| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 6, June 2019, pages 68-99
Bioactives from culinary spices and herbs: a review
Milda E. Embuscado
McCormick & Company, Inc., Hunt Valley, MD 21031, USA. E-mail: Milda_Embuscado@mccormick.com
DOI: 10.31665/JFB.2019.6186
Received: June 15, 2019
Revised received & accepted: June 26, 2019
| Abstract | ▴Top |
Culinary spices and herbs have been used in food and beverages to enhance aroma, flavor, and color. They are rich in phytochemicals that provide significant antioxidant and anti-inflammatory effects. There is growing interest in identifying compounds from spices and herbs responsible for modulating oxidative and inflammatory stress to prevent diet-related diseases. This contribution will provide an overview of culinary spices and herbs, their classification, their sources or origins and more importantly, their chemical composition, antioxidant activity and their impacts on human health based on important and recent studies.
Keywords: Spices; Herbs; Antioxidants; Anti-inflammatory; Bioactives
| 1. Introduction | ▴Top |
Plants are major sources of food bioactives. Spices and herbs are plant materials that provide a wide range of biologically active compounds. In addition to being used as sources of aroma, flavor, and color and as preservatives, spices and herbs have been used for medicinal purposes and for health and wellness for centuries. The term bioactive is derived from the phrase biologically active which indicates that the substance has a positive effect on living organism, tissue or cell. Bioactive molecules are compounds that play an important role in human growth and development and have proven health benefits. They also amend disease risks by easing disease conditions. The National Institutes of Health (NIH) defines bioactive food components as "constituents in foods or dietary supplements, other than those needed to meet basic human nutritional needs, which are responsible for changes in health status." In the classical sense, bioactives are not nutrients. They are not essential for life but likewise perform essential functions: (1) they influence physiological or cellular activities which result in beneficial health effects, (2) they amend disease risk, rather than preventing deficiency diseases, and (3) they act as inducers and inhibitors of enzymes, inhibitors of receptor activity, and inducers and inhibitors of gene expression (Koe et al., 2014). In this respect, the absorption and bioavailability of bioactives present must also be considered (Shahidi and Peng, 2018).
Probiotics, phytosterols, lutein, lycopene, fatty acids and peptides are some examples of bioactive substances. Bioactives also include antioxidants such as polyphenols, carotenoids and non-flavonoid phenolics. Antioxidants protect the human body from oxidation brought about by free radicals, superoxide and other oxygen radicals as well as other substances that trigger oxidation. Bioactives can be classified into several groups based on their function, molecular structure or source. Table 1 summarizes the different groups, examples from each group, food sources, their functions and related publications. Note the diverse health benefits derived from these bioactives, from blocking low-density lipoproteins and cholesterol, antioxidant activity, anticancer to improving cardiovascular, joint and digestive health and strengthening immunity. Three out of seven different groups of bioactives are supplied by spices and herbs. Epidemiological studies indicate that cancer incidence in countries such as India where spices are consumed daily is much lower (94/100,000) than those where spices are not consumed such as United States (318/100,000) suggesting the potential role of spices in cancer prevention (Kunnumakkara et al., 2018). Food bioactives from top spices and herbs will be reviewed in this contribution paper based on significant scientific studies and recent findings.
 Click to view | Table 1. Classification of food bioactives and their associated benefits |
| 2. Spices and herbs | ▴Top |
The value and volume of spices being produced continue to increase by about 5% annually (Fig. 1a). The total world imports from spices and herbs producing countries increased significantly from 1999 to 2017 (Fig. 1b). This is due to the growing popularity of using spices and herbs as natural and clean label ingredients and sources of aroma, flavor and color. Their popularity is also increasing due to the health benefits and therapeutic effects derived by including spices and herbs in personal diets. In fact, the Mediterranean diet includes herbs and spices with the whole grains, fruits, vegetables, beans and healthy fats and oils (e.g., olive oil) (Mayo Clinic, 2019). This provides a well-recognized healthy diet that reduces the risk of heart disease and lowers the level of oxidized low-density lipoprotein (LDL) cholesterol. The Mediterranean diet is also associated with reduced incidence of cancer, Parkinson’s and Alzheimer’s diseases (Mayo Clinic, 2019). Table 2 shows the sources of the spices and herbs in the world and the plant parts where they come from. Asia and the Middle East dominate production of most spices and herbs.
