| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 29, March 2025, pages 1-12
Phytochemistry, biological function and metabolism of Seleno-flavonoids
Ying Nia, b, Wensheng Zhanga, b, *, Jinchao Weic, Youhua Xud
aEngineering Research Center of Natural Medicine, Ministry of Education, Beijing Normal University, Zhuhai, 519087, China
bGuangdong Provincial Observation and Research Station for Coupled Human and Natural Systems in Land-ocean Interaction Zone, Beijing Normal University, Zhuhai 519087, China
cMacau Centre for Research and Development in Chinese Medicine, State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau 999078, China
dFaculty of Chinese Medicine, Macau University of Science and Technology, Macao SAR, PR China
*Corresponding author: Wensheng Zhang, Engineering Research Center of Natural Medicine, Ministry of Education, Beijing Normal University, Zhuhai, 519087, China. E-mail: zws@bnu.edu.cn
DOI: 10.26599/JFB.2025.95029399
Received: February 19, 2025
Revised received & accepted: March 18, 2025
| Abstract | ▴Top |
Selenoflavonoids (SeFs), emerging as a novel class of bioactive compounds that integrate selenium into flavonoid structures, have garnered significant attention due to their enhanced biological properties compared to conventional flavonoids. This review systematically analyzes recent advances in SeFs research, encompassing their structural characteristics, metabolic processes, and diverse biological functions. Studies reveal that selenium incorporation occurs primarily through the formation of covalent bonds between phenolic hydroxyl groups and selenium in its +4 oxidation state, resulting in compounds with superior bioavailability and reduced toxicity compared to inorganic selenium forms. The metabolic fate of SeFs involves complex pathways centered on hydrogen selenide (H2Se) as a crucial intermediate, with subsequent transformations regulated by selenium status and metabolic requirements. Extensive investigations demonstrate that SeFs exhibit remarkable therapeutic potential across multiple biological systems, including enhanced anti-inflammatory and antioxidant activities through NF-κβ pathway modulation and GPx-mimetic properties, neuroprotective effects via regulation of protein aggregation and neuroinflammation, metabolic benefits through modulation of glucose and lipid homeostasis, and antitumor activities targeting multiple cellular pathways. Current challenges in SeFs research include the optimization of isolation techniques, scalable synthesis methodologies, and the need for deeper mechanistic understanding of their biological activities. These findings suggest that SeFs represent promising candidates for therapeutic applications, though further research is needed to fully elucidate their molecular mechanisms and clinical potential.
Keywords: Selenoflavonoids; Structure; Metabolic processes; Biological activities
| 1. Introduction | ▴Top |
Recent studies exploring the interactions between selenium biochemistry and flavonoid compounds have suggested potential implications for pharmaceutical and nutritional research, contributing to our evolving understanding of bioactive compound mechanisms. Selenium, an essential trace element, plays crucial roles in human health through its incorporation into selenoproteins and its involvement in various physiological processes (Zhou et al., 2023). Concurrently, flavonoids, as one of the most diverse and widespread groups of plant secondary metabolites, have demonstrated remarkable biological activities including antioxidant, anti-inflammatory, and anticancer properties (Roy et al., 2022; Pecorini et al., 2023). The emergence of selenoflavonoids (SeFs), compounds that integrate selenium into flavonoid structures, represents a novel class of bioactive molecules that potentially combine and enhance the beneficial properties of both components. This integration has attracted substantial research interest due to several key factors. First, the incorporation of selenium into organic compounds typically results in enhanced bioavailability and reduced toxicity compared to inorganic selenium forms. Second, the resulting SeFs often demonstrate superior biological activities compared to their parent compounds, suggesting synergistic effects between the selenium and flavonoid components.
The understanding of SeFs has advanced significantly, spanning from their natural occurrence in selenium-enriched plants to the sophisticated synthetic methodologies developed for their preparation. The discovery that certain plant species can naturally incorporate selenium into flavonoid structures through specific metabolic pathways has provided valuable insights into the biological synthesis of these compounds. Furthermore, developments in analytical techniques have enabled detailed structural characterization of SeFs, facilitating our understanding of their chemical properties and potential biological mechanisms. The therapeutic potential of SeFs span a broad spectrum of applications, including but not limited to antioxidant, anti-inflammatory, neuroprotective, antidiabetic, and anticancer activities. The molecular mechanisms underlying these effects often involve complex interactions with cellular signaling pathways, suggesting sophisticated modes of action that may offer advantages over conventional therapeutic approaches.
