| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 31, September 2025, pages 101-106
Growth performance and hematological studies on albino rats fed with amylase-treated maize starch (Zea mays)
Oladipo Oladiti Olaniyia, b, *, Oluwashola Esther Owolabia, Tolulope Christianah Oladejoa, Muinat Olanike Kazeemc, Faith Charity Samuel-Osamokad
aDepartment of Microbiology, Federal University of Technology, PMB 704, Akure, Nigeria
bSwammerdam Institute of Life Sciences, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands
cDepartment of Microbiology, University of Ilorin, PMB 1515, Ilorin, Nigeria
dDepartment of Microbiology, Federal University Oye-Ekiti, PMB 373, Nigeria
*Corresponding author: Oladipo Oladiti Olaniyi, Department of Microbiology, Federal University of Technology, PMB 704, Akure, Nigeria. Tel
DOI: 10.26599/JFB.2025.95031424
Received: July 30, 2025
Revised received & accepted: September 10, 2025
| Abstract | ▴Top |
Malnutrition and immune-related disorders remain major concerns in developing countries, where staple diets are dominated by starchy foods with limited digestibility. Developing functional ingredients from local grains may improve nutrient bioavailability. This study evaluated amylase-treated maize starch (MS) as a dietary component in rats. Yeast strains from palm wine were screened for amylase activity, and the most active strain (O1-8, identified as Saccharomyces cerevisiae) produced 1.20 U/mL of amylase. The enzyme, partially purified by ethanol precipitation, was used to hydrolyze maize starch. In a 27-day feeding trial, rats fed enzyme-treated MS attained a mean weight of 79 g, compared with 69 g for untreated MS and 63 g (p < 0.05) for commercial feed. Hematological indices, including packed cell volume, hemoglobin, red blood cells, and white blood cells, improved significantly in the enzyme-treated group. These findings indicate that amylase-treated maize starch improves growth performance and hematological status in albino rats, providing a foundation for future studies, including human trials, to evaluate its potential as a functional dietary ingredient.
Keywords: Palm wine; Saccharomyces cerevisae; Amylase; Maize starch; Albino rats
| 1. Introduction | ▴Top |
In many developing regions, diets rely heavily on starchy foods, whose availability fluctuates with seasonal harvests. This overdependence on starch- and sugar-rich staples often results in nutritional imbalances, particularly among vulnerable populations such as children and the elderly, leading to energy and micronutrient deficiencies (Baptista et al., 2024). Starches, a major class of carbohydrates, are the primary source of energy in these diets, with maize serving as a prominent example. As one of the world’s most important cereal crops, maize is especially critical in developing countries, providing a substantial portion of daily caloric intake (Baptista et al., 2024). However, the nutritional value of maize depends largely on starch digestibility, which is mediated by α-amylase and other pancreatic enzymes, determining the energy yield from the grain. While traditional processing methods such as steaming and flaking can enhance starch digestibility, enzymatic treatments are increasingly preferred due to their efficiency and potential to improve nutrient availability for populations with limited access to diverse diets (Wang et al., 2024).
Enzymatic hydrolysis of starch into disaccharides and monosaccharides mimics natural digestion, facilitating efficient nutrient absorption in the gastrointestinal tract. In humans, α-amylase cleaves polymeric starch into digestible sugars, ultimately yielding glucose for energy metabolism. Despite extensive studies on microbial amylase production, no research has evaluated the effects of amylase-treated maize (Zea mays) on growth and hematological parameters in albino rats. Investigating such effects is particularly relevant for child nutrition and populations with impaired starch digestion, as enhanced starch digestibility could improve energy intake, nutrient absorption, and overall growth. Yeast strains, particularly Saccharomyces cerevisiae, are recognized for their robust hydrolytic capabilities and safety for human consumption, producing no toxic metabolites (Wang et al., 2024). Therefore, this study aimed to hydrolyze maize starch using amylase from S. cerevisiae and evaluate its effects on growth and hematological status in albino rats, providing foundational evidence for potential nutritional benefits in humans.