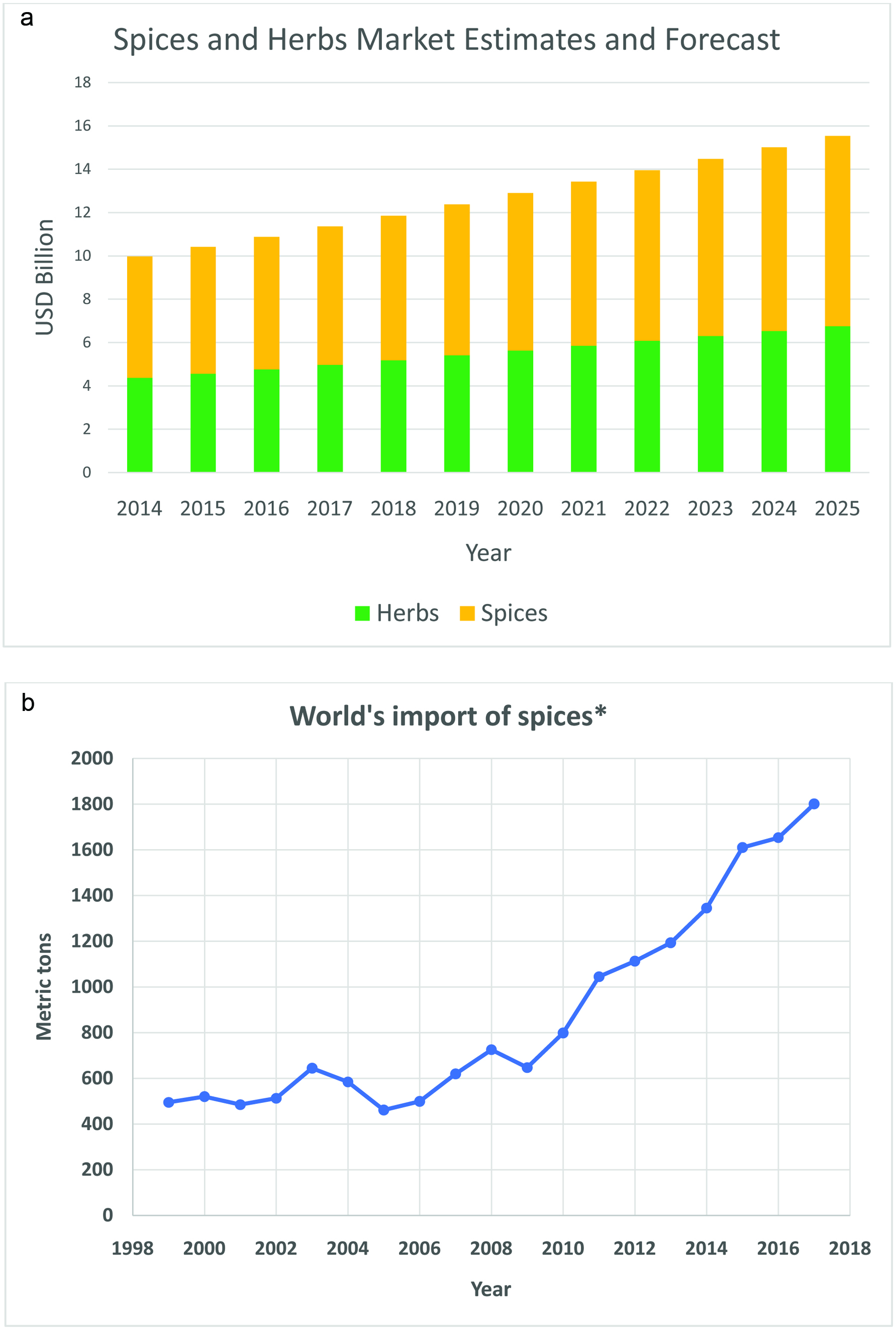 Click for large image | Figure 1. (a) Spices and herbs global market estimates and forecast (Varma, 2019); (b) World’s import of spices (Source: USDA, www.fas.usda.gov). |
 Click to view | Table 2. Major spices for world trade |
| 3. Different product forms of selected spices and herbs | ▴Top |
Spices and herbs are commercially available to consumers in different forms (Table 3). Most spices and herbs when dried are available in ground or powder form. Some are freshly milled before use like whole black pepper when used in some dishes such as salads or soups as the aroma and flavor are retained better when not pre-milled. The white pepper comes from the black peppercorn in which the black pericarp or husk has been removed by soaking and fermenting the mature black pepper in water. This is to differentiate the black pepper from the white pepper which is also a popular spice because of its unique flavor and pungency. Extracts of spices and herbs are also available for specific markets. Some parts of the world like Europe, the Mediterranean region and Asia use fresh herbs in their cuisine.
 Click to view | Table 3. Spices and herbs available to consumers |
 Click for large image | Figure 2. Left: Cinnamomum verum, right: C. burmannii—note the structural and color differences between these two species. |
| 4. Classification of spices and herbs | ▴Top |
Herbs come from leaves of a plant while spices come from different parts of a plant other than the leaves (Table 4). Spices and herbs can be classified into various groups based on flavor/taste, taxonomy or part of the plant where they came from. Based on flavor or taste, spices and herbs can be classified into 4 groups: hot spices (black and white peppers, Cayenne pepper, mustard, chillies), mild flavor spices (paprika, coriander), aromatic spices (clove, cumin, dill fennel, nutmeg, mace, cinnamon) and aromatic herbs and vegetables (thyme, basil, bay leaf, marjoram, shallot, onion, garlic).
 Click to view | Table 4. Sources of spices and herbs |
Based on taxonomic classification, spices and herbs fall under the class Angiospermae or the flowering plants. The taxonomic classification is illustrated in Table 5 and shows the taxonomic relationship of the different spices and herbs. Spices and herbs have been used for medicinal purposes for centuries and are one of the best sources of food bioactives functioning as natural antioxidants because they contain potent compounds that have been shown to impart antioxidative effects when consumed and when added in foods prone to oxidation such as fats and oils.
 Click to view | Table 5. Taxonomic relationship of herbs and spices |
| 5. Phytochemistry | ▴Top |
Phytochemicals constitute a large group of bioactives derived from plants. This group consists of flavonoids, non-flavonoid phenolic compounds, carotenoids, plant sterols, glucosinolates and other sulphur-containing compounds. There are more than 6,000 different flavonoids that have been described and this continues to grow upon discovery of new compounds (Harborne and Williams, 2000). Flavonoids are polyphenolic compounds that consist of 15 carbons, with 2 aromatic rings connected by a 3-carbon bridge (Jaganath and Crozier, 2010). Figure 3 shows the basic molecular structures of flavonoids. Table 6 contains a summary of the different classes within the spice and herb bioactives. Plants produce these substances to defend themselves against various agents in the environment for survival and adaptation. The roles of phenolics and flavonoids include structural roles supporting or protecting tissues, involvement in defense strategies, as attractants for pollinators and seed-dispersing animals, and as allelopathic agents, ultra violet (UV) protectants and signal molecules in the interactions between plants and their environment (Jaganath and Crozier, 2010). The anthocyanins protect chloroplasts from photodegradation by absorbing high-energy quanta, while scavenging free radicals and reactive oxygen species (ROS) (Gould, 2004). These phytochemicals provide similar invaluable benefits to humans by modulating human metabolism in a manner favorable for the prevention or reduction in the risk of degenerative diseases such as cardiovascular diseases, diabetes, obesity and cancer (Anderson et al., 1999).