This comprehensive review aims to analyze the current advances in both chemical and biological studies of SeFs by systematically investigating their structural characteristics, biosynthetic pathways, metabolic processes in the human body, and diverse biological activities, thereby highlighting the current state of knowledge and future research directions in this promising field.
| 2. Sources and accumulation of SeFs | ▴Top |
SeFs are predominantly found in plant species across several families, including Amaranthaceae (formerly Chenopodiaceae), Asteraceae, Brassicaceae, Fabaceae, Orobanchaceae, and Rubiaceae (Bañuelos et al., 2002; Di Gregorio et al., 2006; Statwick et al., 2016). Selenium hyperaccumulators possess unique physiological mechanisms that enable them to absorb and metabolize excess selenium without experiencing stress. This selenium-accumulating capability varies significantly across plant species and has led to the establishment of distinct categories based on accumulation thresholds in natural habitats. According to selenium content in their natural habitats, plants can be classified into three distinct categories: selenium hyperaccumulators (>1,000 mg·kg−1 DW), selenium accumulators (100–1,000 mg·kg−1 DW), and non-selenium accumulators (<100 mg·kg−1 DW) (Schiavon and Pilon-Smits, 2017). The first selenium hyperaccumulators were discovered in the western United States during the 1930s, demonstrating their remarkable ability to thrive in selenium-rich soils (Thornton et al., 1965).
The molecular mechanisms underlying selenium accumulation in plants reveal sophisticated enzymatic pathways that have evolved to manage selenium metabolism. Key differences in these pathways, particularly in enzyme expression and activity levels, help explain why certain plant species can accumulate and tolerate high selenium concentrations while others cannot.
A fundamental example of these specialized mechanisms is found in selenium hyperaccumulator Astragalus bisulcatus, which contains enhanced selenocysteine methyltransferase (SMT) enzyme activity, which catalyzes methylselenocysteine (MeSeCys) synthesis (Sors et al., 2009). This finding has been corroborated in A. thaliana studies, where SMT gene expression significantly increased the accumulation of MeSeCys and γ-glutamyl- methylselenoselenoic acid (Ellis et al., 2004).
In addition, the distribution of selenium within plant tissues exhibits distinct patterns between hyperaccumulators and non-accumulators. In Thlaspi violascens, a selenium hyperaccumulator, selenium-rich regions are concentrated in shoot and root tips and apical meristems, whereas non-accumulating species tend to contain minimal selenium primarily within their vascular systems (Both et al., 2020).
Among all selenium hyperaccumulators, members of the Fabaceae (legume) family not only represent the largest group but are also distinguished by their unique flavonoid metabolic characteristics (Kim et al., 2018; Zhou et al., 2018). This distribution pattern suggests that flavonoid metabolic pathways may play a crucial role in plant selenium accumulation mechanisms. Research has demonstrated that there exists a complex regulatory relationship between selenium accumulation and flavonoid biosynthesis. For instance, Astragalus species cultivated in soil containing 3 mg/kg selenium exhibit remarkable adaptive responses: plants show significantly elevated total flavonoid content, which not only increases the concentration of bioactive compounds but, more importantly, enhances the plant’s capacity for selenium accumulation and transport (Ma et al., 2023). In selenite-treated peanut seedlings, enhanced flavonoid accumulation was observed through the upregulation of the flavonoid branch of the phenylpropanoid pathway, which concurrently promoted selenium accumulation in plant tissues (Wang et al., 2016). This finding suggests that flavonoids may play a synergistic role in selenium metabolism and transport processes.
This synergistic relationship has been corroborated in other selenium hyperaccumulating species. Studies on Cardamine enshiensis have revealed that the activation of flavonoid biosynthetic pathways is closely associated with plant selenium tolerance. Under 400 μM sodium selenate treatment, transcriptome analysis revealed that 175 transcripts exhibited strong correlations with key flavonoid metabolites (Both et al., 2020). The upregulation of glutathione metabolism genes, particularly glucose-6-phosphate dehydrogenase (G6PD) with an 8.5-fold increase in expression, further substantiates that flavonoid-mediated protection against selenium-induced oxidative stress represents a sophisticated adaptive mechanism in selenium hyperaccumulators (Both et al., 2020).