| 2. Materials and methods | ▴Top |
2.1. Sample collection and yeast isolation
Fresh wine obtained from raffia palm was collected in a sterile container from some towns (Ibule, Ado-Ekiti, Osogbo, Ibadan, Iworoko, Igbara-Odo, Ikirun, Ilara, Igbo Oja, Abeokuta, Oba-Ile, Owena, Isarun, Ikogosi, Ikire, Ikere, Ero and Sagamu) in the Southwestern Nigeria and transferred to laboratory immediately. An enrichment technique was adapted for yeasts isolation (Sniegowski et al., 2002). The enrichment medium contains (w/v) per liter, glucose (1%), yeast extract (0.3%), peptone (0.5%), malt extract (0.3%), ethanol (8% v/v), chloramphenicol (200 μg/mL) and 1 M HCl (1 mL). Following 7 days-incubation (28 °C), the medium was serially diluted, pour plated in yeast peptone dextrose agar (YPDA) and incubated at 28 °C. The pure culture was obtained by sub-culturing the distinct colonies. After 48 h, a representative colony of interest from a mixed culture plate was aseptically transferred to an already prepared agar plate. S. cerevisiae was identified by means of morphological examination and sugar fermentation tests. The pure isolates were preserved on YPDA (4 °C) for further studies (Kazeem et al., 2025).
2.2. Fermentative characterization and identification of yeast isolates
The sugar characteristic fermentative ability of the yeast isolates served as guidelines for identification purpose. The fermentative ability was profiled using different sugars involving hexoses (glucose, fructose, galactose), and disaccharides (maltose, lactose, and sucrose). The sugars were separately prepared in a test tube containing 5 ml Phenol red broth medium, inoculated with the isolates accordingly and incubated at 30 °C for 48 h. Afterwards, the tubes were observed for colour change. Colour change from red to yellow is an indication of sugar fermentation and signal reduced pH due to acid production (Barnett et al., 1990).
2.3. Crude amylase production
Crude amylase was produced in a mineral salt medium. Compositionally, the medium had (g/L) Yeast extract (0.03), MgSO4.H2O (0.03), (NH4) SO4 (0.14), MnSO4.H2O (0.016), KH2PO4 (0.2), CaCl2 (0.03), peptone (0.1), ZnSO4.H2O (0.014) and soluble starch (1.5). The medium was autoclaved for 15 min at 121 °C. A 48 h old pure yeast strain (2% v/v) was inoculated into Erlenmeyer flask containing 100 mL of the basal medium and incubated at 30 °C for 48 h. Crude enzyme preparations were separated from yeast biomasses through centrifugation at 5,000 rpm for 20 min at 4 °C in a cool centrifuge. The supernatant was decanted into test tubes and used as the crude extracellular enzyme preparation (Owolabi et al., 2023).
2.4. Enzyme assays
Amylase activity was assayed in a reaction mixture containing 1 ml soluble starch (1%), buffered at pH 6.8 in 50 mM phosphate buffer, and 1 mL crude enzyme preparation at 45 °C for 60 min. The quantity of reducing sugars produced in the reaction mixture was estimated by the dinitrosalicylic acid reagent (DNS) (Miller, 1959). A unit of amylase activity was set as quantity of enzyme that yielded 1 micromole of maltose per minute within standard experimental limits.
2.5. Preparation of cell free extract
A basal medium with the appropriate composition containing the necessary nutrients for amylase production was prepared and autoclaved at 121 °C for 15 min. After autoclaving, the medium was cooled and aseptically inoculated with the best amylase-producing yeast strain. The medium was incubated at 30 °C for 48 h. After the expiration of incubation, culture biomass was separated from the medium and subsequent filtrate spun in a centrifuge at 4,000 rpm for 20 min (Owolabi et al., 2023). The supernatant which contains the enzyme was further partially purified according to Adebiyi et al. (2008). The enzyme was precipitated out of the supernatant with 30% ethanol and pelletized by centrifuged at 4,000 rpm for 20 min. The sediment was taken as the portion containing the enzyme. The pellet was recovered and suspended in distilled water.