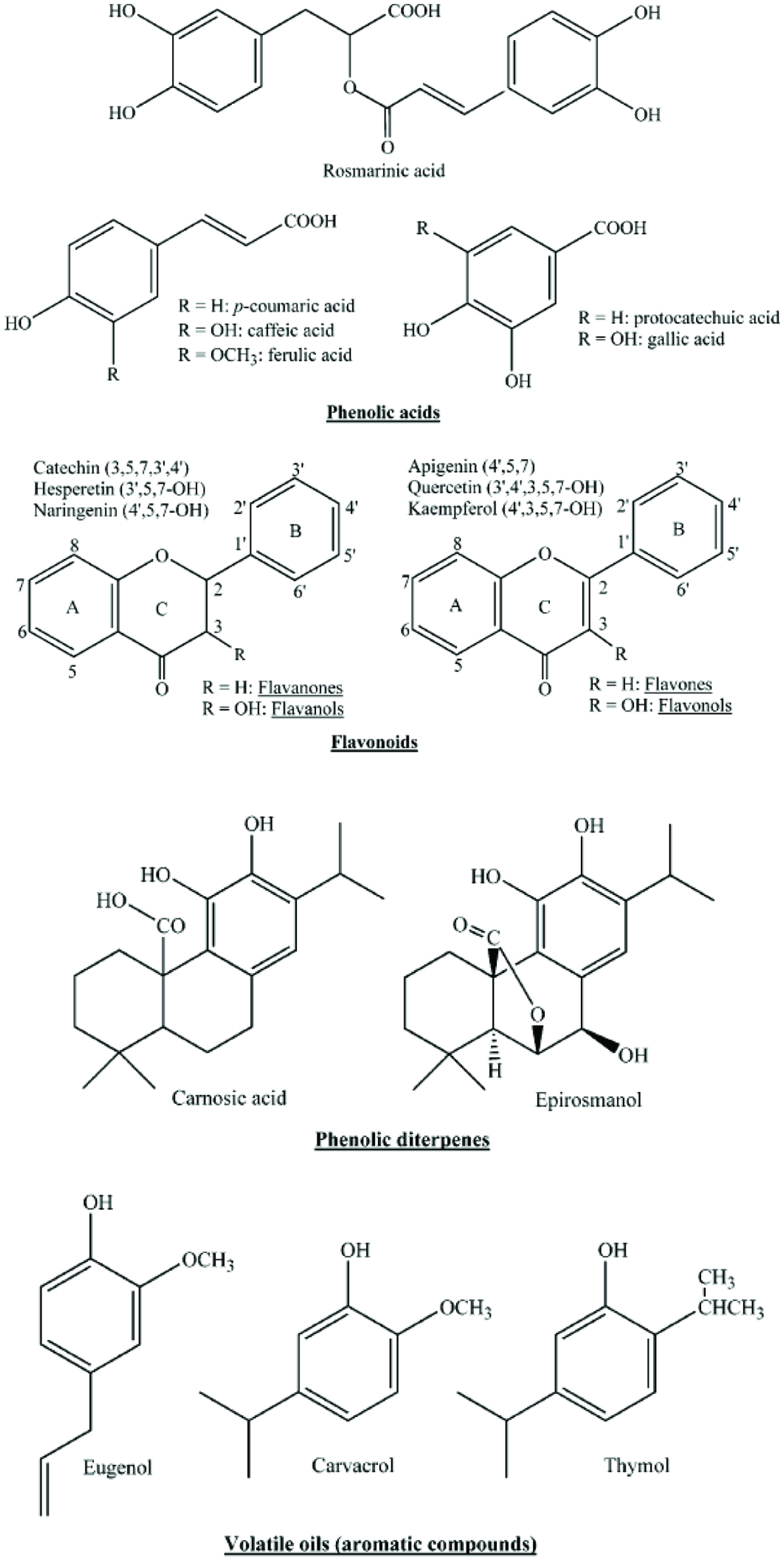 Click for large image | Figure 3. Structures of major phenolic compounds identified in spices and herbs. Reprinted with permission from Shan et al., (2005) American Chemical Society. |
 Click to view | Table 6. Classification of phytochemicals from spices and herbs |
The aromatic herbs in the mint family contain a significant amount of effective antioxidants (Table 7) together with the other herbs in the mint family. In fact, these herbs are used as antioxidants in food to prevent development of rancidity and to improve the shelf life of oils and cosmetics. It is particularly effective in enhancing the stability of omega-3 rich oils. Table 8 summarizes the effectiveness of rosemary or rosemary-derived products in preventing oxidation in foods and production of deleterious compounds during high temperature cooking. This table also listed some of the recent findings on the health benefits of consuming rosemary and other spices and herbs. Since rosemary has been found to be a very effective antioxidant, a number of natural and clean label antioxidants derived from rosemary are now commercially available. It is the main and significant raw material for the manufacture of antioxidants that are used in food and food products. Added benefits of using rosemary or extracts from rosemary are: (1) product is natural or from a natural source (2) clean label (3) long history of safe usage (4) non-GMO and (5) the organic variety is generally available. All these qualities and added benefits from rosemary are what consumers are looking for in food ingredients.
 Click to view | Table 7. Antioxidant compounds identified in rosemary and other aromatic herbs |
 Click to view | Table 8. Antioxidants isolated from herbs and spice* |
| 6. Biological activities | ▴Top |
Figure 4 summarizes the significant effects and mechanism of action of curcumin, a potent bioactive from turmeric. This also sums up and reflects some of the overall health benefits and positive effects of bioactive compounds from spices and herbs on the human body such as an antioxidant, an anti-inflammatory, an anti-amyloidogenic, neuroprotective as well as enhancing effect on cognition (Shahidi and Hossain, 2018).