These discoveries highlight the complexity of plant selenium accumulation mechanisms, indicating that this process depends not only on plant taxonomic position, metabolic enzyme systems, and tissue specificity but also involves a coordinated regulatory network of secondary metabolites, particularly flavonoids. Notably, flavonoids serve not just as characteristic secondary metabolites but potentially as key molecular bridges connecting selenium metabolism and plant stress resistance.
| 3. Chemical components and metabolism of SeFs | ▴Top |
3.1. Structure and classification of SeFs
Flavonoids represent one of the most diverse and widespread groups of polyphenolic compounds in the plant kingdom (Wang et al., 2018). These secondary metabolites constitute the largest class of phenolic compounds, with over 8,000 naturally occurring variants identified to date (Stasińska-Jakubas et al., 2023). As secondary metabolites, phenolic compounds can be broadly categorized into flavonoids and non-flavonoids (such as phenolic acids and hydrolyzable tannins). The fundamental structure of all flavonoids consists of a characteristic diphenylpropane (C6-C3-C6) skeleton, where two aromatic rings are connected by a three-carbon chain. The A-ring, typically derived from phloroglucinol or resorcinol molecules via the acetate pathway, exhibits characteristic hydroxylation patterns at C5 and C7 positions, while the B-ring, originating from the shikimate pathway, commonly displays 4′-hydroxylation, 3′4′-hydroxylation, or 3′4′5′-hydroxylation patterns (Wang et al., 2022). Chemical structure variations give rise to six principal subclasses of flavonoids: flavonols, flavones, flavanones, flavanols (including proanthocyanidins or condensed tannins), anthocyanidins, and isoflavones, each with distinct distribution patterns in plant tissues, shown as Figure 1 (Shen et al., 2022). Flavonols, including compounds such as kaempferol, quercetin, myricetin, and galangin, are widely distributed in common vegetables and fruits, particularly in broccoli, onions, asparagus, lettuce, tomatoes, apples, and grapes (Laoué et al., 2022). Flavones, represented by luteolin and apigenin, are concentrated in bell peppers and celery, while flavanones (dihydroflavones) are characteristic components of citrus fruits, with hesperidin and naringin as notable examples (Herrera-Pool et al., 2021; Zhang et al., 2022; Lin et al., 2024; Peng et al., 2024). Flavanols, especially catechins (tea tannins or catechols), constitute the primary bioactive compounds in tea leaves (Chen et al., 2025). Anthocyanidins serve as natural plant pigments, with varying concentrations across different species, notably abundant in grape skins, peanut skins, and pine bark (Bolling et al., 2011; Zeng et al., 2024). Isoflavones show a more specific distribution pattern, being predominantly found in leguminous plants, particularly in soy and soy-derived products, with additional sources including alfalfa and chickpeas (Kim and Kim, 2020).
 Click for large image | Figure 1. Food sources of flavonoids. Flavonols: Primarily found in vegetables and fruits such as broccoli, onions, apples, grapes, and berries. Flavones: Concentrated in herbs, celery, and bell peppers. Flavanones: Characteristic of citrus fruits like oranges, lemons, and grapefruits. Flavanols: Abundant in tea. Anthocyanidins: Grape skins, peanut skins, and pine bark. Isoflavones: Primarily found in legumes. |
SeFs represent a novel class of selenium-containing compounds formed in selenium-enriched plants through the unique interaction between flavonoids and selenium (Qi et al., 2024). Recent studies have revealed complex interactions between selenium and flavonoid metabolism in plants, which manifest not only at the metabolic regulation level but also through structural modifications and chemical bond reorganization. The incorporation of selenium into flavonoid structures has emerged as a significant area of research, leading to the discovery of various SeFs subclasses with distinct properties and biological activities.
3.1.1. Selenium and flavonol compounds
In-depth research on L. chinense leaves has provided significant insights into selenium’s regulatory effects on specific flavonoid metabolic transformations. Studies have demonstrated that selenium exhibits an inhibitory effect on the conversion of quercetin to rutin, suggesting that selenium might modulate specific flavone glycoside synthesis by influencing glycosyltransferase activity (Dong et al., 2013). This finding has important implications for understanding the molecular mechanisms controlling flavonoid glycosylation in selenium-enriched plants.
