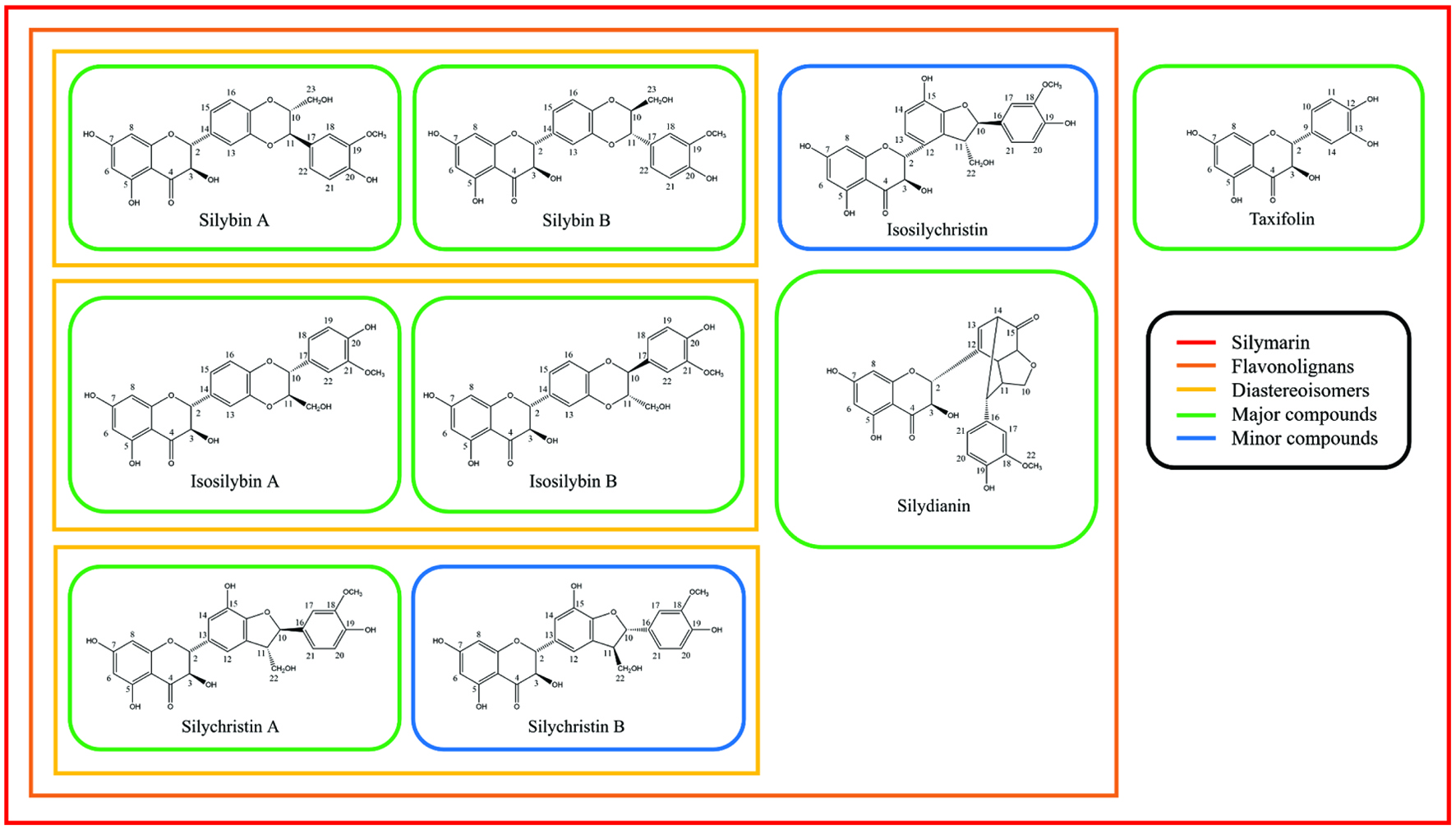
Figure 1. Chemical components of silymarin and their chemical structures.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 10, June 2020, pages 53-63
Total phenolic content, free radical scavenging capacity, and anti-cancer activity of silymarin
Figure

Tables
| Source/Extraction method/Solvent | Extract concentration | Total phenolic content | Reference |
|---|---|---|---|
| Abbreviation: GAE, Gallic acid equivalents. | |||
| Silymarin extract from Darou-Pakhs pharmaceutical company | – | 0.484 ± 0.017 mg GAE/mg of silymarin | Asghar and Masood, 2008 |
| Silymarin powder from Sigma Aldrich | 1, 10, 25, 50, and 100 mg/mL | 560.2 ± 19.5, 6055.2 ± 214, 7255.3 ± 308.6, 13,016.0 ± 538.9, and 23,088.8 ± 1730.3 mg GAE/mg of silymarin powder | Malekinejad et al., 2012 |
| Milk thistle seed/Stirring/Methanol | 1 g/ 100 mL | 18.33 ± 0.16 mg GAE/g of milk thistle seed | Tupe et al., 2013 |
| Milk thistle seed/Soxhlet/Ethanol | 0.5 g of milk thistle seeds/mL | 45.31 mg GAE/g of dry weight milk thistle seed | Ismaili et al., 2016 |
| Milk thistle seed/Shaking/Methanol | 0.25 mg/mL | 29 mg GAE/g dry weight | Mhamdi et al., 2016 |
| Silymarin extract from Berlin pharmaceutical industry/Maceration/Ethanol | – | 233.37 ± 7.53 mg GAE/g of extract | Pientaweeratch et al., 2016 |
| Milk thistle seed | – | 392.1 ± 5.6 mg GAE/100 g | Attia et al., 2017 |
| Source/Extraction method/Solvent | Extract concentration | Experimental methods | Free radical scavenging capacities | Reference |
|---|---|---|---|---|
| Abbreviations: DPPH, 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity assay; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging capacity assay; ORAC, Oxygen radical absorbing capacity assay; HOSC, Hydroxyl radical scavenging capacity assay; TE, Trolox equivalents; FE, Flour equivalents. | ||||
| Silymarin extract purchased from Darou-Pakhs pharmaceutical company | – | DPPH | 1.34 mg/mL (EC50) | Asghar and Masood, 2008 |
| Milk thistle seed powder/Boiling/Water | 30 µg of silymarin/mL | HOSC | 78.5% | Köksal et al., 2009 |
| DPPH | 20.8 µg/mL (EC50) | |||
| ABTS | 8.62 µg/mL (EC50) | |||