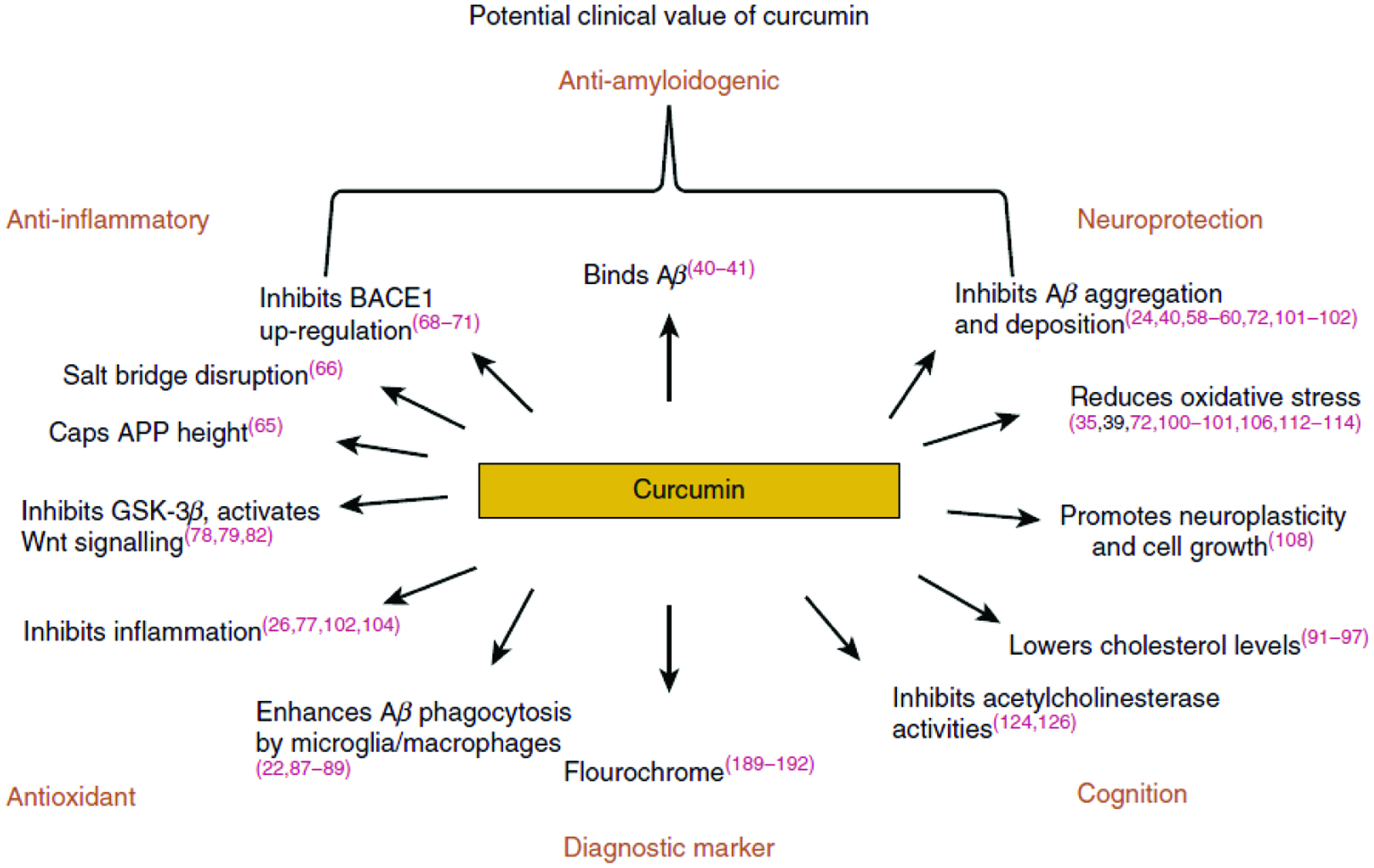 Click for large image | Figure 4. Curcumin reported mechanisms of action. BACE 1, β-APP-cleaving enzyme-1: Aβ, β amyloid; APP, amyloid precursor protein. Source: Gooze et al., 2016, Br. J. Nutr. 115, 455. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. DOI: https://doi.org/10.1017/S0007114515004687 |
| 7. Mechanism of action as an antioxidant | ▴Top |
The free radicals such as R•, RO• (alkoxyl radical), ROO• (peroxyl radical), O2– (superoxide radical anion), H2O2 (hydrogen peroxide), OH− (hydroxyl radical), ROOH (organic hydroperoxide) are constantly being produced in the body due to normal metabolic processes and due to exposure to environmental stressors such as exposure to ozone, industrial chemicals, air pollutants, smoking, UV light and radiation, among others. A appropriate balance between free radicals and antioxidants is necessary for proper physiological function (Lobo et al., 2010). If there is excessive free radicals in the body, then lipids, proteins and DNA are adversely affected which can trigger a number of diseases such as diabetes, cancer, heart disease and neurodegenerative ailments such as Alzheimer’s disease. Spices and herbs contain antioxidants which can ameliorate conditions due to excessive free radicals in the body. An antioxidant is a compound that can donate an electron or a hydrogen atom to a free radical to neutralize it and reduce or prevent cellular damage. There are two mechanisms of action proposed for antioxidants: (1) chain-breaking step by donating an electron to the free radical, and (2) removal of ROS/reactive nitrogen species which initiates the secondary antioxidants by quenching chain-initiating catalysts including electron donation, metal ion chelation, co-antioxidants, or by gene expression regulation (Rice-Evans and Diplock, 1993). Bioactive molecules from spices and herbs act mostly as free radical scavengers and block free radicals by donating a hydrogen atom. Since these antioxidant compounds possess low activation energy, the antioxidant free radicals produced are stabilized by their electrons delocalization which thus would not readily react to propagate additional free radicals. The mechanism of action can be explained by the structures of antioxidant compounds from spices and herbs (Tables 6–8, Fig. 3) in which the presence of aromatic rings and the substituent groups provide for stabilization of the structure even after donation of hydrogen atom. Most antioxidants in dietary plants are phenols, which act as chain-breaking antioxidants because their −OH group scavenges reactive radicals such as peroxyl radicals (ROO•). For example, carnosic acid—a phenolic diterpene is rosemary’s major oil-soluble antioxidant and rosmarinic acid—a caffeic acid dimer is its most powerful water-soluble antioxidant. Allicin, diallyl disulfide and diallyl trisulfide inhibit autoxidation by suppressing the hemolytic decomposition of hydroperoxides (Kim et al., 1997). Extracts of black pepper, nutmeg, rosehip, cinnamon and oregano leaf showed radical-scavenging effects and chelating capacities against Fe2+ and Cu2+ (Su et al., 2007).
The reaction mechanism of lipid oxidation involves 3 stages: initiation, propagation and termination. During the initiation stage, the lipid (RH) molecule through the action of catalysts breaks down to produce free radicals which can react with lipids, proteins or DNA causing cellular damage. More free radicals are formed during the propagation phase resulting in rapid oxidation. These free radicals react with oxygen to produce more free radicals to quickly oxidize lipid or other molecules. To prevent, minimize or slow down the rate of oxidation, oxygen and metal catalysts must be removed, or sequestered to render them unreactive. The antioxidants from spices and herbs can minimize or stop oxidation through their free radical scavenging property at various stages of oxidation: (1) Initiation stage—oxygen scavengers and/or chelating agents; (2) Propagation stage—oxygen and/or free radical scavengers; and (3) Termination stage—free radical scavengers. Through these, the free radicals are neutralized and thus reducing their capacity to damage cellular tissues.