2.6. Preparation of starch
Maize kernels were steeped for 4 h, ground, and the shafts separated by wet sieving. The starch filtrate was left to settle, after which the supernatant was decanted, leaving the starch. The starch was then sun-dried for three consecutive days under consistent conditions. To reduce variability, all batches were dried at the same time of day with uniform sample thickness and exposure to direct sunlight. After drying, the starch was thoroughly mixed to ensure homogeneity before being stored for use (Adebiyi et al., 2008).
2.7. Enzymatic hydrolysis of corn starch
Maize starch hydrolysis was carried out by suspending 12 g of corn starch in 100 mL of water, which contained 0.4 g calcium carbonate and 0.4 g sodium chloride. The suspension was mildly heated in a water bath. At 40 °C (water bath temperature), 5 ml partially purified amylase enzyme was added. The hydrolysis was performed in water bath for 2 h, after which the hydrolyzed starch cooled and sundried for 3 days (Adebiyi et al., 2008).
2.8. Animal care and feeding trial
The feeding trial was performed with a total number of nine male albino rats (young adults) procured in Akure, Ondo State, Nigeria. The animal feeding trial was granted by ethical and school of postgraduate studies advisory committee of the Federal University of Technology Akure, Nigeria. The rats were weighed and randomized into 3 treatment groups. Each group made of three rats was placed in different cage with adequate ventilation and illumination. The rats were firstly acclimatized to the laboratory ambient conditions by feeding with commercial feeds for five days prior to the 27 days feeding trial. Each feeding unit (group) was fed according to their body weight and daily allocated ratio. Regular supplies of potable water and daily cleaning of the cages were also maintained throughout the experiment. The changes in body weight of the animals were monitored throughout the experimental period.
2.9. Experimental diets
The composition of the various diet formulae for the feeding trial were as listed below:
- Diet 1: hydrolyzed maize starch (65.5%); fish meal (21.0%), palm oil (5.0%), cellulose (5.0%), bone meal (2.5%), oyster shell (0.5%), salt (0.25%), and premix (0.25%).
- Diet 2: unhydrolyzed maize starch (65.5%), fish meal (21.0%), palm oil (5.0%), cellulose (5.0%), bone meal (2.5%), oyster shell (0.5%), salt (0.25%), and premix (0.25%).
- Diet 3 (control): Commercial feed obtained from a certified veterinary clinic in Akure, Nigeria, served as the reference diet. The control diet consisted of a commercially packaged standard laboratory rat chow (a certified veterinary clinic, Akure, Nigeria). Detailed proximate composition data were not available from the packaging at the time of purchase. However, the feed is widely used in rodent studies as a standard maintenance diet and served as the reference diet against which maize starch and amylase-treated maize starch diets were compared.
2.10. Effect of formulated diets on the growth and hematological status of albino rats
The daily body weight of the animals in all the feeding treatments were weighed for the 27 days. On the 27th day, the mean average weight of the albino rats in each group (hydrolyzed, unhydrolysed and commercial) was recorded. Thereafter, the rats in each group were anaesthetized with diethyl ether and their blood was sampled by cardiac puncture for hematologic studies. Each group blood was pooled together into an ethylene diaminetetracetic acid (EDTA) tube for packed cell volume (PCV), red blood cells (RBC), Hemoglobin (Hb), erythrocyte sedimentation rate (ESR), mean cell volume (MCV), and total white blood cell (TWBC) determination (Adebiyi et al., 2008).