| Silymarin powder from Sigma Aldrich | 1, 10, 25, 50, and 100 mg/mL | DPPH | 6.0 ± 0.7, 14.1 ± 1.8, 25.7 ± 3.3, 39.7 ± 2.4, and 54.2 ± 5.6% | Malekinejad et al., 2012 |
| Milk thistle seed/Stirring/Methanol | 1 mg/mL | DPPH | 92.45 ± 1.91% | Tupe et al., 2013 |
| ABTS | 53.44 ± 0.71% | |||
| Milk thistle/Soxhlet/Ethanol | 0.5 g of milk thistle seeds/mL | DPPH | 353.89 ± 3.68 mg TE/g dry weight | Ismaili et al., 2016 |
| ABTS | 805.25 ± 16.90 mg TE/g dry weight | |||
| Silymarin extract from Berlin pharmaceutical industry/Maceration/Ethanol | – | DPPH | 27.85 ± 0.98 µg/mL (EC50) | Pientaweeratch et al., 2016 |
| ABTS | 12.34 ± 0.21 µg/mL (EC50) | |||
| Milk thistle seed/Shaking/Methanol | 0.25 mg/mL | DPPH | 39 µg/mL (IC50) | Mhamdi et al., 2016 |
| Milk thistle seed flour/Sonication/50% acetone | 200 mg FE/mL | DPPH | 48.61 ± 6.47 µmol TE/g | Choe et al., 2019 |
| 43 mg FE/mL | ORAC | 633.57 ± 267.17 µmol TE/g | ||
| 40 mg FE/mL | HOSC | 10,420.28 ± 607.58 µmol TE/g | ||
| 30 mg FE/mL | ABTS | 116.16 ± 16.81 µmol TE/g | ||
| Cancer | Experiment subject | Compound used | Reference |
|---|---|---|---|
| Bladder | N-buthyl-N-(4-hydroxybuthyl) nitrosamine (OH-BBN) induced bladder cancer in mice | Silibinin | Tyagi et al., 2007 |
| T24, and UM-UC-3 bladder cancer cell lines | Silibinin | Imai-sumida et al., 2017 | |
| T24 and J82 bladder cancer cell lines and their chemoresistant bladder cancer cell lines, T24R and J82R | Silibinin | Sun et al., 2017 | |
| Breast | MDA-MB 468 breast cancer cell line | Silymarin | Zi et al., 1998 |
| MCF-7 breast cancer cell line | Silibinin | Noh et al., 2011 | |
| MCF-7 breast cancer cell line | Silymarin | Kalla et al., 2014 | |
| Colon | Azoxymethane (AOM) induced colon cancer in rat | Silymarin | Kohno et al., 2002 |
| Fet, Geo, and HCT-116 colon cancer cell lines | Silibinin | Hogan et al., 2006 | |
| HT-29 colon cancer cell line | Silibinin | Akhtar et al., 2014 | |
| HCT-116 and SW480 colon cancer cell lines | Silymarin | Eo et al., 2015 | |
| Gastric | Matrix metallopeptidase 9 (MMP-9) expression levels in SNU216 and SNU688 gastric cancer cell lines induced with tumor necrosis factor alpha (TNF-α) | Silibinin | Kim et al., 2009 |
