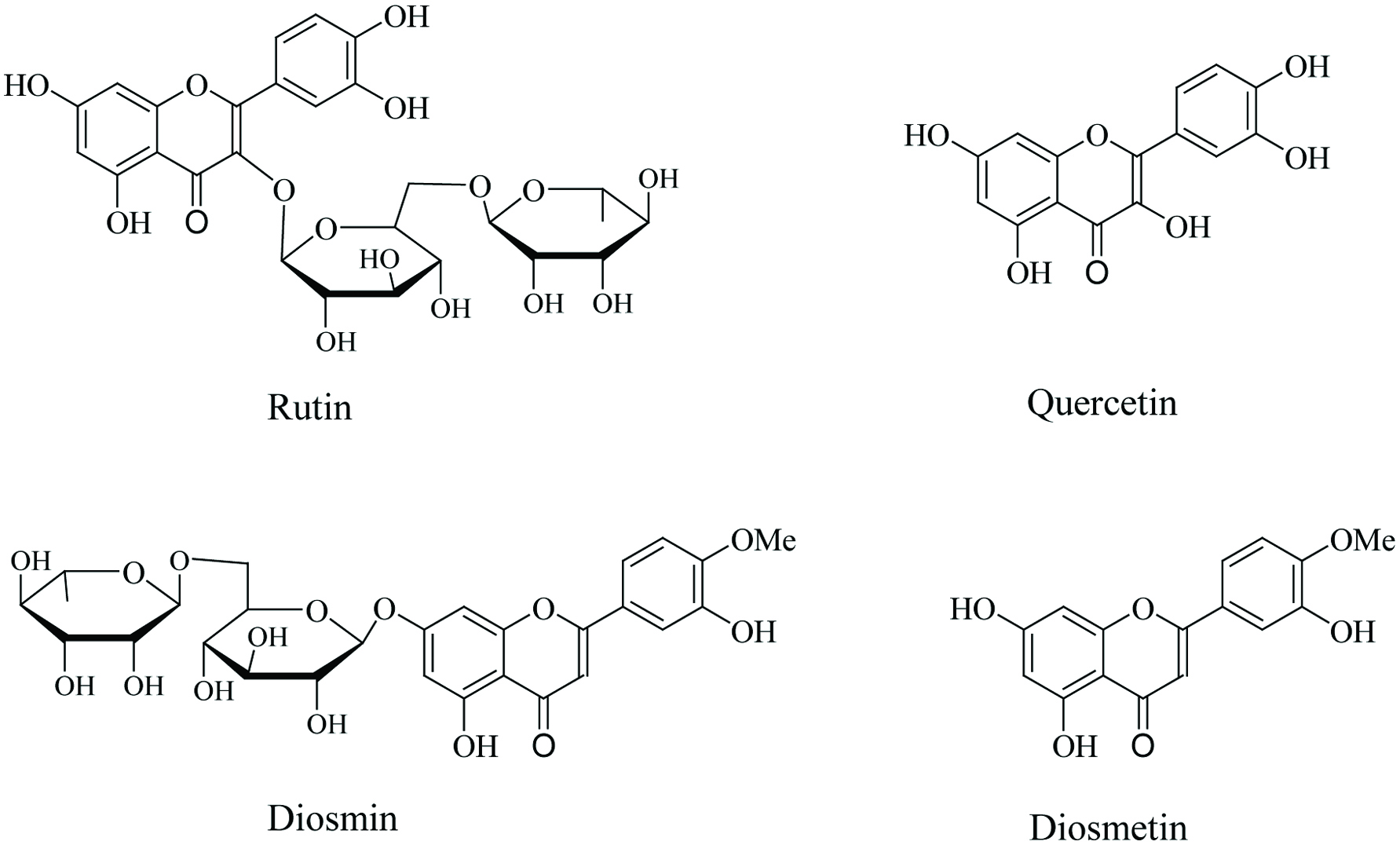
Figure 1. Chemical structures of rutin and diosmin and their aglycones.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 5, March 2019, pages 43-56
The role of rutin and diosmin, two citrus polyhydroxyflavones in disease prevention and treatment
Figure

Table
| Disease | Mechanism | Experimental model | Reference |
|---|---|---|---|
| Rutin | |||
| Cancer | Rutin decreased formation of focal areas of dysplasia via increased apoptosis in colonic crypts | Azoxymethane-induced colon cancer mouse model | (Yang et al., 2000) |
| Rutin increased apoptosis and expression of TNF-α and glycogen synthase kinase-3β | Human lung A549 carcinoma cells | (Wu et al., 2017) | |
| Rutin decreased tumor volume and CEA levels; exerted antioxidant action in vivo and induced apoptosis in MCF-7 and Panc-1 cells | Ehrlich ascites breast cancer mouse model; human breast (MCF-7) and prostate (PANC-1) carcinoma cells | (Saleh et al., 2019) | |
| Rutin inhibited leukemia tumor growth | Leukemia HL-60 xenograft mouse model | (Lin et al., 2012) | |
| Rutin induced apoptosis via mitochondria-mediated pathways through increased Bax/Bcl-2 ratio and activation of caspase-3, -8, -9 and PARP | Human colon cancer HT-29 cells | (Guon and Chung, 2016) | |
| Rutin induced apoptosis via increase of Bax/Bcl-2 ratio and inhibition of TNF-α expression and secretion | Human neuroblastoma LAN-5 cells | (Chen et al., 2013a) | |
| Neurodegenerative disease | Rutin improved memory; decreased oligomeric β-amyloid levels, lipid peroxidation, IL-1β and IL-6; increased antioxidant enzymes and GSH | Alzheimer’s disease mouse model | (Xu et al., 2014) |
| Rutin prevented cognitive deficits and morphological changes in hippocampus; attenuated lipid peroxidation, COX-2, GFAP, IL-8, iNOS and NFκB | Rat model of sporadic dementia | (Javed et al., 2012) | |
| Rutin prevented memory deficits and ameliorated oxidative stress, apoptosis and neurite growth | Rat model for cognitive dysfunction | (Ramalingayya et al., 2017) | |
| Rutin prevented apoptosis via decreased oxidative stress, Bax/Bcl-2, caspase-3 and -9 and c-Jun and p38 phosphorylation | Dopaminergic cell model | (Park et al., 2014) | |
| Rutin decreased oxidative stress and lipid peroxidation by increase of antioxidant enzyme activities and decrease of TNF-α and IL-1β | Alzheimer’s disease cell model | (Wang et al., 2012) | |
| Rutin improved memory; decreased oxidative stress, lipid peroxidation and GFAP; increased antioxidant enzyme and acetylcholine esterase activities | Huntington’s disease rat model | (Suganya and Sumathi, 2017) | |
| Rutin upregulated antiapoptotic and genes relevant in dopamine biosynthesis; decreased caspase-3 and -9 | Parkinson’s disease cell model | (Magalingam et al., 2015) | |
| Rutin improved cognitive deficits and reversed β -secretase, p-STAT3 and post-synaptic density protein 95 to normal levels | High fat diet rat model | (Cheng et al., 2016) | |
| Cardiovascular disease | Rutin induced cardiovascular protection against oxidative stress and apoptosis via decreased Bax/Bcl-2, caspase-3, TNF-α and IL-6 | Streptozotocin-induced diabetic rat model | (Wang et al., 2015) |