2.11. Statistical analysis
All quantitative data were expressed as mean ± standard deviation (SD). Comparisons between groups were performed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons. Differences were considered statistically significant at p < 0.05. Graphs include error bars representing SD, and statistically significant differences between groups are indicated with superscript letters or symbols.
| 3. Result | ▴Top |
3.1. Total yeast counts from palm wine
In Table 1, total yeast counts from palm wine collected from different locations in the Southwestern Nigeria were presented. Palm wine collected from Isarun, Ondo State was observed to give the highest number of yeast population with 9.6 × 109 CFU/mL, while the wine from Iworoko, Ekiti State had the lowest value of 1.0 × 108 CFU/mL.
 Click to view | Table 1. Total yeast counts from palm wine from different locations (CFU/mL) |
3.2. Amylase production by yeast isolates from different palm wine
The quantitative determination of amylase from yeasts isolated from different palm wine is shown in Table 2. All the yeast strains screened for amylase production displayed varied amylase activity. The highest amylase activity of 1.20 U/mL was produced by the isolate designated O1-8 from Ikire, Osun State, while the lowest value of 0.62 U/mL was obtained from isolate H2-3 from Ilara, Ondo State. Therefore, yeast isolate designated as O1-8 was selected for animal feed formulation because of its high amylase production potential.
 Click to view | Table 2. Production of extracellular amylase by yeast isolates from palm wine |
3.3. Tentative identification of yeast code O1-8
The morphologic features of the yeast isolate encoded O1-8 was confirmed by mounting on glass slide and stained with crystal violet. On microscopic examination, ascospores were seen (result not shown). Physiologic sugar fermentative profiling of isolate was carried out with carbon substrates such as galactose, glucose, sucrose, maltose, and raffinose (Table 3). The isolate was tentatively identified as S. cerevisiae grounded on its characteristic cellular morpho-physiologic and sugar fermentative attributes.
 Click to view | Table 3. Fermentation test leading to the tentative identification of yeast code O1-8 |
3.4. Effect of amylase-treated maize starch, untreated maize starch and commercial diets on daily weight of albino rats