A notable advancement in SeFs synthesis has been achieved through the development of an efficient microwave-assisted method. This approach involves protecting hydroxyl groups through methylation using dimethyl sulfate, followed by the transformation of carbonyl groups to selenocarbonyl groups using Woollins’ reagent (WR) as the selenium source. This method has successfully converted natural quercetin (Que) to 3,7,3′,4′-tetramethyl quercetin (SePQue) under specific conditions (acetonitrile, MW, 175 W, 150 °C, 5 min), shown as Figure 2a (Martins et al., 2015).
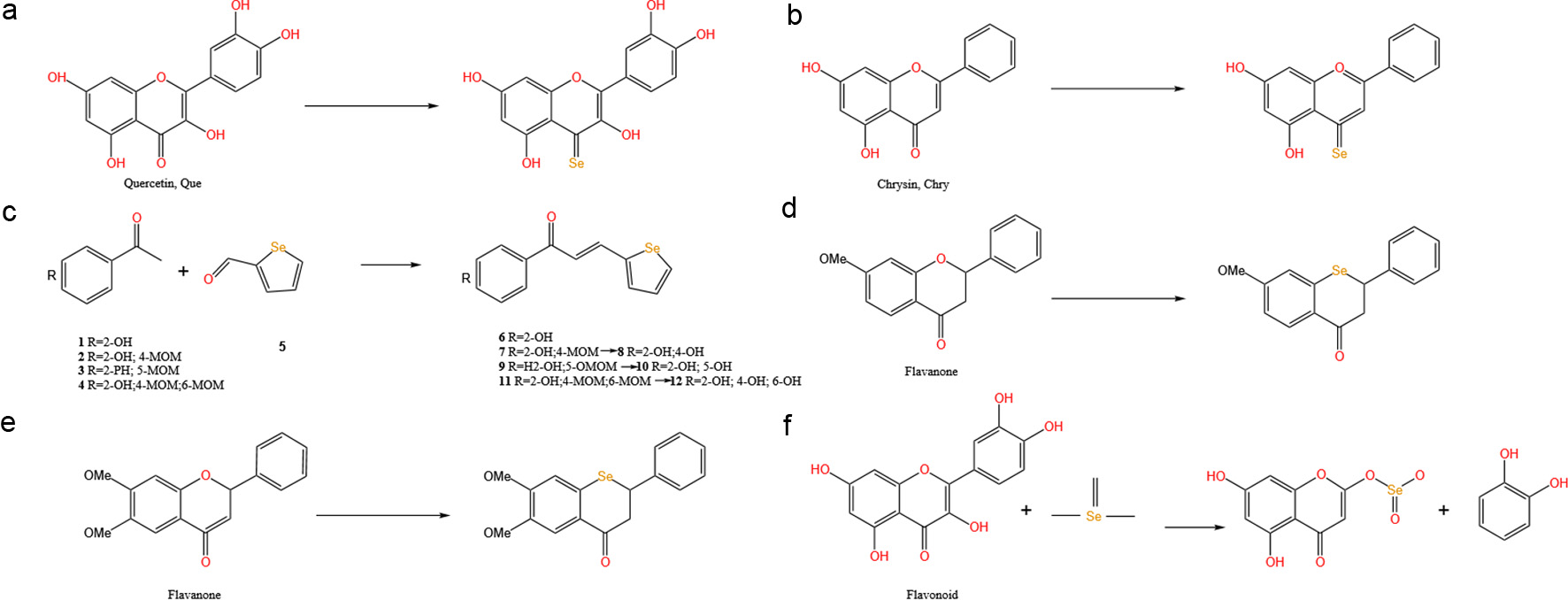 Click for large image | Figure 2. Structure and classification of SeFs. (a) Selenium and flavonol compounds. (b, c) Selenium and flavone compounds. (d, e) Selenium and flavanone compounds. (f) Selenium and Flavan-3-ol compounds. |
3.1.2. Selenium and flavone compounds
In the synthesis of selenium-containing flavone compounds, significant advancements have been made through the application of microwave-assisted methodologies. Under optimized conditions (175 W, 150 oC, 5 min), chrysin (Chry) can be transformed into selenium-containing chrysin (SeChry) using Woollins’ reagent as the selenium source (Figure 2b) (Martins et al., 2015).
