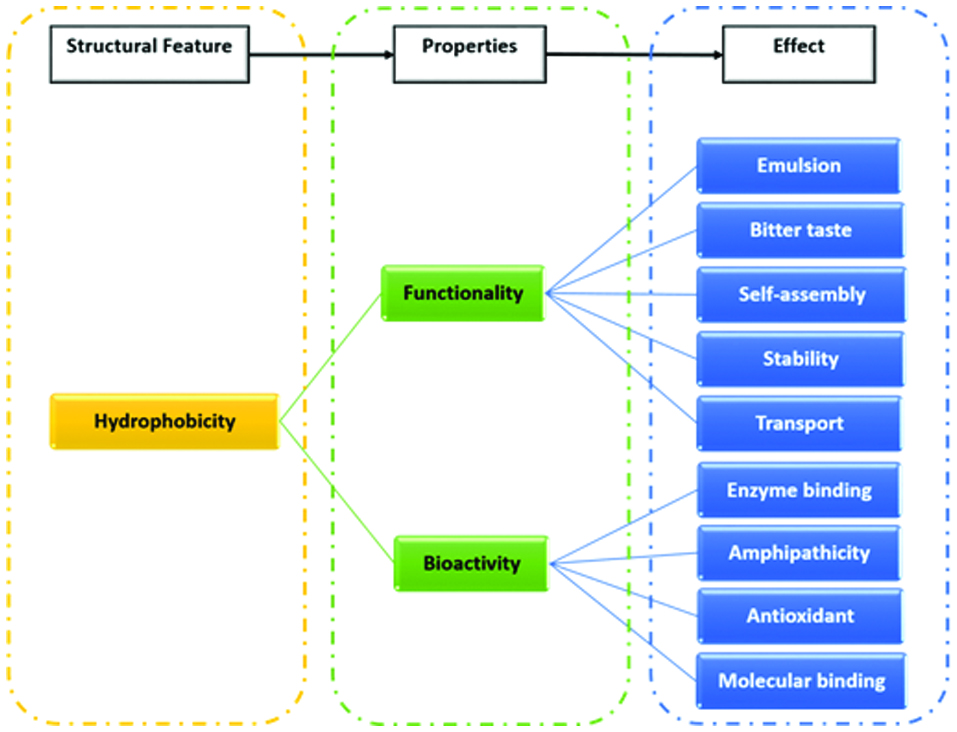
Figure 1.
Role of hydrophobicity in several functionalities and health-related bioactivities of food protein-derived peptides.
| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 4, Number , December 2018, pages 88-98
Role of hydrophobicity in food peptide functionality and bioactivity
Figures

Role of hydrophobicity in several functionalities and health-related bioactivities of food protein-derived peptides.
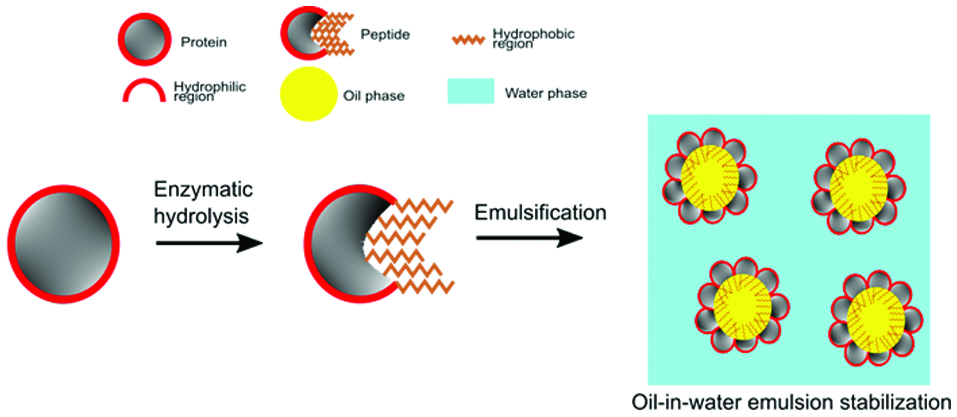
Enzymatic hydrolysis in producing hydrophobic peptides for stabilizing emulsions.