The effects of excessive free radicals in the body is termed as oxidative stress and in the absence of antioxidants to neutralize them are responsible for: (1) Inflammatory diseases—arthritis,vasculitis, glomerulonephritis, lupus erythematous, adult respiratory diseases syndrome; (2) Ischemic diseases—heart diseases, stroke, intestinal ischema; (3) neurological disorder—Alzheimer’s disease, Parkinson’s disease, muscular dystrophy and many other diseases (Lobo, 2010; Shahidi and Ambigaipalan, 2015).
| 8. Anti-inflammatory effects of spices and herbs | ▴Top |
When the human body is exposed to environmental stressors and pathogens, the response is inflammation. There are 2 stages that occur in response to these stimuli (Aggarwal et al., 2009): (1) acute inflammation that is initiated by the immune cells which occurs in a short time; but if the inflammation persists, (2) chronic inflammation is initiated which brings about chronic diseases such as arthritis, cancer, cardiovascular diseases, diabetes, and neurological diseases.
Figure 5 shows the molecular pathway of inflammation linked to chronic diseases (Kunnumakkara et al., 2018).
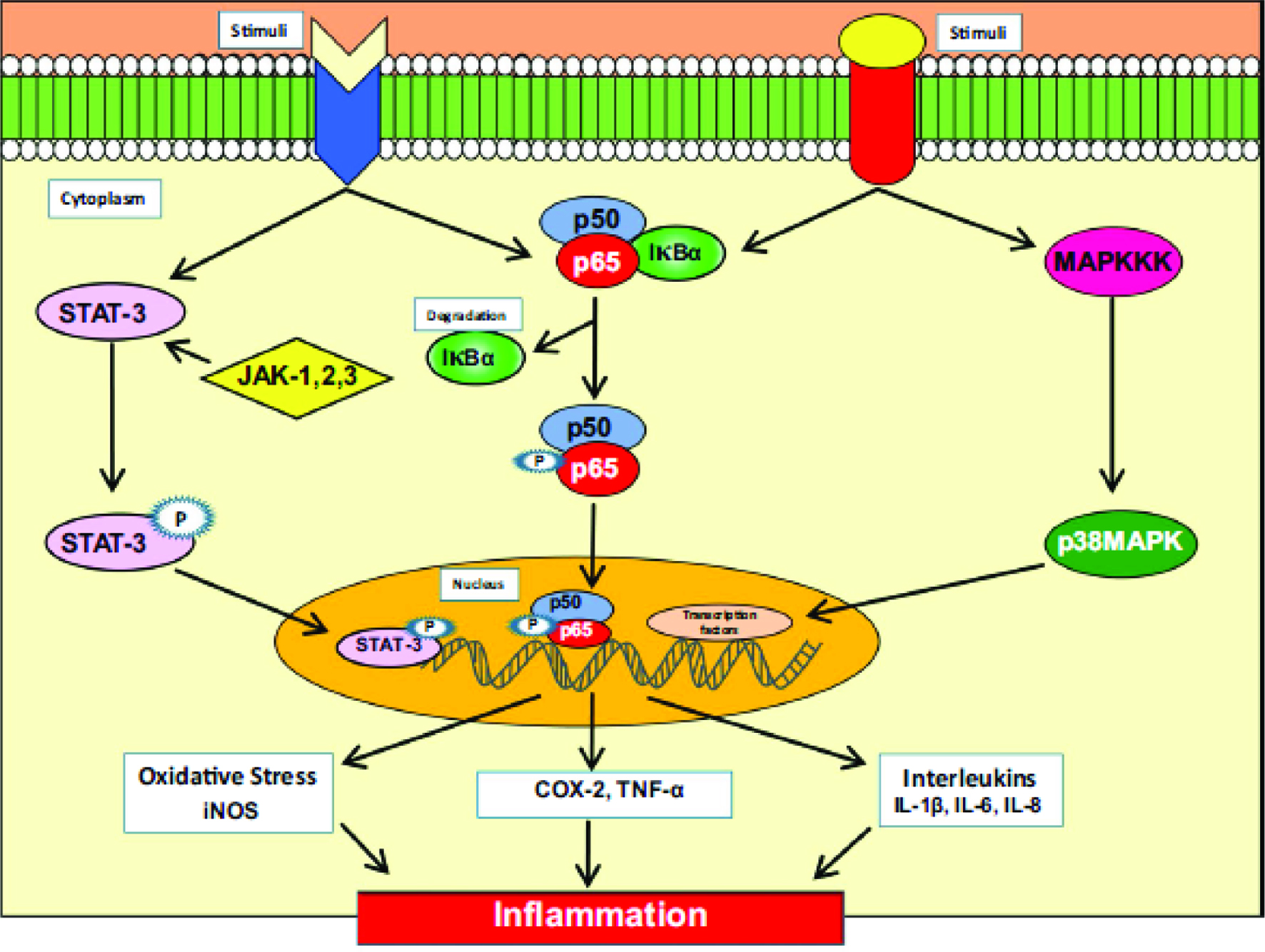 Click for large image | Figure 5. Molecular pathway of inflammation linked to chronic diseases. Source: Kunnumakkara et al., 2018, J. Transl. Med. 16:14. Chronic diseases, inflammation, and spices: how are they linked? BioMed Central. https://doi.org/10.1186/s12967-018-1381-2. http://creativecommons.org/publicdomain/zero/1.0/. |
Based on this diagram, NF-κB and STAT3, inflammatory enzymes such as cyclooxygenase-2 (COX-2), matrix metalloproteinase- 9 (MMP-9), and inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukins (IL) such as IL-1, -6, -8, and chemokines are the main molecular mediators of the immune responses to infection, injury or environmental stressors (Kunnumakkara et al., 2018) . What occurs next is the translocation of subunits p50 and p65 into the nucleus which bind to the promoters regions of various genes and activate more than 400 genes that are involved in inflammation and other chronic diseases (Yadav et al., 2010). When NF-κB is activated, it is known to initiate cancel cell proliferation, survival, invasion, angiogenesis, metastasis, chemoresistance, and radiation resistance and it also regulates the expression of inflammatory mediators such as COX-2, inducible nitric oxide synthase (iNOS), TNF-α, and interleukins (Kawabata et al., 2010). Overexpression of the cytokine, TNF-α, the most potent pro-inflammatory cytokine so far discovered, can lead to various chronic diseases, including cancer, via the activation of NF-κB (Kunnumakkara et al., 2018). There are several potential strategies that can be used for the prevention and management of chronic diseases: (1) Employ blockers of TNF-α, (2) Upregulation of COX-2, iNOS, and aberrant expression of TNF-α and IL-1, IL-6 and IL-8 have been reported to play important roles in oxidative stress that leads to inflammation (Aggarwal 2009; Reuter et al., 2010; Sung et al., 2012; Pandurangan et al., 2015), and (3) Employ the mitogen-activated protein kinase pathway (MPK) as a potential molecular target for the treatment of chronic inflammatory diseases (Liang et al., 2016).