| Rutin showed effects against atherosclerosis by lowering triacylglycerols and cholesterol | Hyperlipidemia rat model | (Santos et al., 1999) | |
| Rutin lowered triacylglycerols but had no effect on total cholesterol and HDL levels | Hypercholesterolemia hamster model | (Kanashiro et al., 2009) | |
| Rutin suppressed mitochondrial-mediated apoptosis via decrease of oxidative stress | Endothelial cell model | (Gong et al., 2010) | |
| Diabetes | Rutin decreased reactive oxygen species, advanced glycation end-product precursors, and inflammatory cytokines; increase of tissue glucose uptake, stimulation of insulin secretion | Obese rat models | (Ghorbani, 2017) |
| Rutin decreased hepatic triacylglycerol, total cholesterol and body fat; decreased oxidative stress via improved antioxidant enzyme activities | High fat diet rat model | (Hsu et al., 2009) | |
| Rutin protected against diabetic cardiomyopathy by decreasing oxidative stress and apoptosis via decreased Bax/Bcl-2, caspase-3, TNF-α and IL-6 | Streptozotocin-induced diabetic rat model | (Wang et al., 2015) | |
| Quercetin | |||
| Cancer | Quercetin decreased formation of aberrant crypt foci which correlated with induction of mitochondrial-mediated apoptosis | Azoxymethane-induced colon cancer rat model | (Volate et al., 2005) |
| Quercetin showed preventive effects against hepatic cancer via a decrease of oxidative stress affecting p53 | N-Nitrosodiethylamine-induced rat hepatocellular carcinoma model | (Seufi et al., 2009) | |
| Quercetin induced apoptosis via Increased cytochrome c release, up-regulation of Bax and activation of caspase-3 | Human lung NCI-H209 carcinoma cells | (Yang et al., 2006) | |
| Quercetin induced apoptosis through mitochondria-mediated pathways via increase of Bax, AIF, caspase-3-, -8, and -9 | Human breast MDA-MB-231 carcinoma cells | (Chien et al., 2009) | |
| Quercetin induced apoptosis via caspase-3 activation and survivin expression | Human renal adenocarcinoma cell line | (Han and Zhang, 2016) | |
| Quercetin induced tumor regression in mice; induced apoptosis in tumor tissues and cancer cell lines via mitochondria-mediated pathways | Mouse model for breast adenocarcinoma and different leukemic and breast cancer cell lines | (Srivastava et al., 2016) | |
| Quercetin induced mitochondria-mediated apoptosis via caspase-3-, -8, and -9 in HL-60 cells and reduced tumor growth in xenografts through ERK activation | Human HL-60 leukemia cells and xenograft mouse model | (Lee et al., 2015) | |
| Quercetin induced apoptosis via inactivation of NFκB and activation of the AP-1/JNK pathway | Human HepG2 hepatoma cells | (Granado-Serrano et al., 2010) | |
| Quercetin induced apoptosis via activation of the apoptosis signal-regulating kinase (ASK-1) and p38 pathway | Human laryngeal squamous carcinoma cells | (Lee et al., 2010) | |
| Neurodegenerative disease | Quercetin attenuated β-amyloid induced lipid peroxidation, protein oxidation and apoptosis in neurons | Alzheimer’s disease cell model | (Ansari et al., 2009) |
