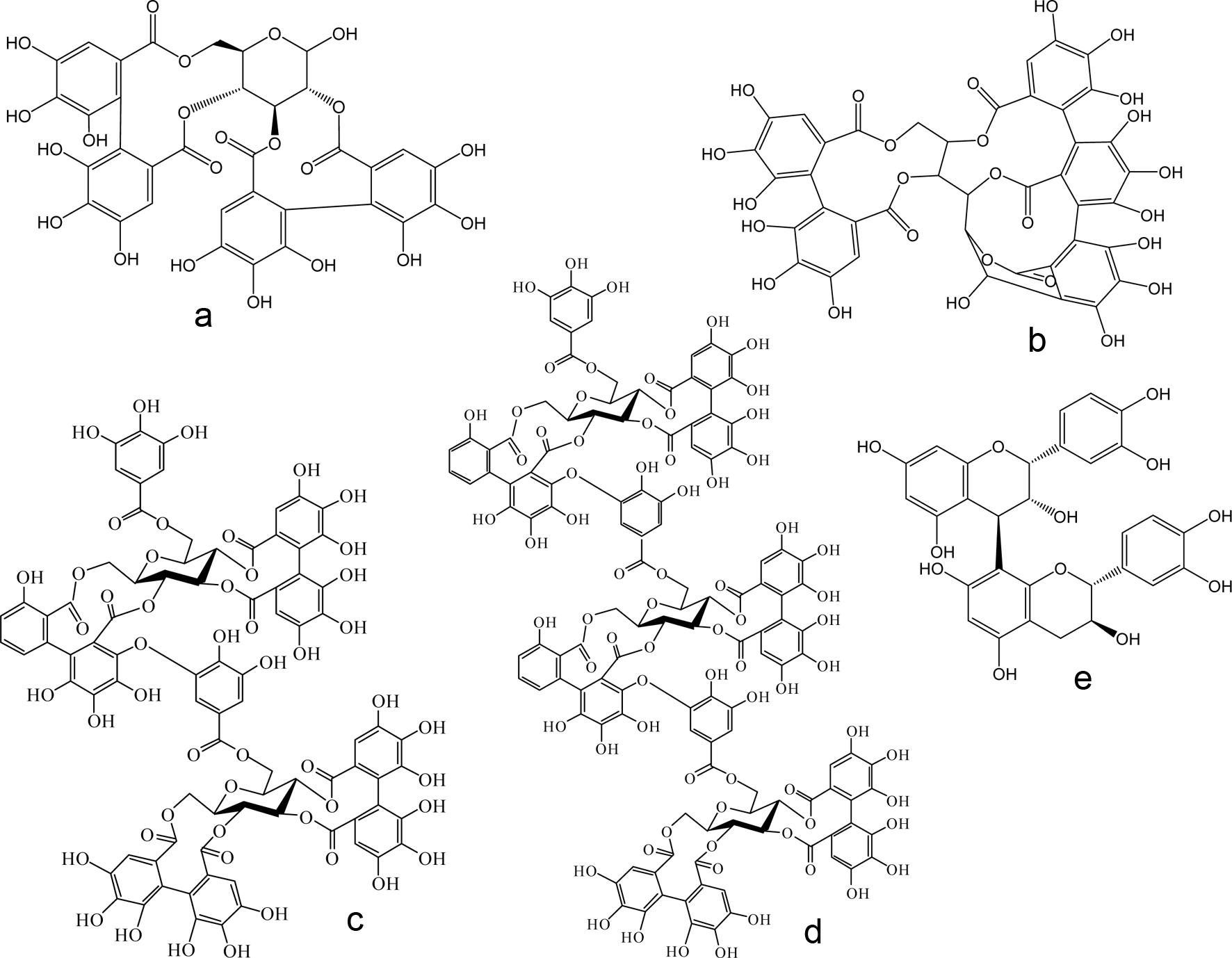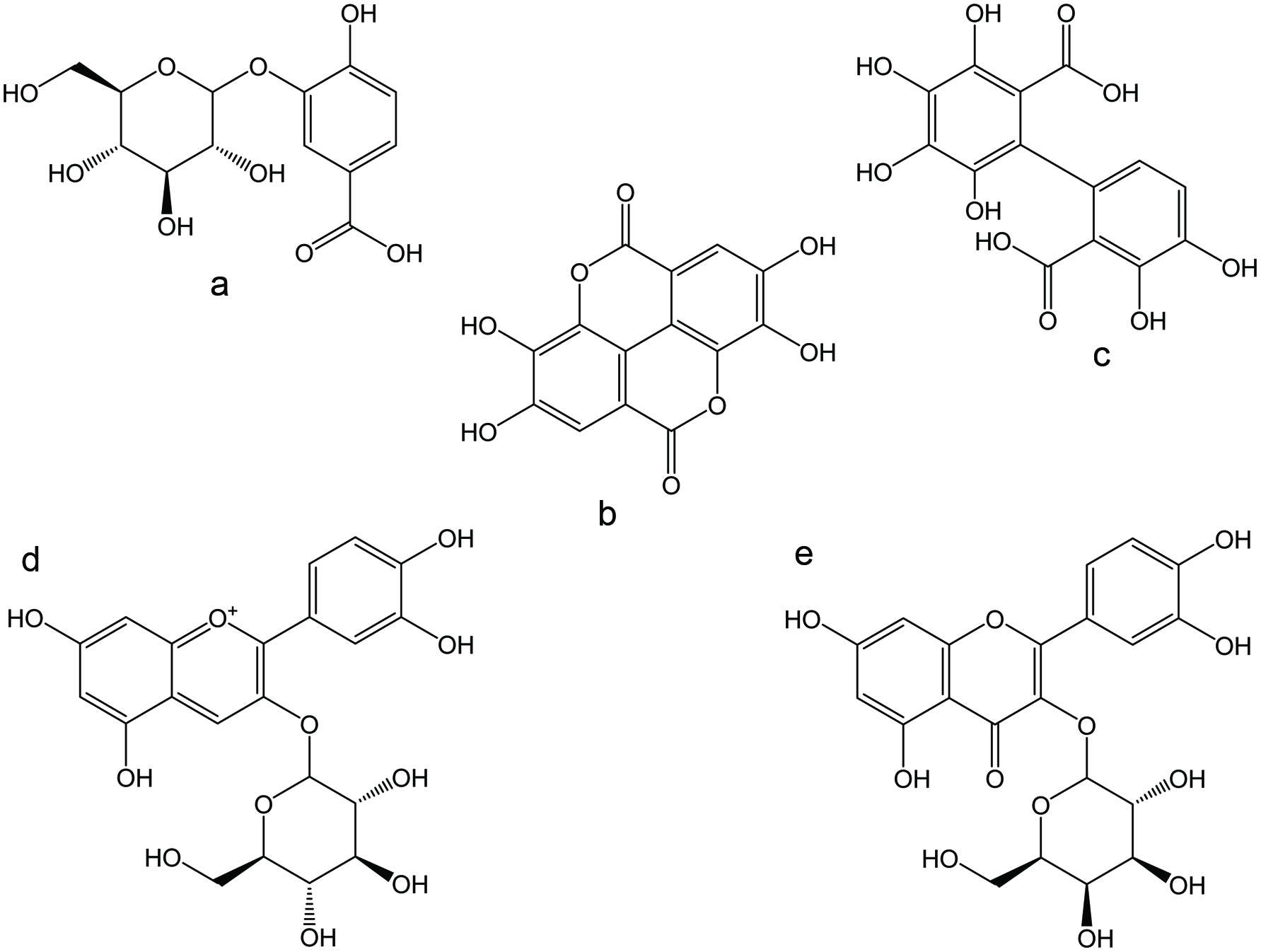
Figure 1. Chemical structures of selected non-tannin polyphenolic antioxidants reported in blackberries. (a) protocatechuic acid glucoside; (b) ellagic acid; (c) hexahydroxydiphenic acid (HHDP); (d) cyanidin-3-O-glucoside (C3G); and (e) quercetin-3-O-galactoside.