Furthermore, an investigation into the synthesis of selenium-containing chalcone derivatives has been conducted through the implementation of Claisen-Schmidt condensation reactions. The reactivity between various acetophenone derivatives (compounds 1–4) and selenophene-2-carbaldehyde (compound 5) has been systematically investigated, and a series of novel selenium-containing chalcone analogs (compounds 6–12) have been successfully obtained (Figure 2c) (Chen et al., 2023). Of particular significance is the biological evaluation of these newly synthesized selenium-containing compounds, which has revealed promising anticancer properties. In vitro studies demonstrated that these compounds exhibit remarkable cytotoxicity against tumor cells, with inhibitory potency comparable to that of the clinically established chemotherapeutic agent 5-fluorouracil (5-FU) (Chen et al., 2023). This observation suggests that the incorporation of selenium into the flavone scaffold may enhance the compound’s therapeutic potential, possibly through modulation of cellular redox processes or other biological pathways associated with cancer cell proliferation and survival.
3.1.3. Selenium and flavanone compounds
The substitution of oxygen atoms with selenium in flavones leads to significant improvements in both physicochemical properties and biological activities. These SeFs exhibit lower topological polar surface area (tPSA) and higher lipophilicity (ClogP) compared to their corresponding flavone analogues, potentially enhancing their bioavailability and therapeutic efficacy (Figure 2d and e) (Jeong et al., 2014).
3.1.4. Selenium and flavan-3-ol compounds
Plants demonstrate distinct interaction patterns between selenium and specific flavonoid compounds. A comprehensive analysis of green tea catechins revealed differential correlations between selenium content and seven different catechin types. Notably, selenium showed strong negative correlations with non-gallated catechins (including gallocatechin (GC), epigallocatechin (EGC), and catechin (C)), while exhibiting strong positive correlations with gallated catechins (including epigallocatechin gallate (EGCG), gallocatechin gallate (GCG), and epicatechin gallate (ECG)) and epicatechin (EC) (Ye et al., 2023).
A groundbreaking discovery in selenium-enriched green tea identified a SeFs compound with remarkably high selenium content (15,690.4 μg L−1). The formation mechanism involves initial substitution of the 3-position hydroxyl group with selenium, followed by C(1′)-C(2) bond cleavage leading to B-ring detachment from the benzopyran structure. The process culminates in an esterification reaction between SeO32−and the alcoholic hydroxyl group of 2-phenyl chromone, forming Se-containing 5,7-dihydroxychromone (Se-DHC), shown as Figure 2f (Fan et al., 2022).
3.1.5. Selenium and Other Flavonoid Compounds
Selenium treatment has been shown to significantly increase flavonoid content in various plants. In strawberries, selenium supplementation leads to elevated levels of anthocyanins and polyphenolic compounds (Mimmo et al., 2017). Similar effects have been observed in other plants, including enhanced naringin chalcone and kaempferol levels in tomato fruits, and increased catechin accumulation in Assam tea (Sae-Lee et al., 2012; Schiavon et al., 2013). However, there remains a notable research gap regarding the interaction between selenium and isoflavone compounds, suggesting an important direction for future investigations.
3.2. In vivo metabolism of SeFs
Selenium, an essential trace element, plays a crucial role in human physiology. Understanding the complex mechanisms of selenium metabolism, particularly in the context of SeFs, is fundamental to appreciating their potential therapeutic applications and biological significance.
The maintenance of optimal selenium status is vital for human health, as both deficiency and excess can lead to significant pathological conditions. Selenium deficiency has been definitively linked to several serious disorders, including Keshan disease and Kashin-Beck disease, which primarily affect cardiovascular and musculoskeletal systems (Amerian et al., 2024; He et al., 2024). Moreover, emerging evidence suggests strong associations between selenium deficiency and various pathological conditions, including myocardial infarction, Alzheimer’s disease, and chronic pancreatitis (Sanmartin et al., 2011; Dabravolski et al., 2023; Ouyang et al., 2024). To prevent these adverse effects, careful attention must be paid to selenium intake, with recommended dietary allowances (RDA) varying based on demographic factors such as age, physiological state, and individual metabolic requirements. The recommended daily intake of selenium is shown in Table 1.
