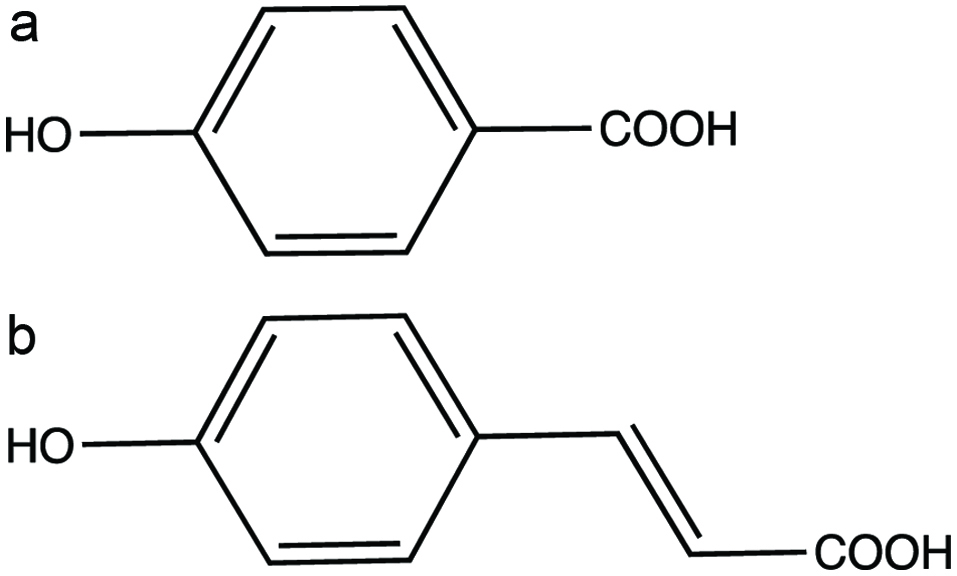
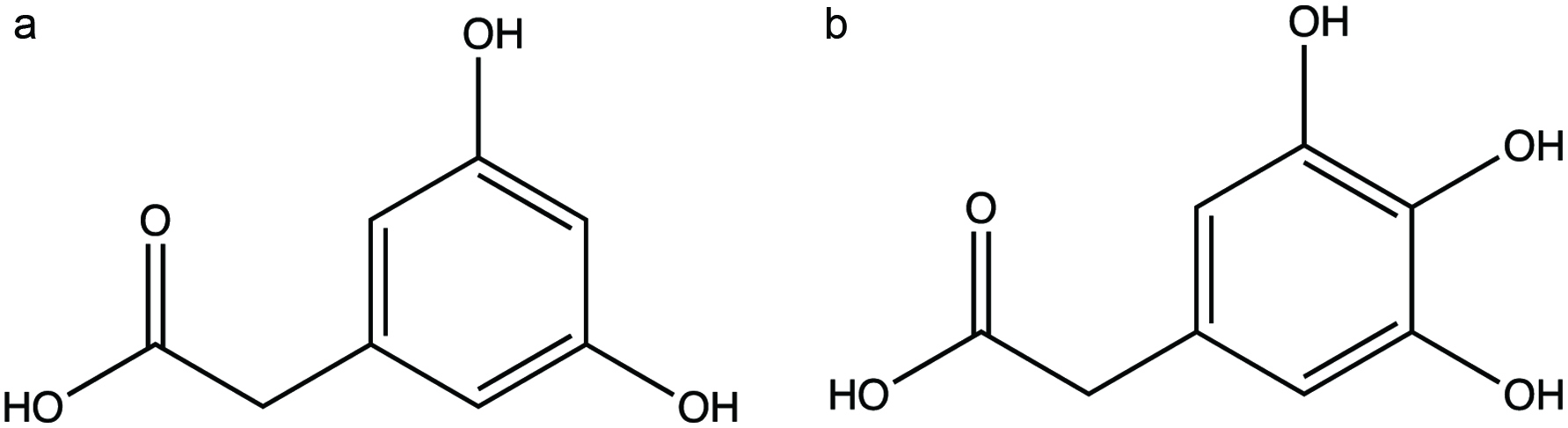
Figure 1. Phenolic acids (a) 4-hydroxybenzoic acid and (b) 4-hydroxycinnamic acid.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 8, December 2019, pages 6-41
Bioavailability and metabolism of food bioactives and their health effects: a review
Figures

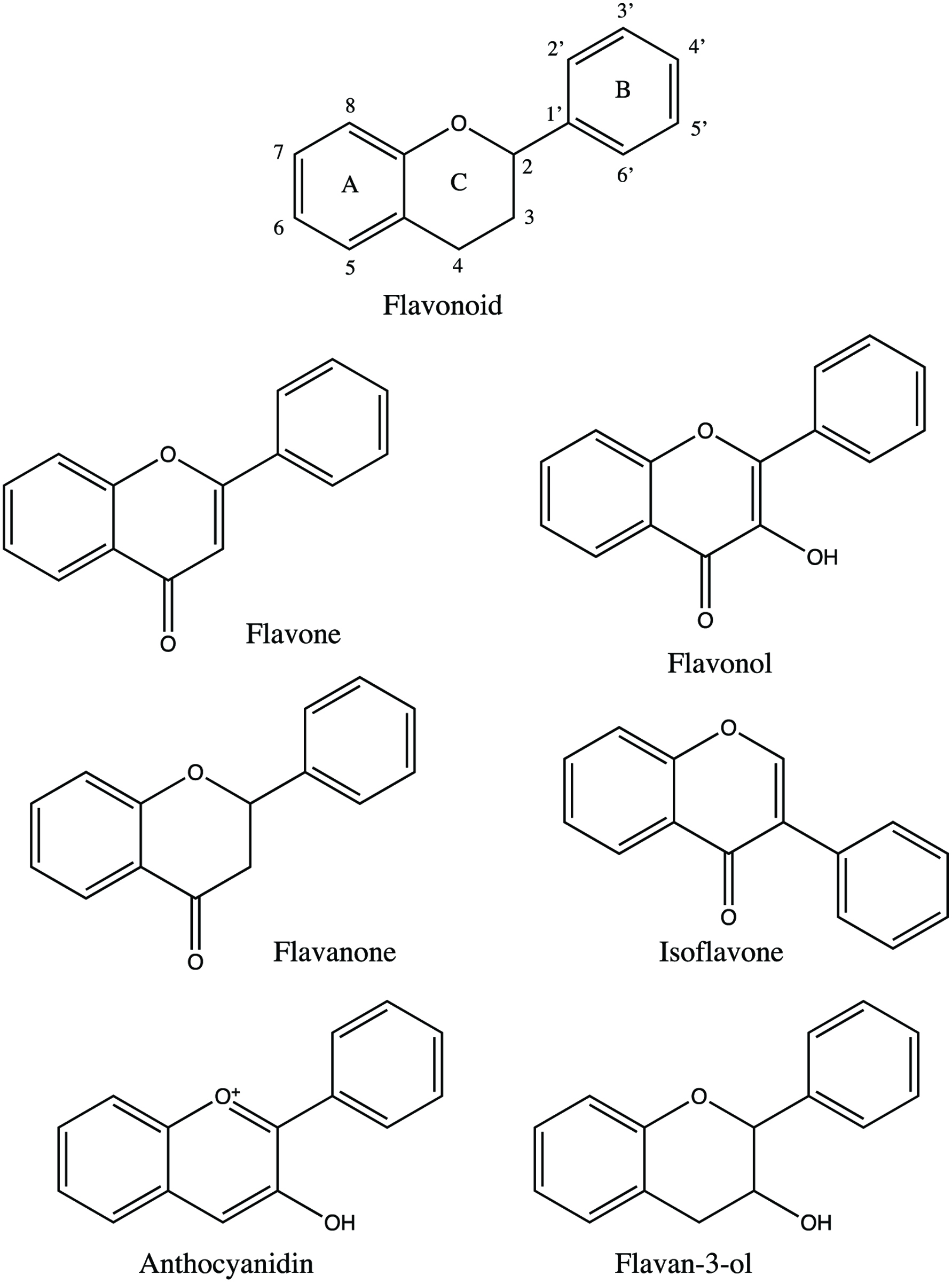
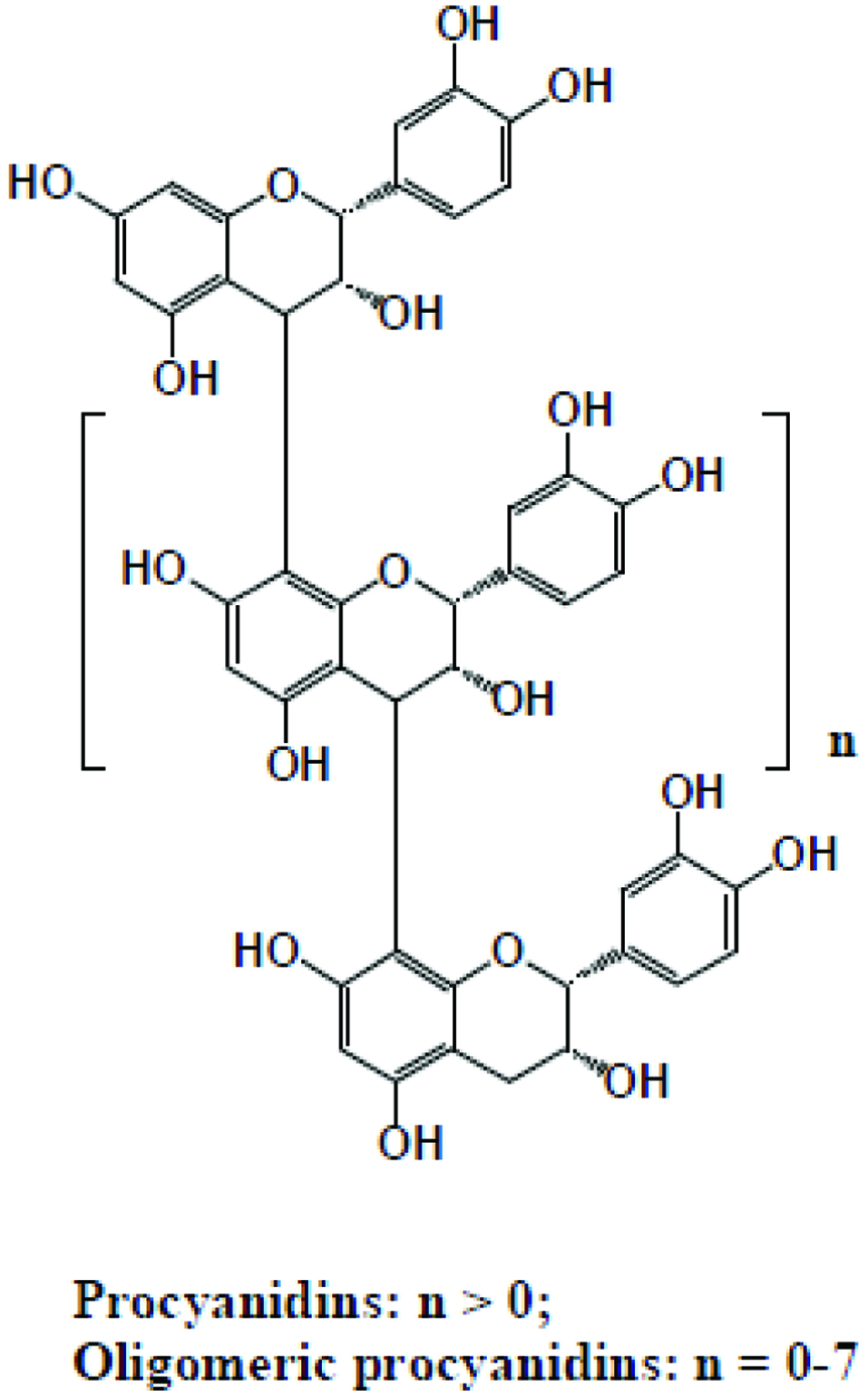
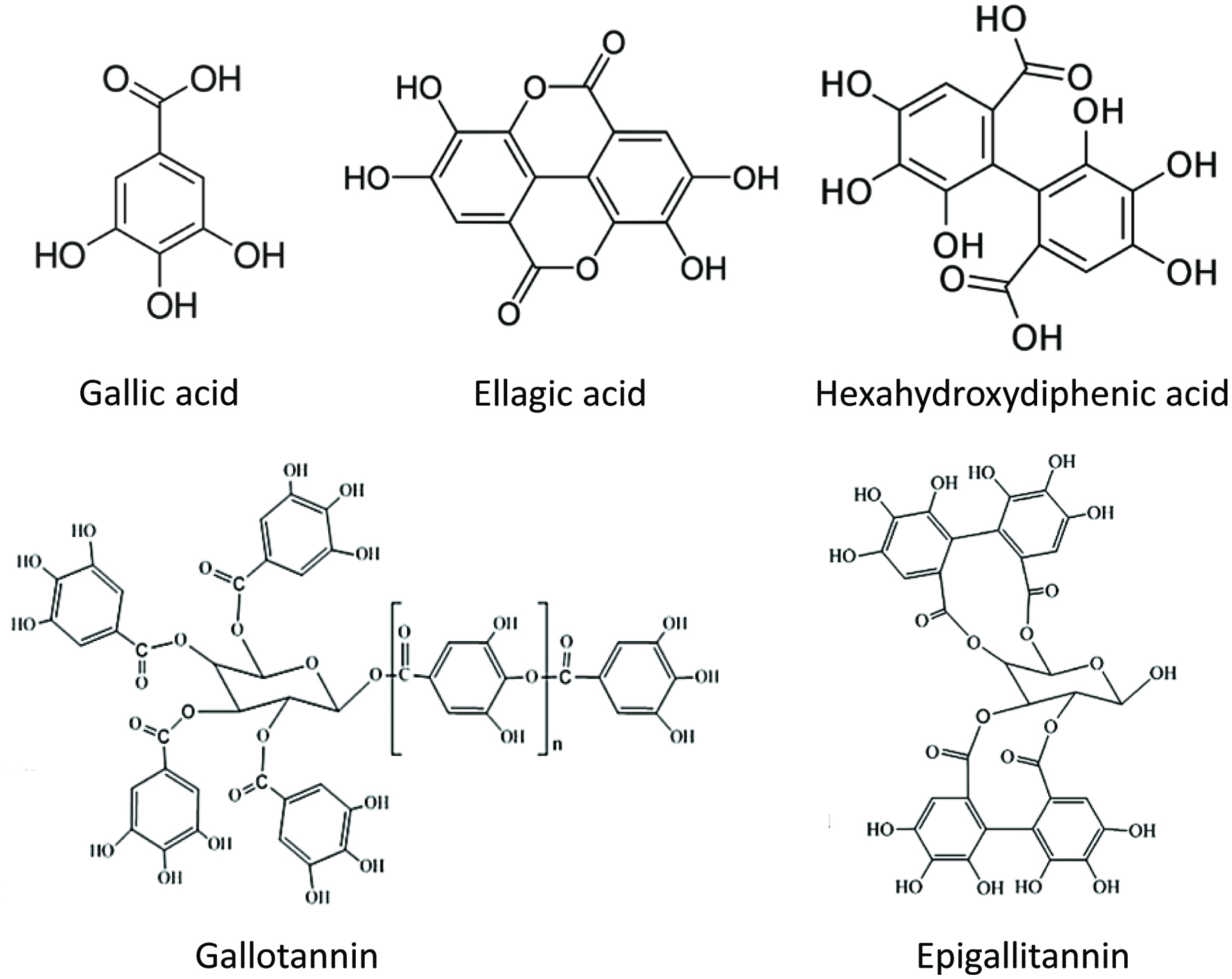
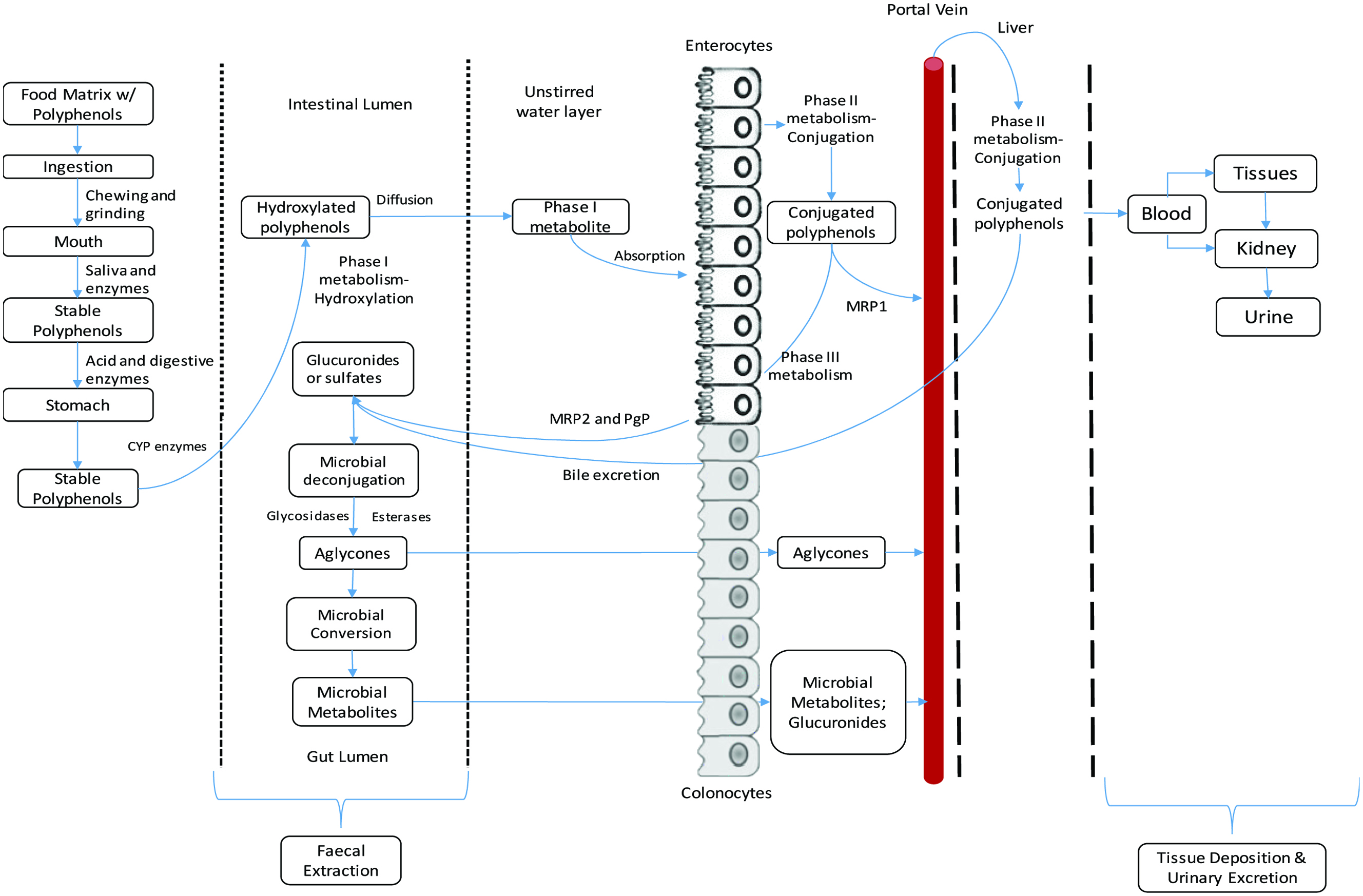
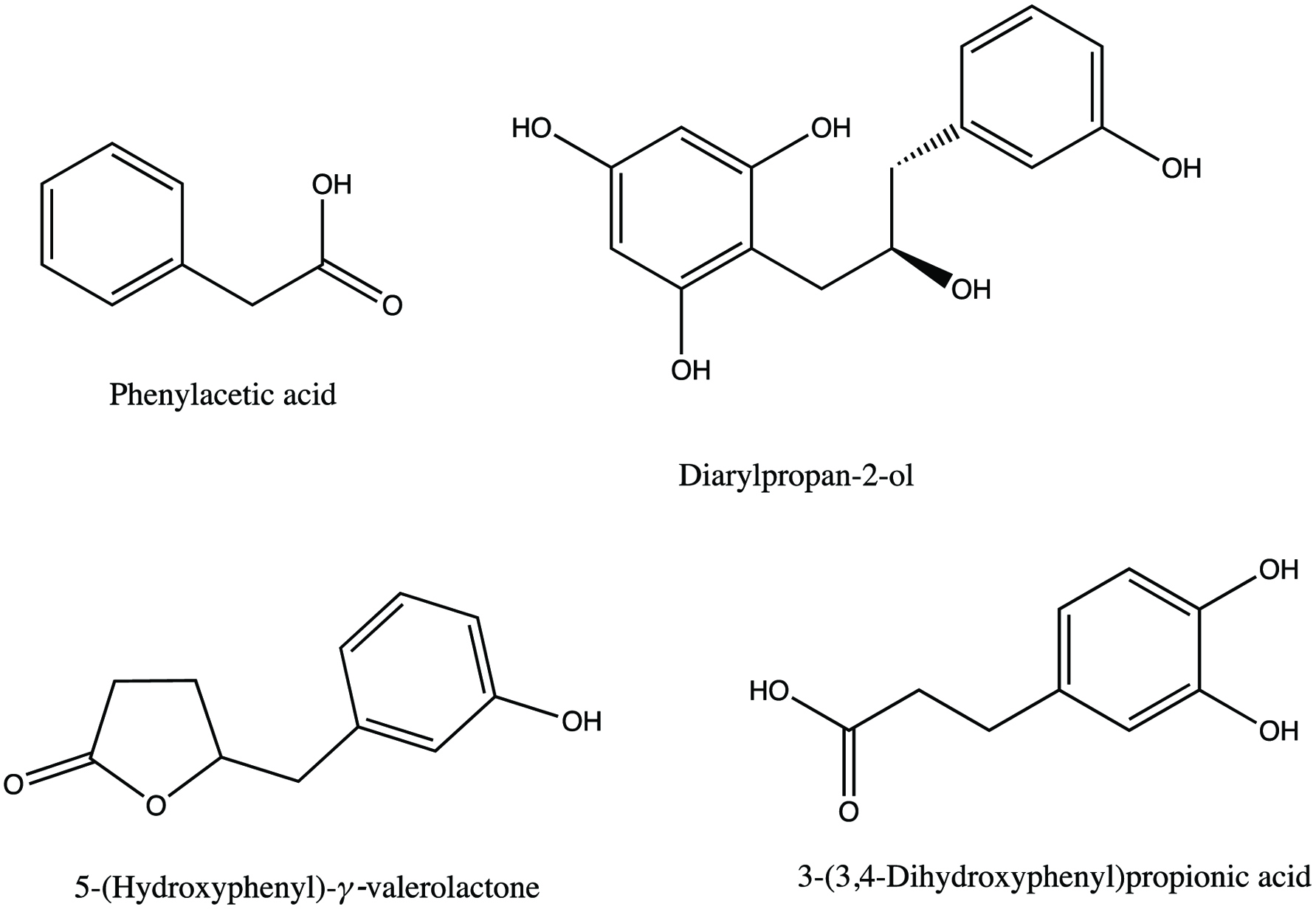
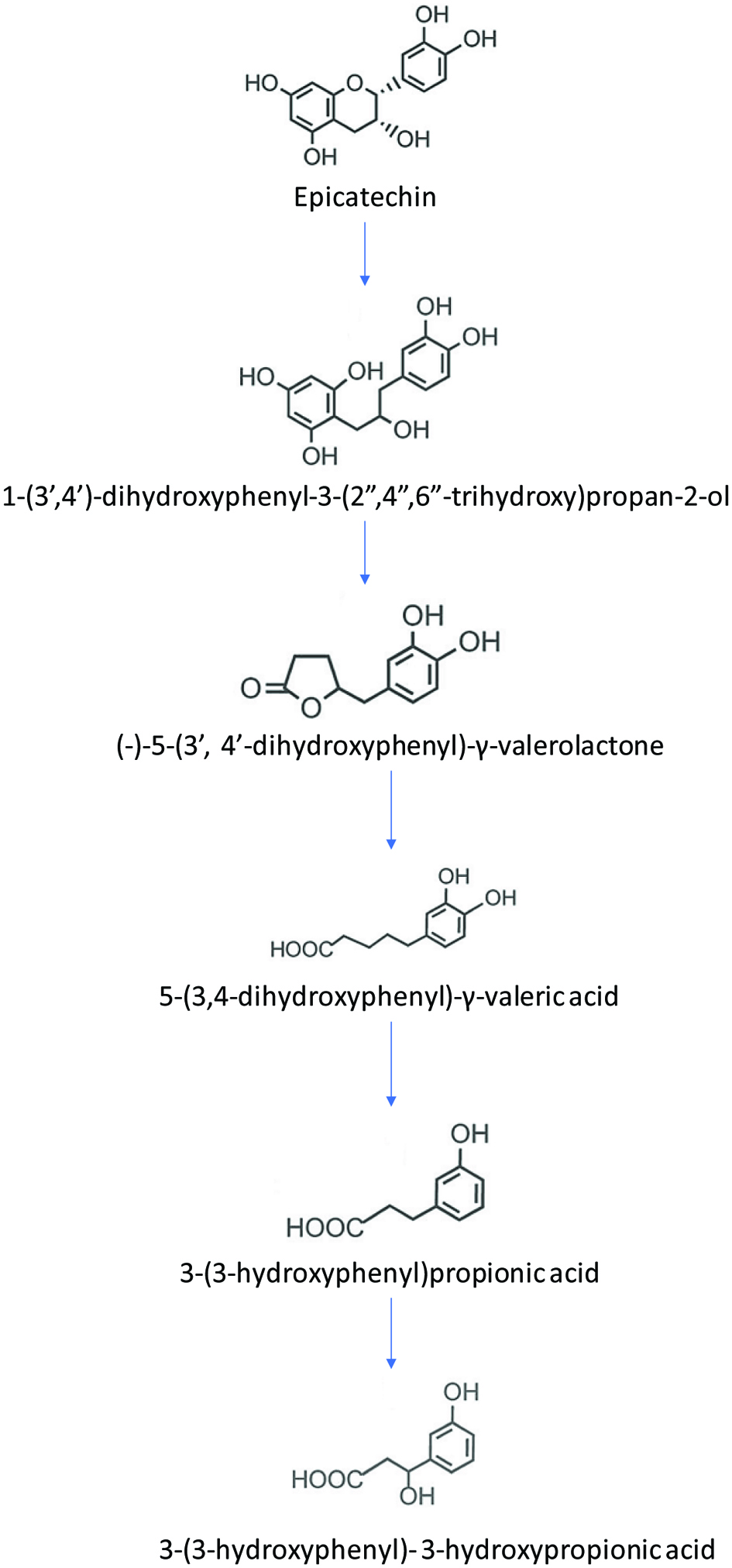
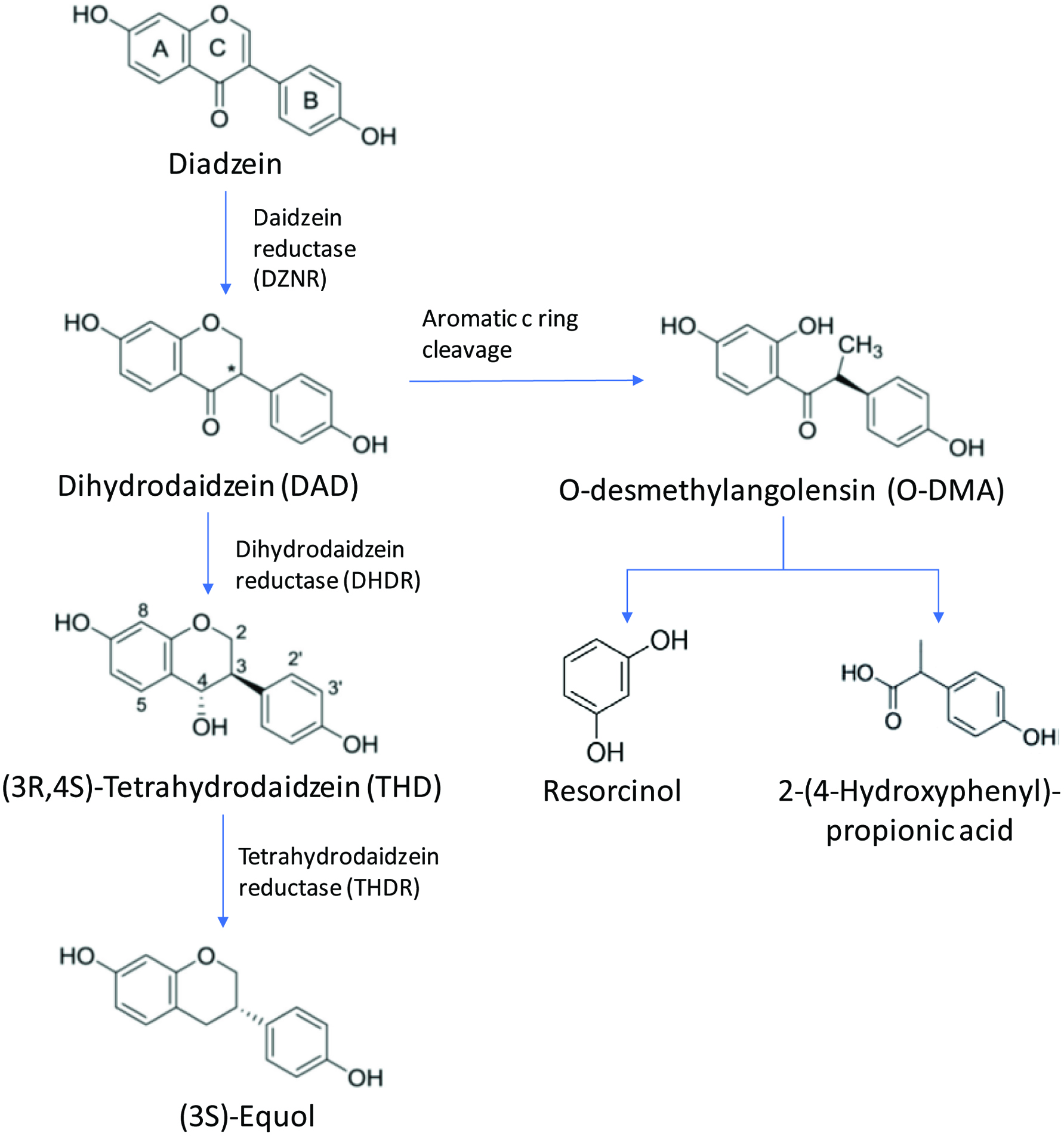
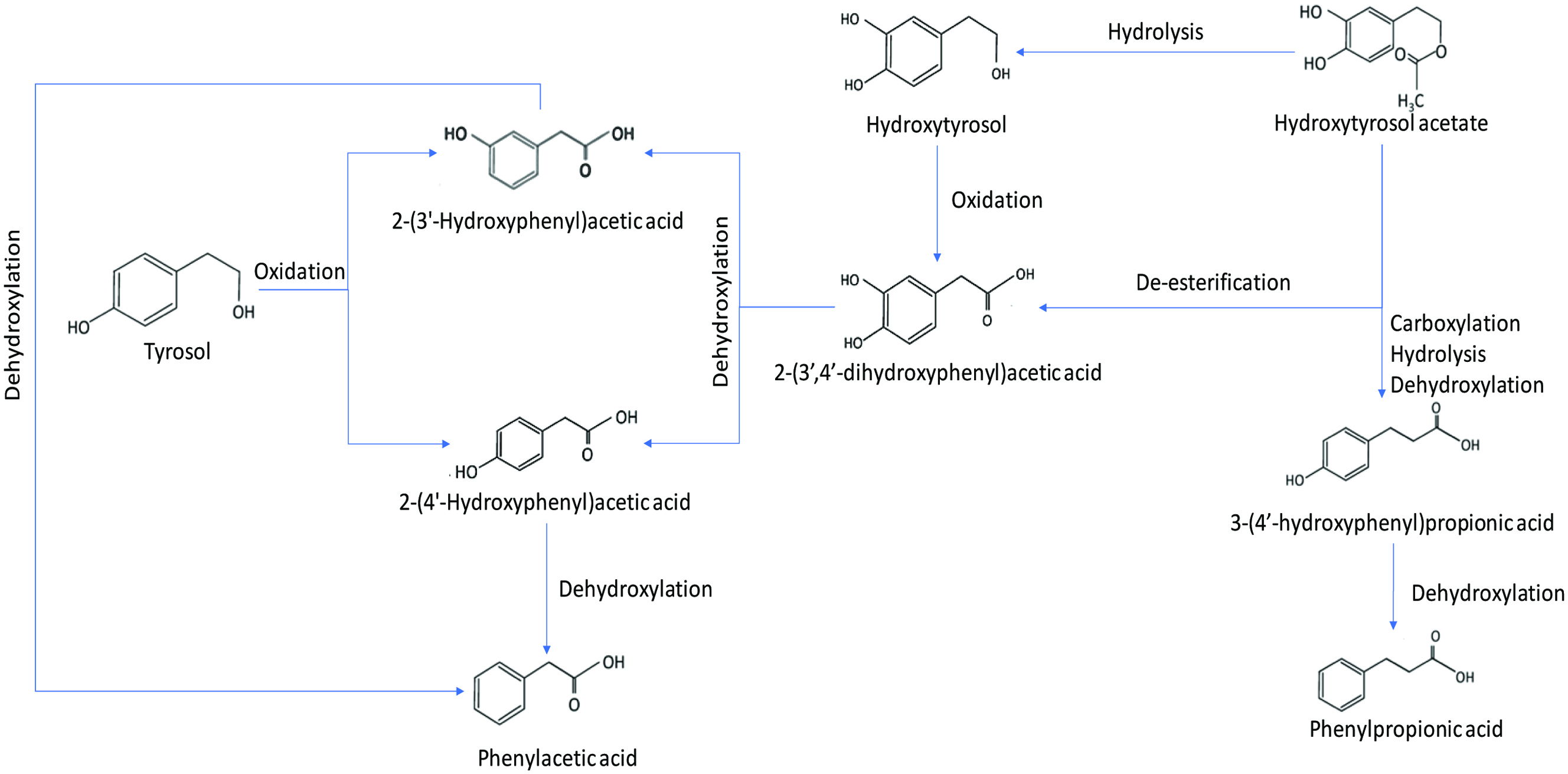
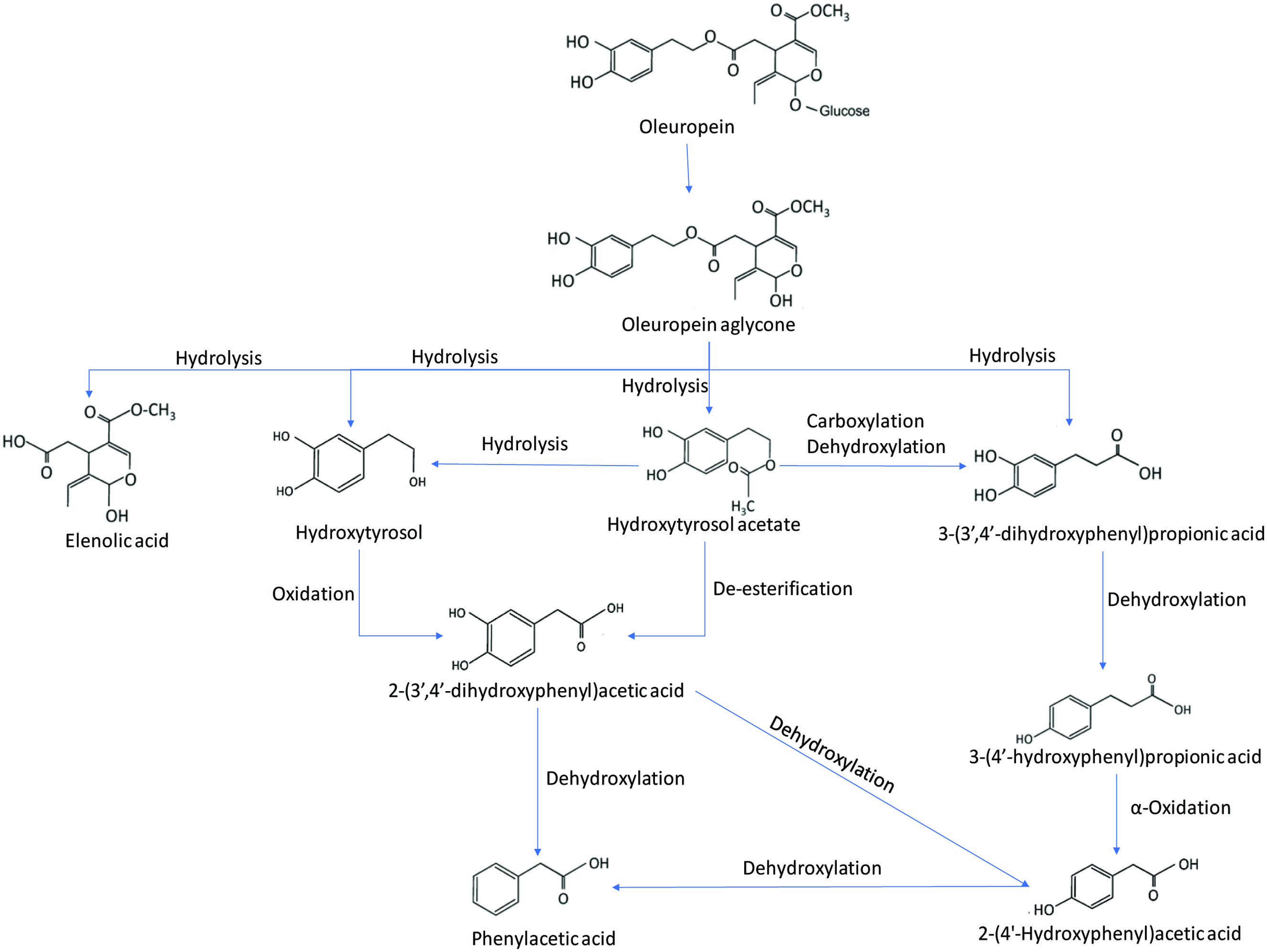
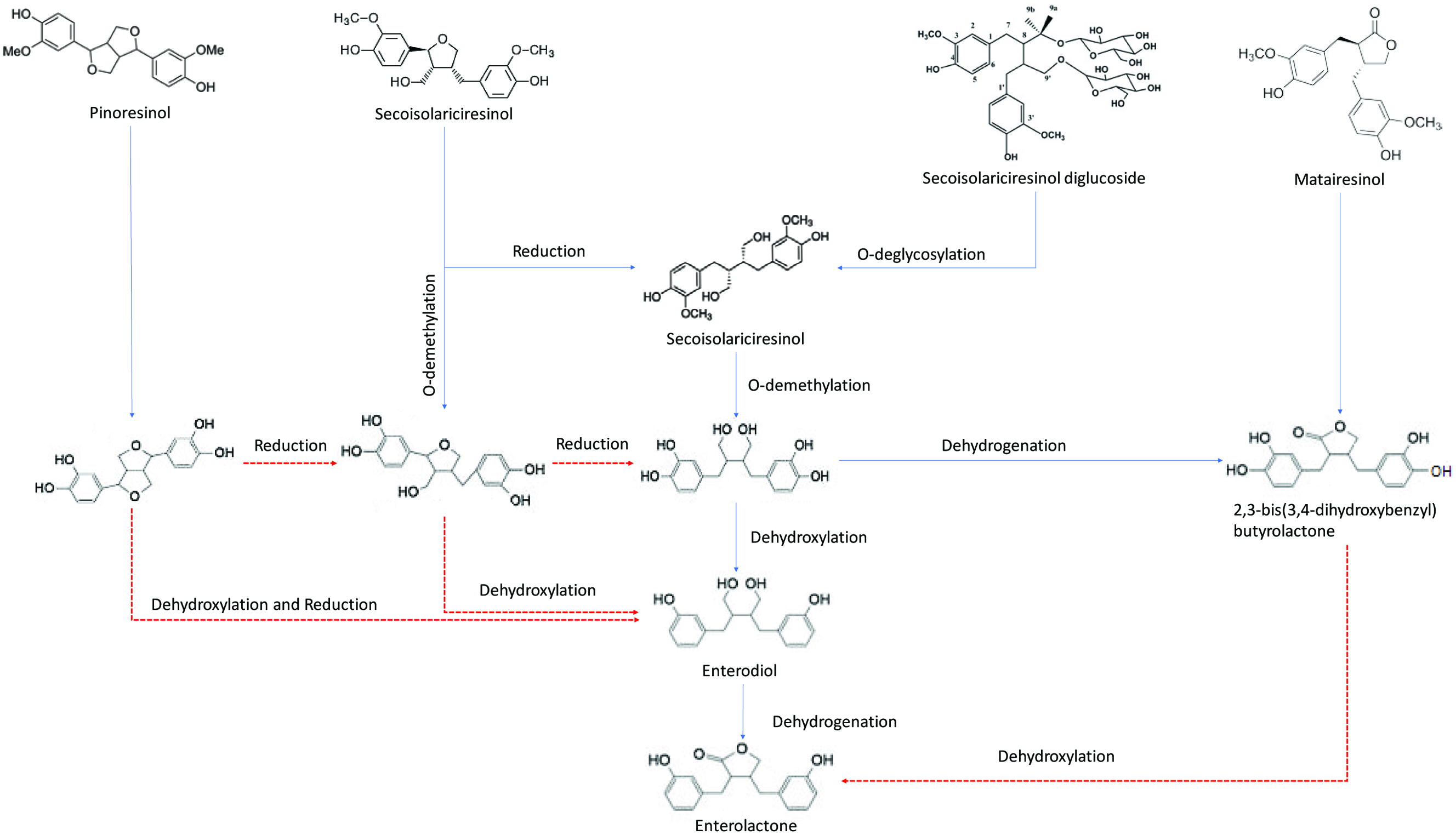
Tables
