ml) were co-incubated with pHrodo-labeled apoptotic thymocytes for 4 h. The proportion of macrophages engulfing apoptotic cells was determined by flow cytometry. (c) To confirm the enhanced efferocytic ability of macrophages induced by SPE, BMDMs engulfing apoptotic cells were detected by immunostaining using F4/80 antibody (green; macrophage marker) and pHrodo-labeled apoptotic thymocytes (red).
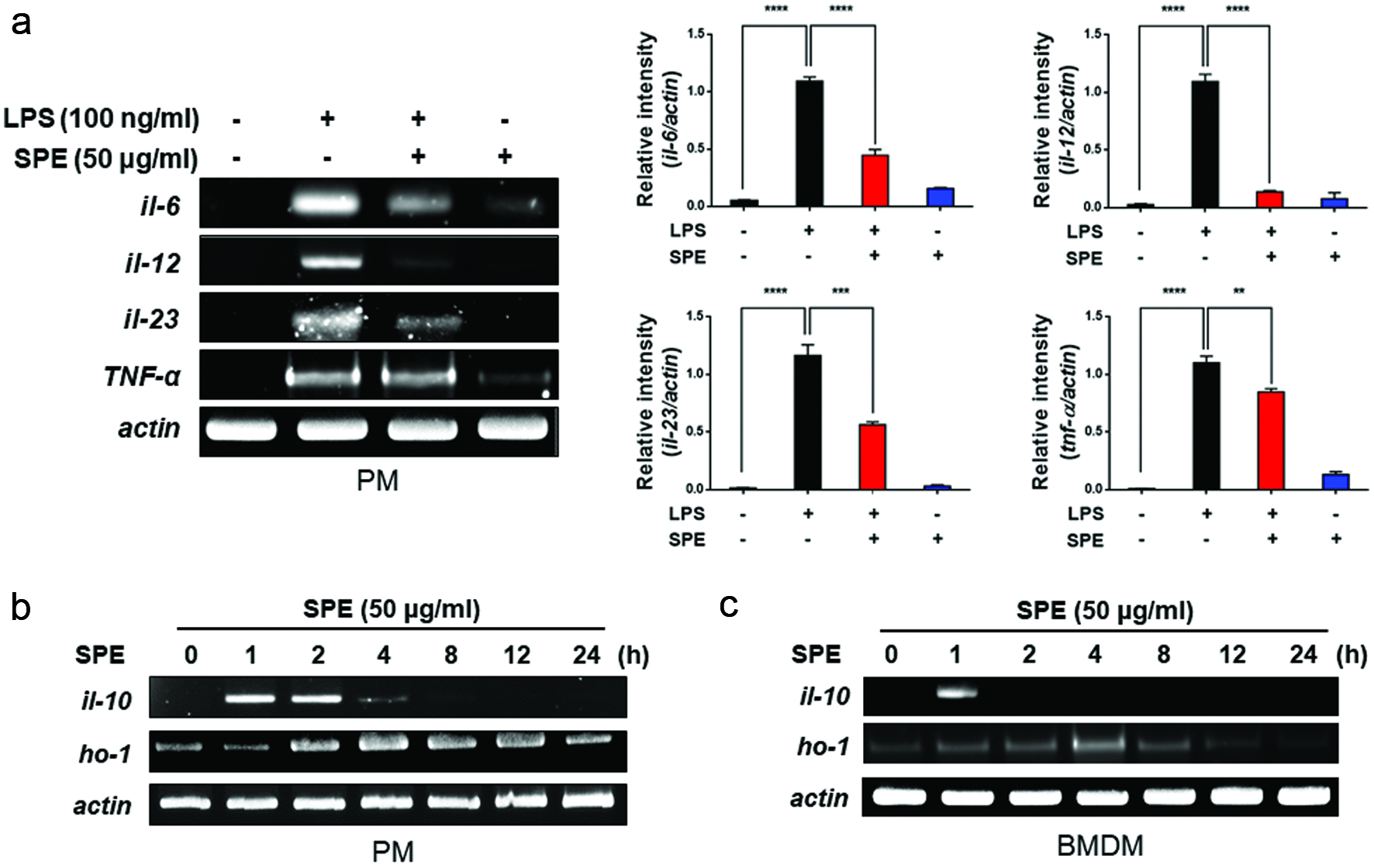
Figure 3. Inhibition of LPS-induced proinflammatory gene expression and induction of anti-inflammatory gene expression by perilla extract. (a) PMs isolated from C57BL/6 mice were treated with SPE (50 μg/ml) for 4 h followed by exposure to LPS (100 ng/ml) for additional 1 h. The expression of pro-inflammatory cytokines in PMs was measured by RT-PCR as described in Materials and methods. Data are expressed as means ± standard deviation. (b) PMs were treated with SPE (50 μg/ml) for the indicated time periods, and mRNA levels of IL-10, HO-1 and Nrf2 were determined by RT-PCR. Data are expressed as means ± standard deviation. (c) After SPE (50 μg/ml) treatment of BMDMs, anti-inflammatory and antioxidant gene expression was determined by RT-PCR