 Click to view | Table 1. Recommended daily intake of selenium (Kipp et al., 2015). |
The bioavailability of selenium compounds exhibits marked variation depending on their chemical form, with organic selenium compounds generally demonstrating superior absorption characteristics compared to their inorganic counterparts (Davis et al., 2017). This makes organic forms preferentially utilized in selenoprotein biosynthesis. Following ingestion, selenium compounds undergo distinct absorption processes in the gastrointestinal tract before being transported to the liver, the primary site of selenium metabolism. Organic selenium compounds, particularly selenomethionine (SeMet) and selenocysteine (SeCys), are absorbed through transcellular pathways, utilizing the same transport proteins as their sulfur-containing analogues (Nickel et al., 2009). SeMet demonstrates remarkable metabolic flexibility, being processed through multiple pathways including sodium-dependent transport process, undergo non-specific incorporation into proteins at methionine residues, or enter the trans-selenation pathway for conversion to selenocysteine (Suzuki and Ogra, 2002; Roman et al., 2014). When proteins containing SeMet undergo degradation, the released SeMet enters the trans-selenation pathway, representing an important selenium recycling mechanism. This process highlights the body’s sophisticated approach to maintaining selenium homeostasis. In cases of excess selenium intake, SeMet can undergo direct methylation through β-lyase-mediated reactions, facilitating its excretion and preventing potential toxicity (Suzuki et al., 2006). SeCys, released during selenoprotein catabolism, follows a cyclic conversion pathway to hydrogen selenide (H2Se) (Roman et al., 2014).
Selenium enters the body in various chemical forms, each following distinct initial metabolic routes that ultimately converge on a central metabolic intermediate: H2Se (Hu et al., 2021). This convergence point represents a crucial regulatory hub in selenium metabolism, directing selenium either toward essential biological functions or elimination pathways depending on the body’s needs. When selenium levels are within the normal physiological range, H2Se is primarily converted to selenocysteinyl-tRNA, an essential component for selenoprotein synthesis (Bodnar et al., 2016). However, when selenium intake exceeds the requirements for selenoprotein synthesis, several detoxification pathways become activated. H2Se undergoes sequential methylation, first forming methylselenol, which notably possesses anti-cancer properties (Nicastro and Dunn, 2013). With increasing selenium levels, methylselenol is further methylated to produce dimethylselenide and trimethylselonium ions, which are eliminated through respiration and urinary excretion, respectively (Hu et al., 2021). Additionally, H2Se can be converted to selenosugars, providing another pathway for urinary selenium excretion.