The average daily body weight of the rats throughout the 27-day feeding trial is shown in Fig. 1. Rats fed Diet 1 and Diet 2 exhibited increases in body weight during the trial period. The final average body weight of rats fed enzyme-treated and untreated maize starch (Diets 1 and 2) was higher than that of rats fed Diet 3 (commercial feed). In the Diet 3 group, mean body weight increased until day 18, with values of 62 g and 64 g on days 12 and 15, respectively, followed by a decrease to 61 g on day 27. Daily body weight measurements for the hydrolyzed maize starch group (Diet 1) are shown in Fig. 1.
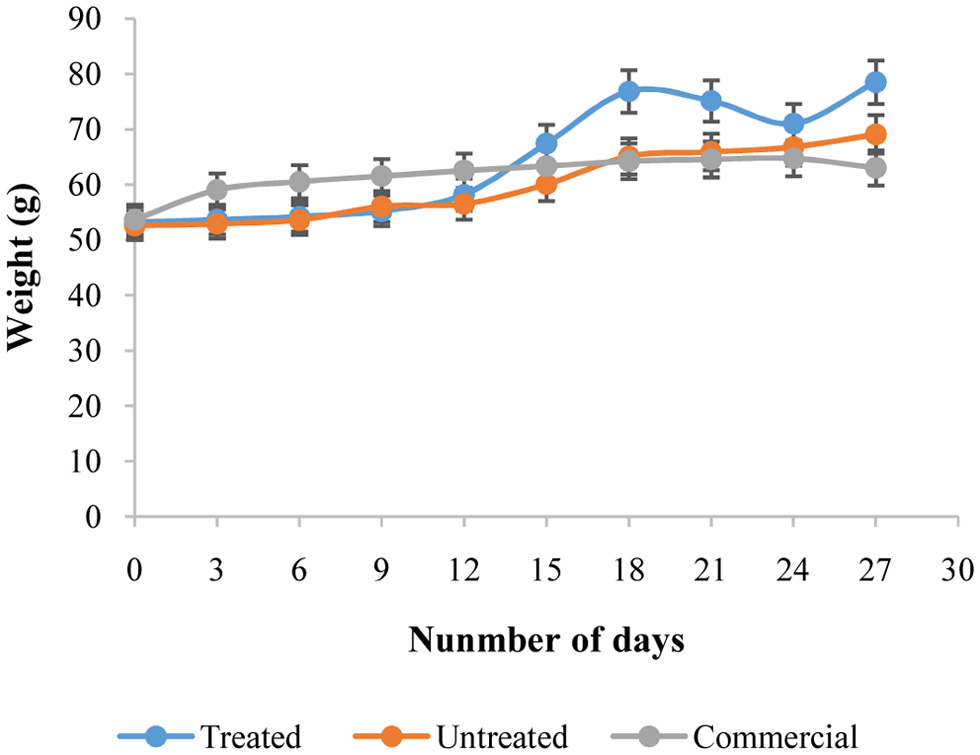 Click for large image | Figure 1. Average daily body weight of albino rats fed amylase-treated maize starch (Diet 1), untreated maize starch (Diet 2), or commercial feed (Diet 3) over 27 days. Data represent mean ± SD of n = 3 rats per group. Different letters indicate statistically significant differences between groups (p < 0.05). |
3.5. Hematologic properties of amylase-treated-corn-starch fed albino rats
Fig. 2a show the hematologic condition of rats fed with different diet (control, amylase treated and untreated diets). In Fig. 2a, the rats fed with enzyme-treated corn had the highest packed cell volume (PCV) of 38% while lowest value (33% PCV) was observed in the control group. Also, highest hemoglobin count (Hb) (12.7 g/dl) was obtained in the rats fed with enzyme-treated diet while the group fed with the control (Diet 3) had the least value (11.0 g/dl) (Fig. 2b). The red blood cell (RBC) count was highest (9.6 × 105 µl−1) in rats fed with the enzyme-treated corn but least (6.4×105µl−1) in control group (Fig. 2c). The erythrocyte sedimentation rate (ESR) was 2.0 mm/h in the rats fed with the untreated feed but had equal value of 0.5 mm/h in other groups (Fig. 2d). The total white blood count (TWBC) was 9,350 mm3 in the rats fed with enzyme treated feed but least in the control group (7,025 mm3) (Fig. 2e). However, the commercial diet fed rats had the highest mean cell volume (MCV) value of 47.4 µ3 while the lowest value (42.2 µ3) was recorded in enzyme-treated diet fed rats (Fig. 2f).