Spices and herbs contain bioactives that can interact with multiple targets and alter dysregulated inflammatory pathways and mediators associated with chronic diseases (Kunnumakkara, et al., 2018, Figure 6). A summary of these bioactives and their potential mechanisms for mitigating chronic diseases are summarized in Table 9.
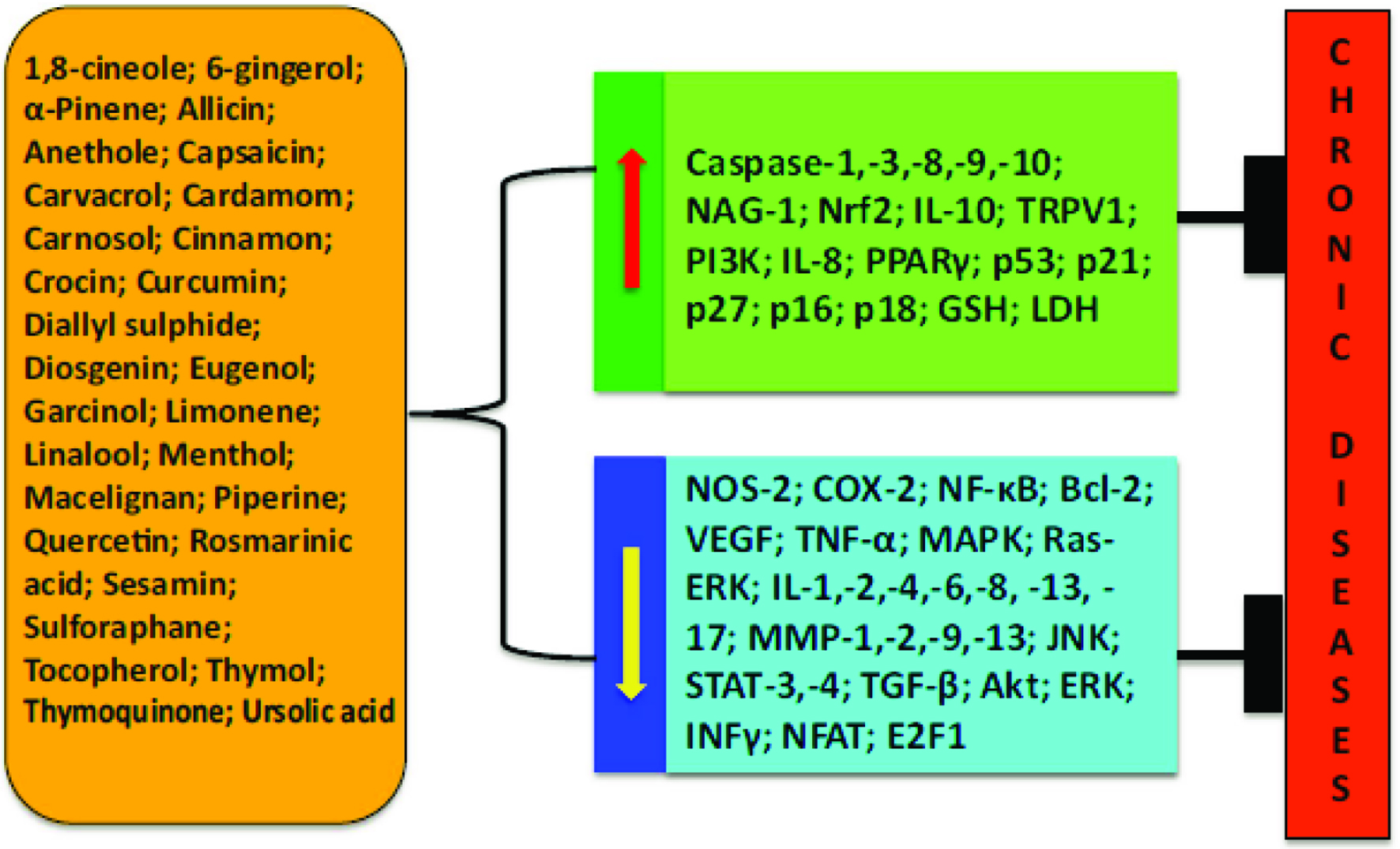 Click for large image | Figure 6. Different bioactive components of spices and their molecular mechanisms against chronic diseases. Source: Kunnumakkara, et al., 2018, J. Transl. Med. 16:14. Chronic diseases, inflammation, and spices: how are they linked? https://doi.org/10.1186/s12967-018-1381-2. http://creativecommons.org/publicdomain/zero/1.0/. |
 Click to view | Table 9. Bioactive compounds from selected spices and herbs and their potential mechanisms |
| 9. Anticarcinogenic and chemopreventative activities associated with spices | ▴Top |
DNA damage has been associated with aging and cancer. Pathways associated in the development of cancer includes oxidative DNA damage brought about by redox activity of endogenous and exogenous species such as caused by active oxygen species (ROS). Jebalan et al. (2015) reported 11 aqueous and non-aqueous extracts from anise (Pimpinella anisum), coriander (Coriandrum sativum var. vulgare), cumin (Cuminum cyminum), dill (Anethum graveolens), fennel (Foeniculum vulgare var. vulgare), caraway (Carum carvi), celery (Apium graveolens) parsley (Petroselinum crispum), carrot seeds (Daucus carota), angelica (Angelica archangelica), and ajowan (Carum copticum) all from the Apiaceae family. Jebalan et al. (2015) found that aqueous (5 mg/ml) and non-aqueous extracts (6 mg/ml) substantially inhibited (83–98%) formation of DNA adducts in the microsomal reaction but only aqueous extracts showed the inhibitory activity (83–96%) in non-microsomal reaction. Adduct inhibition was also observed at 5-fold lower concentrations of aqueous extracts of cumin (60%) and caraway (90%), and 10-fold lower concentrations of carrot seeds (76%) and ajowan (90%) and these results suggests the presence of two groups of phytochemicals—polar compounds that have free radical-scavenging activity, and lipophilic compounds that selectively inhibit P450 activity associated with estrogen metabolism (Jebalan et al., 2015). These are significant findings which demonstrate that these Apiaceae species may be potentially protective against estrogen-mediated breast cancer.
