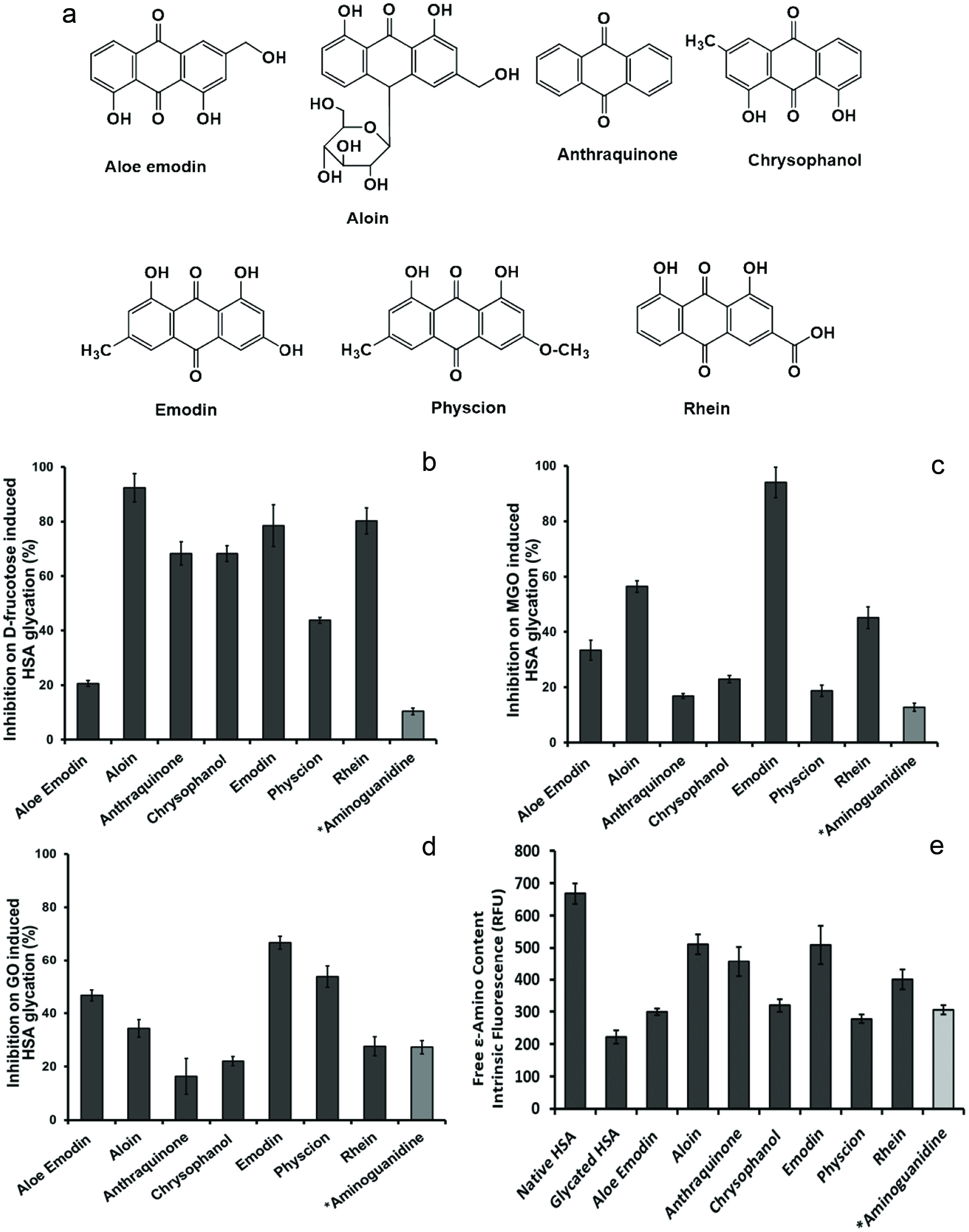
Chemical structures of anthraquinone derivatives (a). The intrinsic fluorescence characterization of effects of the anthraquinones (at 100 µM) on the formation of HSA AGEs with different inducers including fructose (b), methylglyoxal (MGO) (c), and glyoxal (GO) (d). The inhibitory effects of the anthraquinones (at 100 µM) on HSA side chain modification induced by fructose (e). All data points represent the average of triplicate measurements with the bars at each point representing the respective standard derivation. *Aminoguanidine, AG (100 µM), served as a positive control.