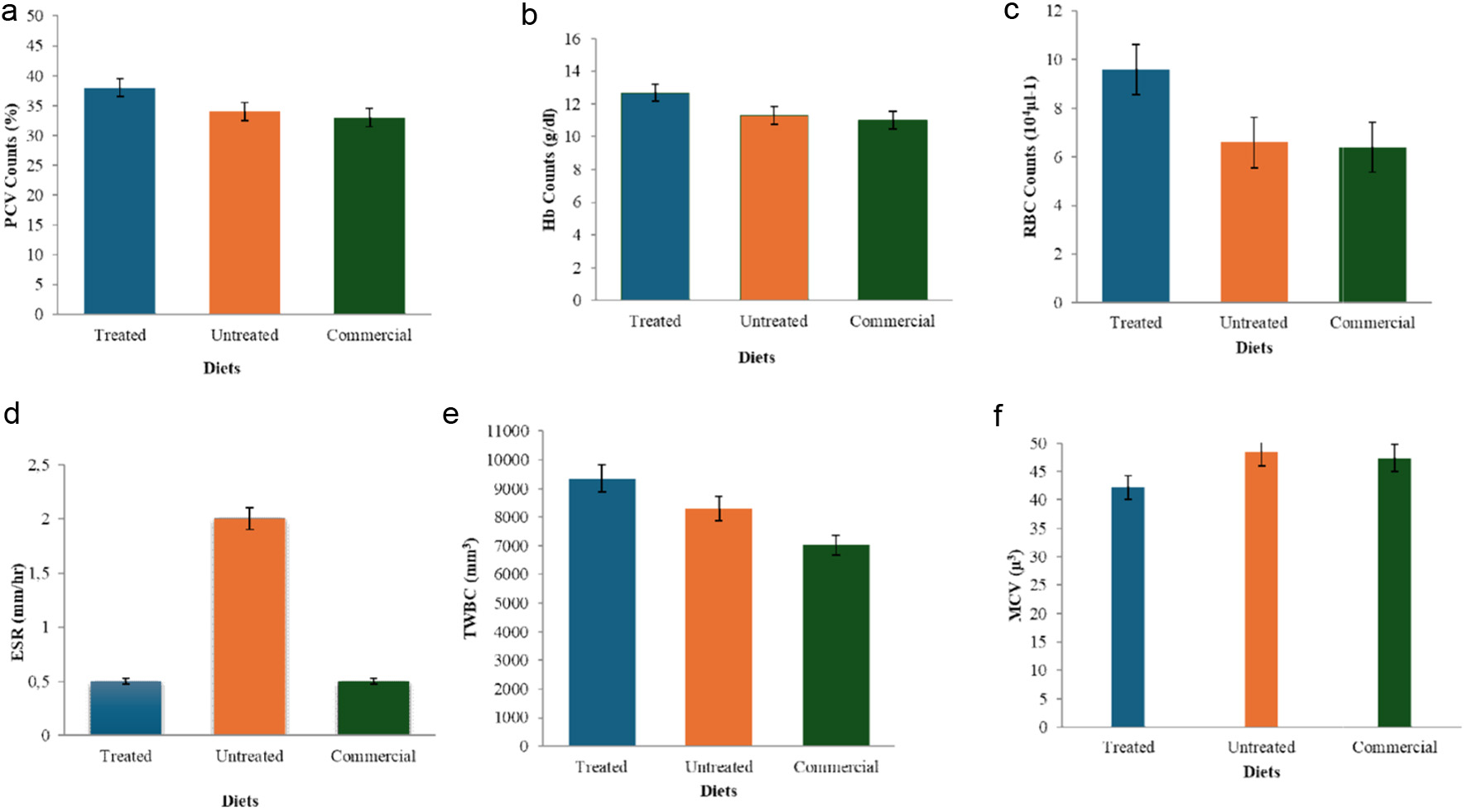 Click for large image | Figure 2. Effects of enzyme-treated, untreated and commercial diets on the PCV (a), hemoglobin counts (b), red blood cell count (c), erythrocyte sedimentation rate (d), total white blood count (e), and mean cell volume of the albino rats (f). |
| 4. Discussion | ▴Top |
In this study, yeast was deliberately selected and isolated using YPDA medium supplemented with 1 M HCl and 8% ethanol to favor the growth of extremotolerant strains. This isolation methodology aligns with Kumar et al. (2011) and Osilo et al. (2024). The high yeast population in palm wine collected from Isarun may be attributed to uncontrolled fermentation and inoculum sources from unsterilized containers previously used for sap or wine collection (Tapsoba et al., 2014; Djeni et al. 2022). Techniques applied during palm wine tapping and collection have been reported to influence microbial properties (Djeni et al. 2022), highlighting the need for proper hygienic procedures to prevent contamination. Variations in yeast counts among samples might also be influenced by environmental factors, handling processes, packaging materials, and human interference. The presence of yeast further supports the richness of fermentable sugars in palm wine, which allow yeast to thrive and produce acid during fermentation (Essien et al., 2011; Djeni et al. 2022).
The observed differences among strains’ amylase production potentials may relate to their sources and genetic pedigrees (Olaniyi and Akinyele, 2018). Variations in protein content among strains could result from the production of non-target hydrolytic enzymes such as cellulases, xylanases, and proteases. Other cellular metabolites might interfere with amylase production, contributing to fluctuations in measured protein contents, which reflect total protein rather than enzyme-specific proteins (Olaniyi and Akinyele, 2018). The capacity to degrade starch is widely distributed among bacteria and fungi (Fogarty and Kelly, 2004; Radzlin et al. 2024), and starch degradation is a complex process requiring multiple microbial enzymes. Amylase activity is influenced by the nature of the substrate, with soluble starch commonly used for screening microbial amylase producers. In our study, physiologic sugar fermentative profiling of the isolate showed a color change from red to yellow after 48 h, indicating acid production, consistent with Kumar et al. (2011).