In addition, certain selenium compounds follow unique metabolic routes. For instance, Se-methyl-selenocysteine (SMC) and its dipeptide form γ-glutamyl-Se-methylselenocysteine (GGSMC) are absorbed in the gastrointestinal tract (Dong et al., 2001). GGSMC undergoes hydrolysis by γ-glutamyl transpeptidase to release SMC for tissue distribution. Unlike other selenium compounds that must first be converted to H2Se, SMC can be directly methylated by β-lyase to form methylselenol, providing a more direct route to this important metabolite (Figure 3) (Suzuki et al., 2006).
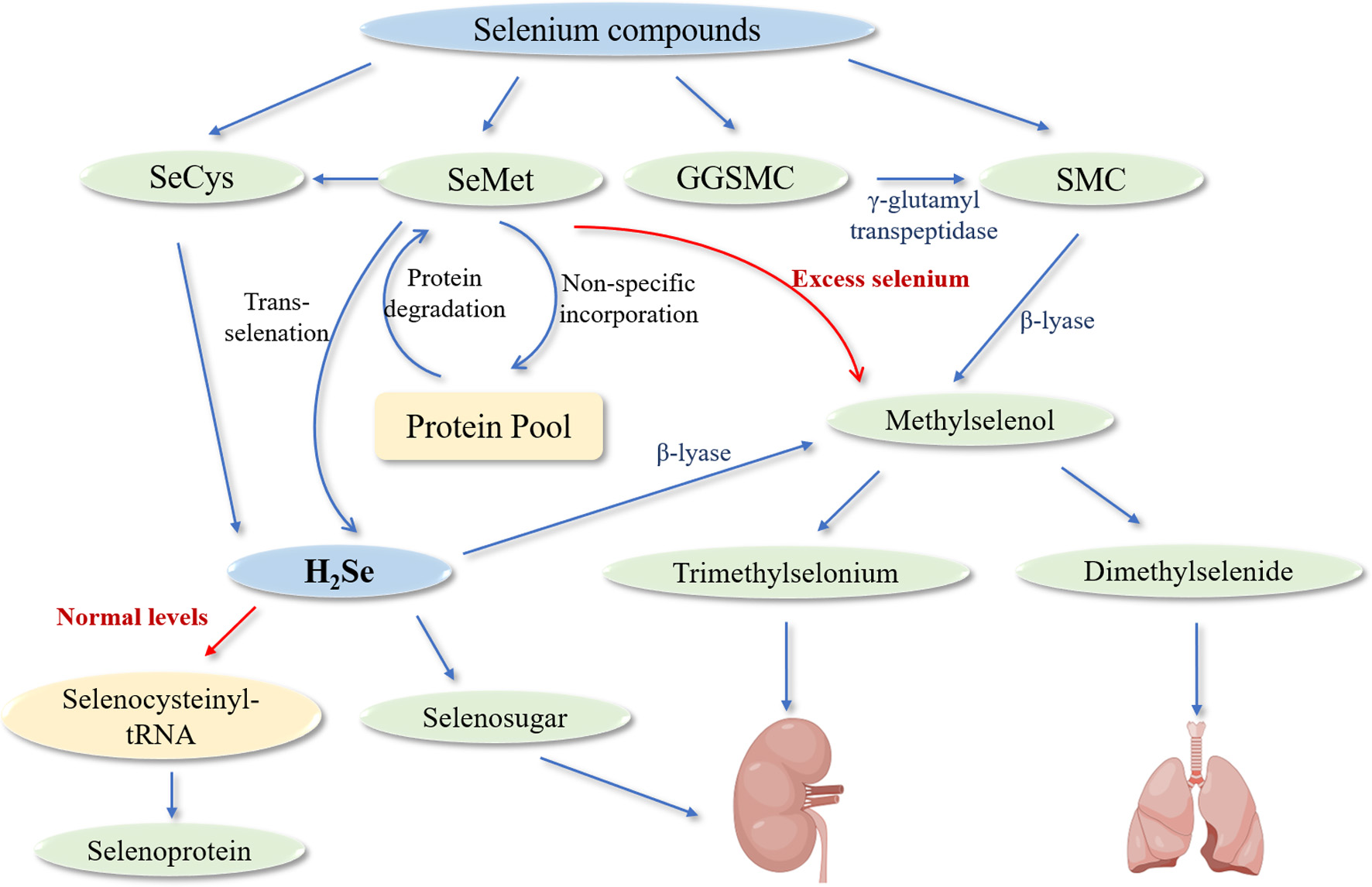 Click for large image | Figure 3. Metabolic pathways of selenium compounds in biological systems. Abbreviations: SeCys (selenocysteine) - an organic selenium-containing amino acid released during selenoprotein catabolism; SeMet (selenomethionine) - an organic selenium compound that can follow multiple metabolic pathways; GGSMC (γ-glutamyl-Se-methylselenocysteine) - a dipeptide form of selenium that undergoes hydrolysis; SMC (Se-methylselenocysteine) - an organic selenium compound that can be directly converted to methylselenol; H2Se (hydrogen selenide) - the central metabolic intermediate in selenium metabolism; β-lyase (beta-lyase) - an enzyme that catalyzes the conversion of selenium compounds to methylselenol; γ-glutamyl transpeptidase - an enzyme that hydrolyzes GGSMC to release SMC. The diagram depicts two primary metabolic fates: under normal selenium levels (indicated by red arrow), H2Se is primarily directed toward selenocysteinyl-tRNA for selenoprotein synthesis; under excess selenium conditions (indicated by red arrow), detoxification pathways are activated, leading to the formation of methylated selenium compounds (methylselenol, dimethylselenide, and trimethylselonium) that are subsequently excreted. Blue arrows represent metabolic conversion pathways between compounds. The protein pool represents the non-specific incorporation of SeMet into proteins and its subsequent release through protein degradation, an important selenium recycling mechanism. |
This intricate network of metabolic pathways ensures optimal selenium utilization while preventing accumulation to toxic levels. Understanding these processes provides crucial insights for developing effective selenium-enriched supplements and therapeutic interventions, particularly in the context of SeFs compounds. The complex interplay between selenium metabolism and flavonoid biochemistry opens new avenues for research into the potential health benefits and applications of these unique compounds.
| 4. Biological activities of SeFs | ▴Top |
The incorporation of selenium into organic compounds represents a significant advancement in the development of bioactive molecules, particularly in the context of SeFs. Organic selenium compounds demonstrate superior bioavailability and reduced toxicity compared to their inorganic counterparts, while exhibiting a broad spectrum of biological activities. These properties have garnered increasing attention, especially given that Ebselen remains the only selenium-containing drug approved for clinical use, primarily due to its exceptional glutathione peroxidase-mimetic properties (Rieder et al., 2023).