| Class | Subgroup | Example |
|---|---|---|
| Phenolic acid | Benzoic acid | Protocatechuic acid |
| Vanillic acid | ||
| Gallic acid | ||
| Cinnamic acid | p-Coumaric acid | |
| Caffeic acid | ||
| Chlorogenic acid | ||
| Sinapic acid | ||
| Stilbene | Resveratrol | |
| Pterostilbene | ||
| Flavonoid | Flavonol | Quercetin |
| Kaempferol | ||
| Myricetin | ||
| Flavones | Apigenin | |
| Acacetin | ||
| Luteolin | ||
| Flavanone | Naringenin | |
| Hesperetin | ||
| Flavanol | Catechin | |
| Epicatechin | ||
| Epigallocatechin | ||
| Epigallocatechin gallate | ||
| Anthocyanidin | Cyanidin | |
| Malvidin | ||
| Petunidin | ||
| Isoflavones | Genistein | |
| Daidzin | ||
| Lignan | Secoisolariciresinol | |
| Matairesinol | ||
| Syringaresinol | ||
| Tannin | Hydrolyzable | Gallotannin |
| Ellagitannin | ||
| Tannic acid | ||
| Condensed | Proanthocyanidin |
| Ring position | 3 | 5 | 7 | 3′ | 4′ | 5′ |
|---|---|---|---|---|---|---|
| 1Narayana et al., 2001; 2Graf et al., 2005; 3Shahidi and Naczk, 2003. -O-Me, Methoxy; -O-Glu, Glucosyl; -O-R, Alkoxy; and G, Gallate. | ||||||
| Flavonols1 | ||||||
| Kaempferol | OH | OH | OH | H | OH | H |
| Myricetin | OH | OH | OH | OH | OH | OH |
| Quercetin | OH | OH | OH | OH | OH | H |
| Flavonones1 | ||||||
| Hesperetin | H | OH | OH | OH | O-Me | H |
| Naringin | H | OH | O-R | H | OH | H |
| Flavones1 | ||||||
| Apigenin | H | OH | OH | H | OH | H |
| Luteolin | H | OH | OH | OH | OH | H |
| Anthocyanidins2 | ||||||
| Cyanidin | OH | OH | OH | OH | OH | H |