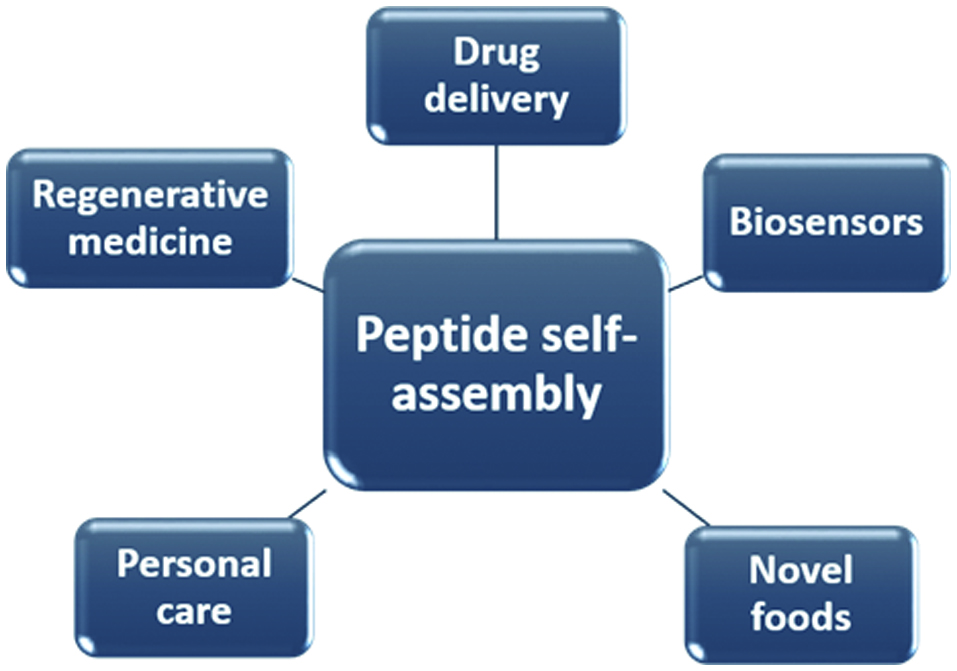
Various applications of peptide self-assembly.
Tables
| Amino acid | Symbol | Hydrophobicity Scale | |||||
|---|---|---|---|---|---|---|---|
| Kyte and Doolittle (1982) | Eisenberg et al. (1984) | Wolfenden et al. (1983) | Cornette et al. (1987) | Wimley and White (1996) | Hopp and Woods (1983) | ||
| Isoleucine | Ile (I) | 4.50 | 1.38 | 0.88 | 4.80 | 0.31 | −1.80 |
| Valine | Val (V) | 4.20 | 1.08 | 0.86 | 4.70 | −0.07 | −1.50 |
| Leucine | Leu (L) | 3.80 | 1.06 | 0.85 | 5.70 | 0.56 | −1.80 |
| Phenylalanine | Phe (F) | 2.80 | 1.19 | 0.88 | 4.40 | 1.13 | −2.50 |
| Cysteine | Cys (C) | 2.50 | 0.29 | 0.91 | 4.10 | 0.24 | −1.00 |
| Methionine | Met (M) | 1.90 | 0.64 | 0.85 | 4.20 | 0.23 | −1.30 |
| Alanine | Ala (A) | 1.80 | 0.62 | 0.74 | 0.20 | −0.17 | −0.50 |
| Glycine | Gly (G) | −0.40 | 0.48 | 0.72 | 0.00 | −0.01 | 0.00 |
| Threonine | Thr (T) | −0.70 | −0.05 | 0.70 | −1.90 | −0.14 | −0.40 |
| Serine | Ser (S) | −0.80 | −0.18 | 0.66 | −0.50 | −0.13 | 0.30 |
| Tryptophan | Trp (W) | −0.90 | 0.81 | 0.85 | 1.00 | 1.85 | −3.40 |
| Tyrosine | Tyr (Y) | −1.30 | 0.26 | 0.76 | 3.20 | 0.94 | −2.30 |
| Proline | Pro (P) | −1.60 | 0.12 | 0.64 | −2.20 | −0.45 | 0.00 |
| Histidine | His (H) | −3.20 | −0.40 | 0.78 | 0.50 | −0.96 | −0.50 |
| Glutamic acid | Glu (E) | −3.50 | −0.74 | 0.62 | −1.80 | −2.02 | 3.00 |
| Glutamine | Gln (Q) | −3.50 | −0.85 | 0.62 | −2.80 | −0.58 | 0.20 |
| Aspartic acid | Asp (D) | −3.50 | −0.90 | 0.62 | −3.10 | −1.23 | 3.00 |
| Asparagine | Asn (N) | −3.50 | −0.78 | 0.63 | −0.50 | −0.42 | 0.20 |
| Lysine | Lys (K) | −3.90 | −1.50 | 0.52 | −3.10 | −0.99 | 3.00 |
| Arginine | Arg (R) | −4.50 | −2.53 | 0.64 | 1.40 | −0.81 | 3.00 |
| Bioinformatic tools | Platform provider | Website |
|---|---|---|
| Peptide 2.0 | Peptide 2.0 Inc. | https://www.peptide2.com/N_peptide_hydrophobicity_hydrophilicity.php |
| Peptide property tool | Genescript | https://www.genscript.com/tools/peptide-property-calculator |
| Peptide analysing tool | ThermoFisher Scientific | https://www.thermofisher.com/ca/en/home/life-science/protein-biology/peptides-proteins/custom-peptide-synthesis-services/peptide-analyzing-tool.html |
| Peptide property calculator | Biosynthesis | https://www.biosyn.com/peptidepropertycalculator/peptidepropertycalculator.aspx |
| PepCalc.com | Innovagen | https://pepcalc.com/ |
| Prot pi peptide tool | Prot pi | https://www.protpi.ch/Calculator/PeptideTool |
| Peptide property calculator | Northwestern University | http://biotools.nubic.northwestern.edu/proteincalc.html |
| PLATINUM | Laboratory of Biomolecular Modelling, Russian Academy of Sciences | https://model.nmr.ru/platinum/ |
| Peptide Library Design and Calculator Tool | Millipore Sigma | https://www.sigmaaldrich.com/technical-documents/articles/biology/peptide-library-design-and-calculator-tool.html |