| Quercetin decreased expression of IL-1β, IL-4, IL-6 and TNF-α and apoptosis in brain tissue | Rat model of intracerebral hemorrhage | (Zhang et al., 2015) | |
| Quercetin attenuated mitochondrial-mediated apoptosis by a decrease of oxidative stress, cytochrome c translocation, Bax/Bcl-2 ratio, p53 and caspase-3 | Rat model for neurodegeneration | (Sharma et al., 2016) | |
| Quercetin ameliorated β-amyloid induced learning and memory deficits and reduced scattered senile plaques and mitochondrial dysfunction; increased AMPK activity in hippocampus | Mouse model for Alzheimer’s disease | (Wang et al., 2014) | |
| Quercetin inhibited okadaic acid-induced inflammasome activation leading to attenuated tau phosphorylation in neuroblastoma cells; increased AMPK activity and improved cognitive disorder paralleled with a decrease in tau phosphorylation in mice exposed to high fat diets | Cell and mouse model for Alzheimer’s disease | (Chen et al., 2016a) | |
| Quercetin protected against hydrogen peroxide and to a lesser degree against β-amyloid induced neurotoxicity by preventing mitochondrial dysfunction in hippocampal neurons | Mouse model for Alzheimer’s disease | (Godoy et al., 2017) | |
| Cardiovascular disease | Quercetin inhibited apoptosis by suppressing of oxidative stress via NO-guanylyl cyclase pathway | Endothelial cell models | (Perez-Vizcaino et al., 2006) |
| Quercetin reduced activation of NFκB via iκB stabilization and decreased ERK and p38 phosphorylation | LPS-stimulated RAW 264.7 macrophages | (Cho et al., 2003) | |
| Quercetin inhibited doxorubicin-induced apoptosis | Rat H9C2 cardiomyocytes | (Chen et al., 2013b) | |
| Diabetes | Quercetin decreased weight of whole body, liver and adipose tissue; attenuated lipid peroxidation, cholesterol, triglycerides via altered expression profiles of several lipid metabolism-related genes | High fat diet rat model | (Jung et al., 2013) |
| Quercetin decreased plasma levels of glucose, triacylglycerols, cholesterol and TBARS; increased plasma HDL, adiponectin and activities of antioxidant enzymes | Obese type 2 diabetes mouse model | (Jeong et al., 2012) | |
| Quercetin attenuated adipogenesis via decreased expression of adipogenesis-related factors and enzymes through AMPK signaling; induced apoptosis via decreased ERK and JNK phosphorylation | 3T3-L1 preadipocyte model | (Ahn et al., 2008) | |
| Diosmin and Diosmetin | |||
| Cancer | Diosmin induced genotoxicity and apoptosis via generation of oxidative stress | Human DU145 prostate carcinoma cells | (Lewinska et al., 2015) |
| Diosmin inhibited inflammatory markers (e.g., TNF-α, COX-2), oxidative stress and caspase-3 expression | Acetic acid-induced ulcerative colitis rat model | (Shalkami et al., 2018) | |
| Diosmin reduced oxidative stress via decreased expression of cell proliferation biomarkers and declined incidence of squamous cell and esophageal carcinoma | N-methyl-N-amylnitrosamine-induced esophageal carcinogenesis rat model | (Tanaka et al., 1997a) | |