| Malvidin | OH | OH | OH | OCH3 | OH | OCH3 |
| Petunidin | OH | OH | OH | OCH3 | OH | H |
| Flavanols3 | ||||||
| Catechin/Epicatechin | OH | OH | OH | OH | OH | H |
| Gallocatechin | OH | OH | OH | OH | OH | OH |
| Epigallocatechin | OH | OH | OH | OH | OH | OH |
| Epigallocatechin gallate | G | OH | OH | OH | OH | OH |
| Isoflavones3 | ||||||
| Genistein | – | OH | OH | H | OH | H |
| Daidzin | – | H | O-Glu | H | OH | H |
| Daidzein | H | H | OH | H | OH | H |
| Food sample | Metabolites | Parent ion [M-H]− (m/z) | Fragment ions (m/z) |
|---|---|---|---|
| Data extracted from Dall’Asta et al. (2012). | |||
| Raspberries | protocatechuic acid | 153 | 109 |
| benzoic acid | 121 | 77 | |
| Blueberries | gallic acid | 169 | 125 |
| coumaric acid | 163 | 119 | |
| protocatechuic acid | 153 | 109 | |
| quinic acid | 191 | 85 | |
| dihydrocaffeic acid | 181 | 59, 137 | |
| hydroxybenzoic acid | 137 | 93 | |
| Blackberries | protocatechuic acid | 153 | 109 |
| Strawberries | gallic acid | 169 | 125 |
| quinic acid | 191 | 85 | |
| Onion | (3,4-dihydroxyphenyl)acetic acid | 167 | 123 |
| phloroglucinol | 125 | 51 | |
| Oat bran | dihydroferulic acid | 195 | 136 |
| dihydrosinapic acid | 225 | 151 | |
| Wheat bran | dihydroferulic acid | 195 | 136 |
| dihydrosinapic acid | 225 | 151 | |
| Flaxseed | protocatechuic acid | 153 | 109 |
| dihydroferulic acid | 195 | 136 | |
| homovanillic acid | 181 | 137 | |
| enterodiol | 301 | 253 | |
| enterolactone | 297 | 253 | |