| GPMAW lite | Alphalyse Inc. | https://www.alphalyse.com/customer-support/gpmaw-lite-bioinformatics-tool/start-gpmaw-lite/ |
| R-software | R Core Team | https://www.r-project.org/contributors.html |
| Sequence | Bioactivity | Experimental analysis | Reference |
|---|---|---|---|
| Phe-Asp-Ser-Gly-Pro-Ala-Gly-Val-Leu | Antioxidant capacity | In vitro analysis with human lung fibroblasts | Mendis et al. (2005) |
| Asn-Gly-Pro-Leu-Gln-Ala-Gly-Gln-Pro-Gly-Glu-Arg | |||
| Gly-Val-Ser-Asn-Ala-Ala-Val-Val-Ala-Gly-Gly-His | Antioxidant capacity | In vitro and ex vivo analysis on mouse fibroblasts | Coda et al. (2012) |
| Asp-Ala-Gln-Glu-Phe-Lys-Arg | |||
| Tyr-Ala | Antioxidant capacity | In vitro analysis using standard methods involving ABTS, DPPH and superoxide anion | Tang et al. (2010) |
| Glu-Gln-Arg-Pro-Arg | Anticancer activity | In vitro analysis in diverse normal and cancer cell cultures | Kannana et al. (2010) |
| Gly-Gly-Arg-Lys-Gln-Gly-Gln-His-Gln-Gln-Glu-Glu | Immunostimulating | In vitro and in vivo analysis in cell cultures and mice models | Gusa and Tani (2009) |
| Val-Ile-Lys | Anti-inflammatory activity | In vitro analysis in murine macrophage cell lines | (Dia et al (2014) |
| Tyr-Val-Pro-Gly-Pro | Anticancer activity | In vitro analysis on prostate cancer cells | Wu et al. (2018) |
| Ile-Pro | DPP-IV inhibition | In vitro analysis | Hatanaka et al. (2012) |
| Leu-Pro | |||
| Lys-Ala | DPP-IV inhibition | In vitro analysis | Gallego et al. (2014) |
| Ala-Ala-Ala-Thr-Pro | |||
| Arg-Trp | Antimicrobial activity | Increase in antimicrobial activity and hemolysis of red blood cells with increasing peptide chain length | Liu et al. (2007) |
| Arg-Trp-Arg-Trp | |||
| Arg-Trp-Arg-Trp-Arg-Trp | |||
| Arg-Trp-Arg-Trp-Arg-Trp-Arg-Trp | |||
| Arg-Trp-Arg-Trp-Arg-Trp-Arg-Trp-Arg-Trp | |||
| Val-Gln-Trp-Arg-Ile-Arg-Val-Ala-Val-Ile-Arg-Lys | Antimicrobial activity | 60 mg/kg dose shown to be as effective as vancomycin in rats with Staphylococcus aureus | Wu et al. (2014) |
| Val-Leu-Leu-Val-Thr-Leu-Thr-Arg-Leu-His-Gln-Arg-Gly-Val-Ile-Tyr-Arg-Lys-Trp-Arg-His-Phe-Ser-Gly-Arg-Lys-Tyr-Arg | Antimicrobial activity | Antimicrobial studies with Escherichia coli DH5α, Bacillus subtilis AZ54, Staphylococcus aureus ATCC 6538 P and Pseudomonas aeruginosa strains | Pane et al. (2017) |
| Glu-Lys-Glu-Arg-Glu-Arg-Gln | ACE inhibition | Oral administration to spontaneously hypertensive rats (SHR) at a dose of 10 mg/kg body weight | Katayama et al. (2008) |
| Lys-Arg-Gln-Lys-Tyr-Asp-Ile | |||
| Lys-Arg-Val-Ile-Gln-Try | ACE inhibition | Oral administration to SHR at a dose of 10 mg/kg body weight | Muguruma et al. (2009) |
| Arg-Pro-Arg | ACE inhibition | Oral administration to SHR at a dose of 1 mg/kg body weight | Escudero et al. (2012) |
| Lys-Ala-Pro-Val-Ala | |||
| Pro-Thr-Pro-Val-Pro |