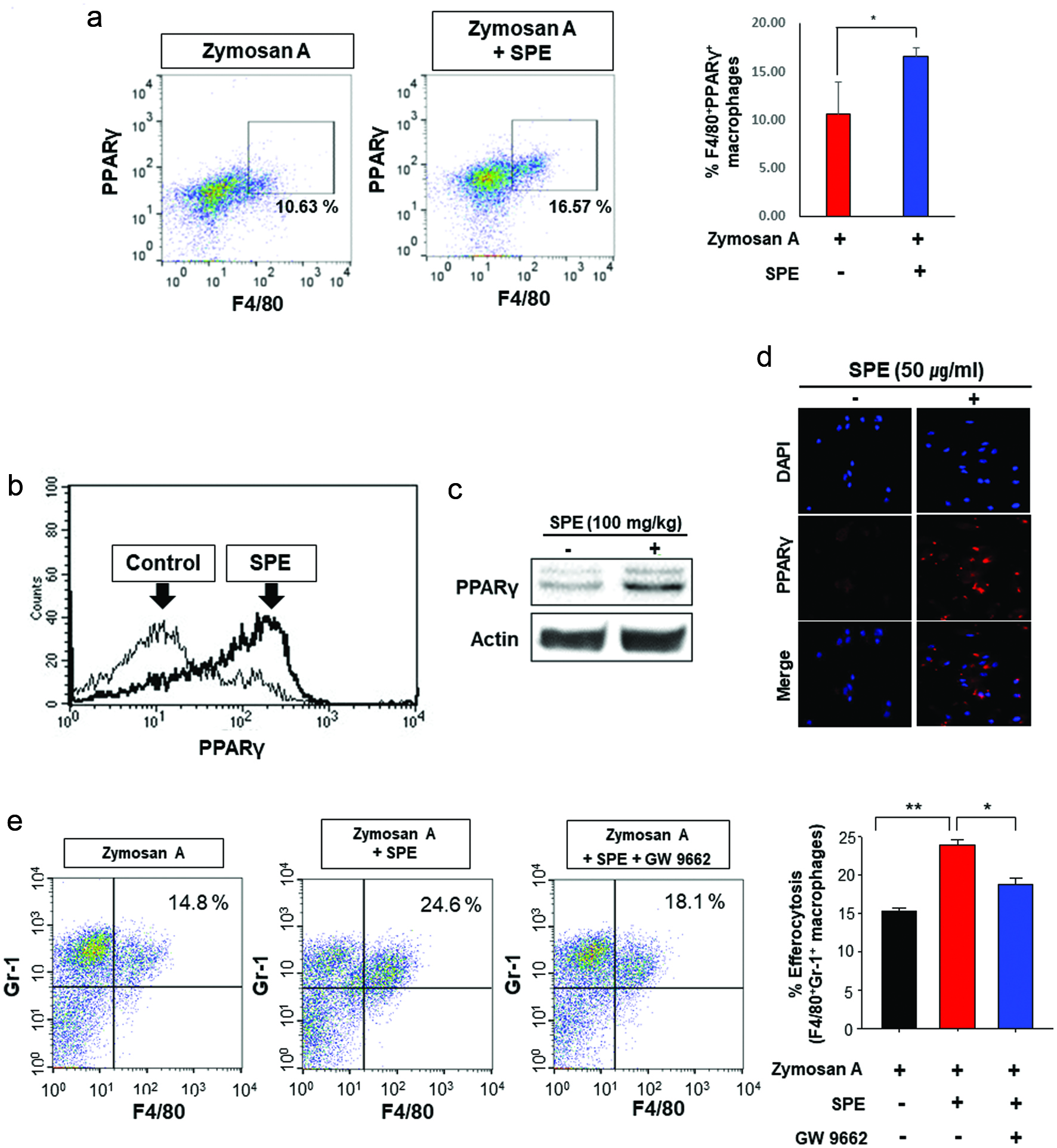
Figure 4. Role of PPARγ in efferocytosis stimulated by perilla extract in the zymosan-induced murine peritonitis model. (a) The PPARγ expression of PMs from mice was measured by flow cytometry. (b, c) Upregulation of PPARγ expression by SPE in PMs. SPE (100 mg/kg) was co-injected into mouse peritoneal cavity with thioglycollate. After 4 days, the PMs were isolated and subjected to flow cytometry (b) and Western blot analysis (c). (d) The thioglycolate-elicited murine PMs were treated with PE (50 µg/ml) for 4 h. The PPARγ expression of macrophages was examined by immunofluorescence staining. (e) Mice injected with zymosan A (30 mg/kg) for 6 h were given a single intraperitoneal injection of vehicle or GW9662 (3 mg/kg) 1 h before SPE (100 mg/kg) treatment. The efferocytotic activity of macrophages was measured by flow cytometry.