In another study on the in vitro evaluation on prostate cells, Lackova et al. (2017) showed that the extracts from black pepper and caraway seed gave the strongest inhibitory effect on prostatic cells using the Cell-Line Proliferative Activity Testing (MTT Assay). This is used to evaluate the cells’ metabolic activity and provide the cytotoxicity of the tested compounds. The activity of the black pepper extract was postulated to be due to 3,4-dihydroxybenzaldehyde and naringenin chalcone and from caraway seeds, neochlorogenic acid and apigenin. Lackova et al. (2017) identified naringenin chalcone to be the most potent growth inhibitor of prostate cell.
Vanilla is one of the most important spices and flavorings. Vanillin which is responsible for the desirable aroma and flavor in vanilla extract has been shown to suppress metastasis in a mouse model (Jantaree et al., 2017) using the Transwell invasion assay but the homodimer of vanillin (divanillin, also a vanilla extract compound) exhibited a potency higher than vanillin and apocynin which was attributed to inhibiting phosphorylation of FAK and Akt and divanillin’s stronger binding to the Y397 pocket of the FAK FERM domain based on molecular docking studies.
A review on spices for prevention and treatment of cancers by Zheng et al. (2016) evaluated more than 250 scientific papers on the subject. They concluded that numerous studies have documented the antioxidant, anti-inflammatory and immunomodulatory effects of spices might be related to prevention and treatment of several cancers and that several spices are potential sources of bioactive compounds for these effects: Curcuma longa (tumeric), Nigella sativa (black cumin), Zingiber officinale (ginger), Allium sativum (garlic), Crocus sativus (saffron), Piper nigrum (black pepper) and Capsicum annum (chili pepper). These spices contain several important bioactive compounds, such as curcumin, thymoquinone, piperine and capsaicin the mechanisms of action include inducing apoptosis, inhibiting proliferation, migration and invasion of tumors, and sensitizing tumors to radiotherapy and chemotherapy.
| 10. Blood glucose control of spices and herbs | ▴Top |
Bioactives from chili pepper, cinnamon, ginger, turmeric, fenugreek, rosemary, and garlic maybe beneficial in the control of glucose intolerance and diabetes (Srinivasan, 2005; Chase and McQueen, 2007; Magistrelli and Chezem, 2012; Bayan et al., 2014; Upsani et.al., 2014; Heshmati and Namazi, 2015; Rashmi and Shilpy, 2016; Bi and Lim, 2017; Yasnin et al., 2017; Ge et al., 2017; Ranasinghe et al., 2017; Byrne et al., 2017 ). Figure 7 shows that associated with spices, it was found that diabetes mellitus, inflammation and carcinogenesis have the highest number positive associations and that the spices have a preventive role in various cancers (Rakhi et al., 2018). Chili pepper was associated with a decrease in insulin levels and healthy insulin in human trials while the doubly-linked phenol type-A polymers in cinnamon is the bioactive responsible for the control of glucose intolerance and potentiates insulin action. Ginger on the other hand can prevent and/or inhibit protein glycation which has been implicated in diabetes. Turmeric has been shown to improve glucose indices and decrease glucose in serum. An extract of fenugreek seed improved insulin signaling and sensitivity and was comparable with metroformin, a drug used to treat high blood sugar. The soluble fiber in fenugreek helps maintain healthy glucose absorption. Rosemary activates PPARgamma leading to lower levels of glucose and inhibitor of α-glucosidase which may help reduce sugar absorption. Garlic powder tablets (Allicor) lowered fasting blood glucose.