| dihydrocaffeic acid | 181 | 59, 137 | |
| Dark chocolate | 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone | 207 | 163 |
| (3,4-dihydroxyphenyl)acetic acid | 167 | 123 | |
| protocatechuic acid | 153 | 109 | |
| hydroxybenzoic acid | 137 | 93 | |
| salicylic acid | 137 | 93 | |
| Orange juice | dihydroferulic acid | 195 | 136 |
| sinapic acid | 223 | 149, 208 | |
| protocatechuic acid | 153 | 109 | |
| Apple juice | quinic acid | 191 | 85 |
| 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone | 207 | 163 | |
| dihydrocaffeic acid | 181 | 59, 137 | |
| protocatechuic acid | 153 | 109 | |
| Pomegranate juice | gallic acid | 169 | 125 |
| pyrogallol | 125 | 51, 41 | |
| phlorogucinol | 125 | 51 | |
| syringic acid | 197 | 153, 182 | |
| protocatechuic acid | 153 | 109 | |
| Black tea | phlorogucinol | 125 | 51 |
| pyrogallol | 125 | 51, 41 | |
| coumaric acid | 163 | 119 | |
| gallic acid | 169 | 125 | |
| 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone | 207 | 163 | |
| 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone | 223 | 179 | |
| 5-(3′-hydroxyphenyl)-γ-valerolactone | 191 | 147 | |
| protocatechuic acid | 153 | 109 | |
| dihydrocaffeic acid | 181 | 59, 137 | |
| homovanillic acid | 181 | 137 | |
| quinic acid | 191 | 85 | |
| Coffee | caffeic acid | 179 | 135 |
| dihydroferulic acid | 195 | 136 | |
| quinic acid | 191 | 85 | |
| dihydrocaffeic acid | 181 | 59, 137 | |
| ferulic acid | 193 | 134 | |
| protocatechuic acid | 153 | 109 | |
| hydroxybenzoic acid | 137 | 93 | |
| Green tea | phloroglucinol | 125 | 51 |
| pyrogallol | 125 | 51, 41 | |
| gallic acid | 169 | 125 | |
| 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone | 207 | 163 | |
| 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone | 223 | 179 | |
| protocatechuic acid | 153 | 109 | |
| dihydrocaffeic acid | 181 | 59, 137 | |
| 5-(3′-hydroxyphenyl)-γ-valerolactone | 191 | 147 | |
| quinic acid | 191 | 85 | |
| Red wine | 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone | 207 | 163 |
| 3-(3-hydroxyphenyl) propionic acid | 165 | 121 | |
| protocatechuic acid | 153 | 109 | |
| gallic acid | 169 | 125 | |
| pyrogallol | 125 | 51, 41 | |
| phloroglucinol | 125 | 51 | |
| coumaric acid | 163 | 119 | |
| quinic acid | 191 | 85 | |