| MGC803 gastric cancer cell line | Silibinin | Wang et al., 2014 | |
| Kidney | Caki-1 renal carcinoma cell line | Silibinin | Li et al., 2008 |
| ACHN, OS-RC-2, and SW839 renal carcinoma cell lines | Silibinin | Liang et al., 2012 | |
| Lung | SHP-77 and A-549 lung cancer cell lines | Silibinin | Sharma et al., 2003 |
| H1299, H460, and H322 lung cancer cell lines | Silibinin | Mateen et al., 2010 | |
| A549, H1299, and H460 lung cancer cell lines | Silymarin | Singh et al., 2016 | |
| Oral | HSC-4, YD15, and Ca9-22 oral cancer cell lines and HSC-4 injected xenograft model in rats | Silymarin | Won et al., 2018 |
| Ovarian | A2780 ovarian cancer cell line | Silibinin | Scambia et al., 1996 |
| A2780 and PA-1 ovarian cancer cell lines | Silymarin | Fan et al., 2014 | |
| Prostate | DU145 prostate cancer cell line | Silymarin | Zi et al., 1998 |
| LNCaP prostate cancer cell line | Silibinin | Zi and Agarwal, 1999 | |
| PC-3 prostate cancer cell line | Silibinin | Zi et al., 2000 | |
| LNCaP prostate cancer cell line | Silibinin | Zhu, 2001 | |
| DU145 prostate cancer cell line | Silymarin | Bhatia and Agarwal, 2001 | |
| LNCaP and DU145 prostate cancer cell lines | Silymarin | Sharma et al., 2001 | |
| DU145 prostate cancer cell line | Silibinin | Dhanalakshmi et al., 2002 | |
| DU145 prostate cancer cell line | Silibinin and Doxorubicin | Tyagi et al., 2002 | |
| DU145 xenograft model in mice | Silibinin | Singh et al., 2002 | |
| DU145 prostate cancer cell line | Silibinin and cisplatin | Dhanalakshmi et al., 2003 | |
| LNCaP, DU145, and PC-3 prostate cancer cell lines | Each flavonolignan found in silymarin | Davis-Searles et al., 2005 | |
| LNCaP and 22Rv1 prostate cancer cell lines | Isosilybin A and isosilybin B | Deep et al., 2007 | |
| ARCaP prostate cancer cell line | Silibinin | Wu et al., 2010 | |
| Skin | UVB and 7, 12-dimethylbenz[a]anthracene-induced tumor in mouse skin model | Silymarin | Kativar et al., 1997 |
| A431 human epidermoid carcinoma cell line | Silymarin | Ahmad et al., 1998 | |
| SKH-1 hairless mice | Silibinin | Dhanalakshmi et al., 2004 | |
| JB6 mouse epithelial cell model | Silibinin | Singh et al., 2006 |