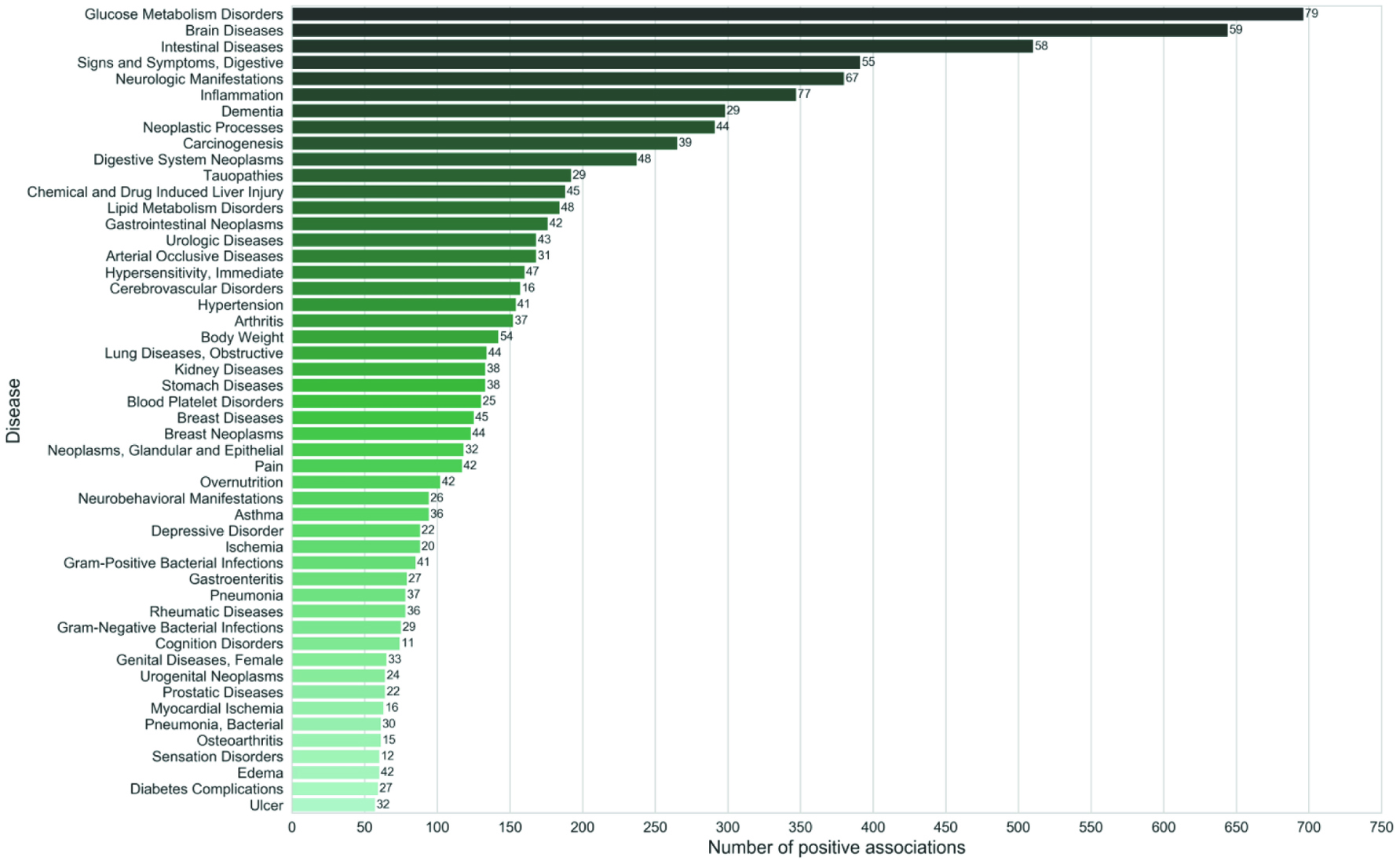 Click for large image | Figure 7. Top diseases (Third level of MeSH hierarchy) ranked according to their total number of positive associations. Numbers shown against the bars indicate the ‘number of spices’ involved in the associations. Source: Rakhi et al., 2018, PLoS ONE 13(5): e0198030. Data-driven analysis of biomedical literature suggests broad-spectrum benefits of culinary herbs and spices. (MeSH—Medical Subject Headings is a controlled vocabulary of biomedical terms curated and developed by National Library of Medicine. It organizes terms hierarchically from general to more specific.). www.PLOS.org. https://doi.org/10.1371/journal.pone.0198030. |
There are other benefits of bioactives from spices and herbs such as enhancing nutrient bioavailability (piperine), weight management and satiety (chili pepper, ginger, fenugreek, piperine), gut health (chili pepper, turmeric), anti-bacterial and anti-fungal (eugenol and cinnamaldehyde from cinnamon), hepatoprotective effect against CCl4 (ethanol extract of cinnamon), neuroprotective (cinnamaldehyde from cinnamon), reduce nausea and vomiting during pregnancy or after chemotherapy (ginger), joint and muscle health (ginger), digestion aid (black pepper), lipid metabolism and vascular health (fenugreek, rosemary), chemopreventive and anti-carcinogenic (rosemary), antithrombotic and anticoagulant (garlic), and immunomodulatory activity (garlic). Prevalence of cardiovascular disease (CVD) as correlated to diets in which spices play an important role was summarized by Tsui et al. (2018). Based on this review incidence of CVD increases when spices are not used in preparation of food as follows (1) Western diet (spice-free with salt and sugar): 11–15%, (2) Arabic diet (saffron, peppers, allspice, turmeric, garlic, cumin, cinnamon, parsley and coriander): 7–12%, (3) Indian diet (cardamom, clove, cassis, peppers, cumin coriander, nutmeg, mustard seed, fenugreek, turmeric, saffron and garlic): 7–11% (4) Chinese diet (Cardamom, cinnamon, cumin, cloves, peppers, nutmeg, peppercorns, fennel, star anise, garlic, ginger and chili peppers): 5%, and (5) Mediterranean diet (Anise, basil, bay leaf, cardamom, cinnamon chervil, chilis, chives, cloves, cumin, coriander, dill, fennel, fenugreek, garlic, mace, marjoram, mint, nutmeg, oregano, peppers, rosemary, saffron, sage, savory, sumac, tarragon and thyme): 1.5–3.2% (Tsui et al., 2018). Table 10 provides the health benefits of spices and herbs in greater detail based on various studies including the referenced scientific publications.
 Click to view | Table 10. Health benefits of spices and herbs |
| 11. Summary | ▴Top |
For millennia, spices and herbs have been used to flavor food but are also sought after for their medicinal power. In fact, as early as the 1st through the 4th centuries, Arabian developed techniques to distill essential oils from aromatic plants and around the 9th century, Arab physicians used spices and herbs to formulate syrup and flavoring extracts (Rosengarten, 1969) for homeopathic remedies. Today, there is a large volume of evidence that spices and herbs can help alleviate conditions linked with specific diseases as well as prevent or reduce risks associated with degenerative diseases such as cardiovascular diseases, diabetes, obesity and cancer. This is also supported by findings on meta-analysis of more than 1.5 million healthy adults that following a Mediterranean diet which included spices and herbs was associated with a reduced risk of cardiovascular mortality as well as overall mortality (Mayo Clinic, 2019). Scientific studies provided proof that spices and herbs from black pepper to vanilla contain phytochemicals that show strong antioxidant and anti-inflammatory activities both in vitro and in vivo and in clinical studies. Spices and herbs have shown therapeutic and protective potential against chronic disorders due to their anti-inflammatory, antiproliferative and pharmacological activities. There are still potential areas for future research that include concentration effects on treatment and acceptability, differences in reaction of humans to different phytochemicals as well as employing nutrigenomics to understand how the compounds in the diet (e.g., from spices and herbs) affect health by altering the expression of genes and the structure of an individual’s gene at a molecular level.
| References | ▴Top |