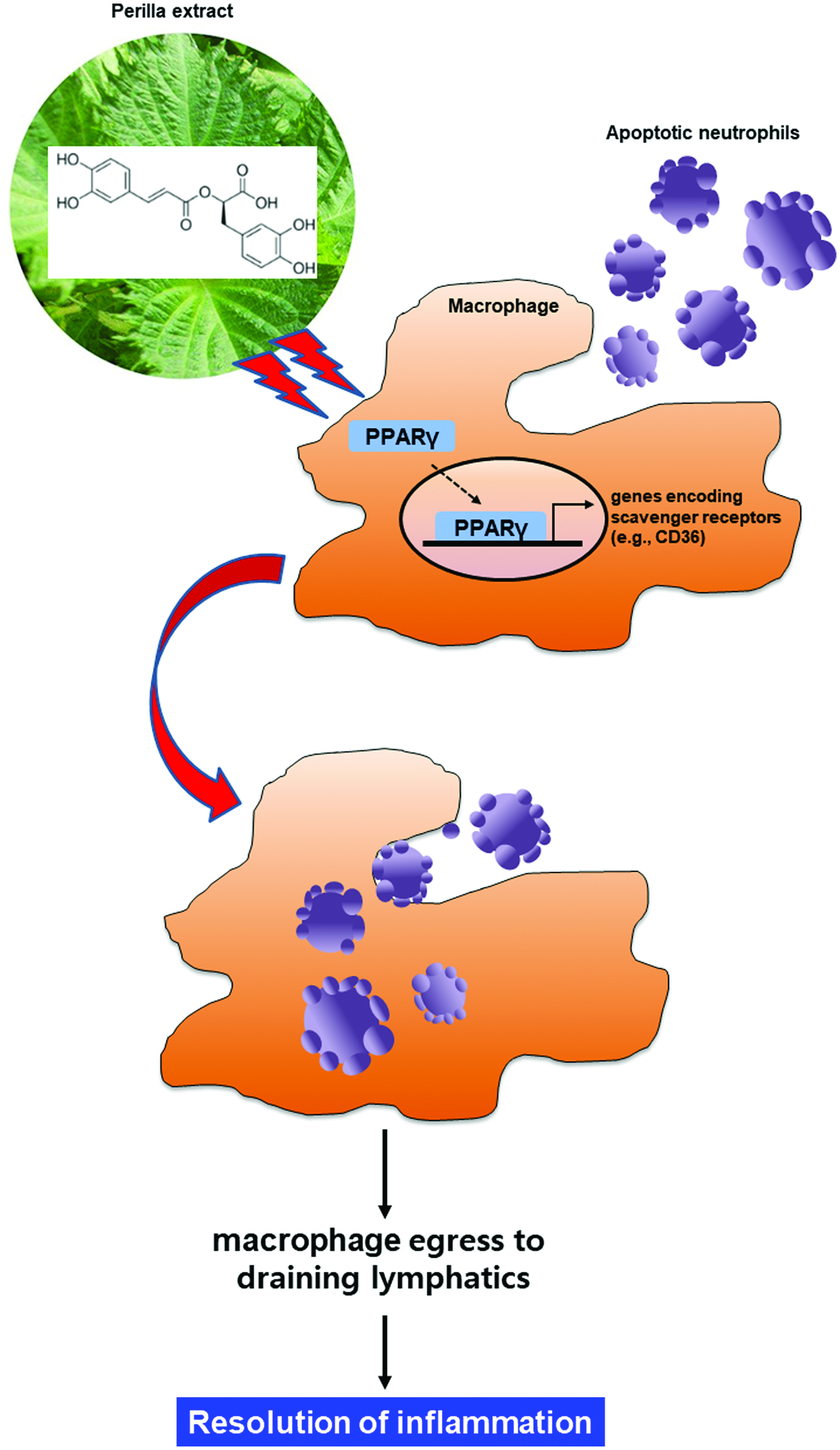
Figure 5. Proposed scheme illustrating the stimulatory effects of perilla extract and its ingredient rosmarinic acid on macrophage-mediated phagocytic removal of apoptotic neutrophils (efferocytosis). SPE activates the PPARγ in macrophages, which upregulates the expression of CD36, a scavenger receptor involved in the recognition of phosphatidylserine epitopes exposed on the surface of apoptotic cells. This will facilitate the phagocytic removal of dying neutrophils, thereby completing the resolution of acute inflammation.