| Diosmin reduced incidence and multiplicity of adenocarcinoma and aberrant crypt foci as well as cell proliferation biomarkers declined | Azoxymethane-induced colon carcinogenesis rat model | (Tanaka et al., 1997b) | |
| Diosmin reduced the frequency of tongue carcinoma cell proliferation biomarkers | 4-nitroquinoline 1-oxide-induced oral carcinogenesis rat model | (Tanaka et al., 1997c) | |
| Diosmin reduced bladder lesions, cell-proliferation activity and frequency of bladder carcinoma and preneoplasia | N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary-bladder carcinogenesis mouse model | (Yang et al., 1997) | |
| Diosmin and diosmetin worked as agonist of the hydrocarbon receptor; only diosmetin inhibited carcinogen activation via decreased CYP1A1 enzyme activity | Human MCF-7 breast epithelial carcinoma cells | (Ciolino et al., 1998) | |
| Neurodegenerative disease | Diosmin alleviated neurological deficits; upregulated the expression of pJAK2, pSTAT3 and Bcl-2 and downregulated Bax | Mouse cerebral ischemia/reperfusion model | (Liu et al., 2014) |
| Diosmin reduced cognitive impairment; decreased γ-secretase activity, β-amyloid generation and tau hyperphosphorylation | Alzheimer’s disease mouse model | (Sawmiller et al., 2016) | |
| Cardiovascular disease | Diosmin improved cardiac functional recovery, antioxidant enzyme activities and Bcl-2 expression; lowered lipid peroxidation | Ischemia/reperfusion ex vivo heart rat model | (Senthamizhselvan et al., 2014) |
| Diosmin lowered hypertension and related biomarkers; decreased oxidative stress via increased antioxidant enzyme activities | Deoxycorticosterone-induced hypertension rat model | (Silambarasan and Raja, 2012) | |
| Diosmin reduced pancreatic injury and decreased inflammation (e.g., TNF-α, IL-1β, IL-6, iNOS and NFκB) | Cerulein-induced acute pancreatitis mouse model | (Yu et al., 2014) | |
| Diosmin reversed pathological alterations and decreased oxidative stress via activation of antioxidant defenses and stimulation of PPAR-γ expression | Radiation-induced hepatic fibrosis rat model | (Hasan et al., 2017) | |
| Diosmin lowered symptoms of chronic venous insufficiency by decreasing oxidative stress | Clinical study with CVI patients | (Feldo et al., 2018) | |
| Diosmin reduced edema and pain symptoms of chronic venous insufficiency | Clinical study with CVI patients | (Batchvarov et al., 2010) | |
| Diabetes | Diosmin increased antioxidative enzymes and levels of antioxidants and decreased lipid peroxidation | Streptozotocin-induced diabetic rat model | (Srinivasan and Pari, 2012) |
| Diosmin decreased cholesterol, triacylglycerols, free fatty acids, LDL and increased HDL | Streptozotocin-induced diabetic rat model | (Srinivasan and Pari, 2013) | |
| Diosmin decreased plasma glucose and HbA1c and increased plasma insulin | Streptozotocin-induced diabetic rat model | (Pari and Srinivasan, 2010) | |
| Diosmin increased antioxidative enzymes and levels of antioxidants and decreased lipid peroxidation | Alloxan-induced diabetic rats | (Michael et al., 2013) | |
| Diosmin decreased levels of advanced glycation end products (AGEs) | Hyperglycemia-induced lens model | (Patil et al., 2016) | |