Notably, this study highlights a novel application of S. cerevisiae isolated from palm wine as a source of amylase for functional feed formulation, which has not been previously reported. The increased body weight observed in rats fed diet 1 (amylase-formulated maize starch) compared with other groups may be attributed to enhanced nutrient bioavailability, ease of absorption, and efficient utilization due to enzymatic preprocessing of starch into readily digestible disaccharides and oligosaccharides. Hydrolyzed starches are increasingly valuable in functional food development due to their low glycemic impact and enhanced digestibility (Baptista et al., 2024; Radzlin et al. 2024), making them potentially useful in baby foods, specialty foods, and diabetic-friendly formulations (Radzlin et al. 2024; Chang et al., 2025). Hydrolyzed starch products may also benefit confectionery formulations (Chang et al., 2025).
The increased body weight observed in rats fed diet 1 (amylase-formulated maize starch) may be associated with the enzymatic hydrolysis of starch into more readily digestible disaccharides and oligosaccharides. Hydrolyzed starches are increasingly valuable in functional food development due to their low glycemic impact and digestibility (Baptista et al., 2024). Improved hematological parameters in rats fed enzyme-treated maize starch likely reflect the nutritional effects of the diet, including the potential contribution of partially hydrolyzed starch products to overall nutrient utilization (Böswald et al., 2023). Increased in RBC and hemoglobin (Hb) concentrations might indicate improved oxygen transport capacity (Adebiyi et al., 2008), with packed cell volume (PCV) also influenced by starch hydrolysis products such as fructooligosaccharides (Mikulic et al., 2024; Husmann and Zimmermann, 2022). These results indicate that enzyme-hydrolyzed starch may have potential benefits in food and feed production. The enzyme-formulated diet also led to higher white blood cell counts, suggesting an effect on immune parameters. Although partially hydrolyzed feed ingredients are recommended for partially breastfed infants at risk of allergic disease (EAACI–ESPGHAN consensus, 2020), our findings in rats only suggest that enzymatic starch hydrolysis may influence nutrient utilization and hematological outcomes. Further studies are necessary to assess the safety and efficacy of such diets in humans before considering applications in children, diabetic patients, or specialty foods.
One limitation of this study is the lack of detailed proximate composition for the commercial control feed, as it was supplied without nutritional labeling. While this feed is a standardized laboratory chow, the absence of nutritional data may limit the precision of comparisons. Another limitation is the small sample size (n = 3 per group), which may reduce statistical power and generalizability. Despite these limitations, the findings provide preliminary evidence supporting the use of yeast-derived amylase to enhance starch digestibility and promote growth and hematological health.
Overall, these findings demonstrate that locally sourced yeast-derived amylase can convert maize starch into a functional dietary ingredient with measurable benefits on growth and hematology, providing a foundation for future functional food and feed applications.
| 5. Conclusion | ▴Top |
Saccharomyces cerevisiae isolated from palm wine effectively hydrolyzed maize starch, and feeding enzyme-treated starch to albino rats improved growth and key hematological parameters. This study demonstrates the novel use of locally sourced yeast to create a functional dietary ingredient. While the findings suggest potential applications in functional foods and specialized diets, further studies are needed to assess safety and efficacy in humans before considering use in pediatric nutrition or populations with impaired starch digestion. These results provide a foundation for integrating traditional microbial resources with modern nutritional strategies.
Acknowledgments
The technical supports from the Department of Microbiology, Federal University of Technology, Akure, Nigeria was highly appreciated. This research is self-sponsored.
| References | ▴Top |