SeFs represent a unique class of compounds that combine the structural features of flavonoids with the biological properties of selenium. The integration of selenium into the flavonoid scaffold occurs through a specific chemical mechanism: the phenolic hydroxyl groups form covalent bonds with selenium in its +4 oxidation state. This bonding involves the formation of a shared electron pair between selenium’s d orbital and the p orbital of the oxygen anion (Cheng et al., 2023). This molecular architecture confers distinctive biological properties that differentiate SeFs from their conventional flavonoid counterparts, as shown in Table 2.
 Click to view | Table 2. Summary of SeFs mechanisms of action across different biological systems |
4.1. Anti-inflammatory and antioxidant activities
Recent investigations have revealed the anti-inflammatory and antioxidant capabilities of SeFs through multiple mechanistic pathways. In a study using selenium-enriched rice grass extract, researchers demonstrated its ability to modulate inflammatory responses in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages without inducing cytotoxicity. The extract’s bioactive components were identified as a complex mixture of flavone glycosides, including quinic acid, phenolic glycosides (protocatechuic glucoside and 1-o-sinapoyl-β-d-glucose), 3-o-feruloyl-quinic acid, tricin, swertisin, and tricin-7-o-β-d-glucopyranoside (Chomchan et al., 2018). The anti-inflammatory mechanism appears to operate through dual pathways. First, the compounds inhibit inflammatory mediator synthesis by suppressing the NF-κβ pathway, leading to reduced expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Second, the selenium component, as an integral part of glutathione peroxidase (GPx), contributes to the anti-inflammatory effect by modulating iNOS expression and subsequent nitric oxide production. Investigation into selenium modification of auraptene, a naturally occurring coumarin abundant in citrus fruits, has revealed enhanced biological properties of its selenium-containing derivative (selenoauraptene). Notably, selenoauraptene demonstrates superior in vitro antioxidant capacity and free radical scavenging activity compared to its parent compound auraptene, highlighting the potential of selenium incorporation in enhancing the biological efficacy of natural compounds (Fiorito et al., 2021). Furthermore, comprehensive chemical analysis of selenium-enriched mung bean fermentation broth (Se-MBFB) has identified a complex bioactive profile predominantly consisting of polyphenols, peptides, and γ-aminobutyric acid (GABA). This unique compositional profile contributes to its multifaceted biological activities, including significant free radical scavenging properties and tyrosinase inhibition, which effectively reduces melanin synthesis (Wei et al., 2022).
In hepatoprotective investigations, selenium-enriched radish sprouts have demonstrated remarkable therapeutic efficacy against carbon tetrachloride (CCL4) induced liver injury in mouse models, whereby the treatment significantly enhanced hepatic antioxidant capacity while simultaneously attenuating inflammatory responses and cellular apoptotic processes (Jia et al., 2019). Similarly, selenium-treated fava beans showed promising results in an ulcerative colitis model, where increased phenolic and flavonoid content correlated strongly with improved antioxidant parameters in colonic tissue, including enhanced superoxide dismutase (SOD) activity and glutathione (GSH) levels (El-Sayed et al., 2022). Furthermore, in the context of acute lung injury (ALI) induced by oxidative stress and inflammatory dysregulation, researchers have developed an innovative therapeutic approach utilizing selenium nanoparticles (Se NPs) encapsulated with chlorogenic acid to generate CHSe NPs, which demonstrate remarkable dual-enzymatic properties mimicking both superoxide dismutase (SOD) and glutathione peroxidase (GPX) activities (Xing et al., 2025). The therapeutic efficacy of these nanostructures is attributed to their sophisticated mechanism of action, wherein they effectively eliminate diverse reactive oxygen species (ROS) while simultaneously suppressing inflammatory responses in macrophages. This dual functionality is achieved through the coordinated modulation of both MAPK and PI3K-Akt signaling pathways, resulting in synergistic antioxidant and anti-inflammatory effects.
These findings collectively highlight the multifaceted nature of SeFs biological activities, where the synergistic effects of selenium and flavonoid components contribute to enhanced therapeutic potential. The diverse mechanisms of action, ranging from direct antioxidant effects to complex signaling pathway modulation, underscore the versatility of these compounds as potential therapeutic agents.
4.2. Neuroprotective effects
Neurodegenerative disorders present complex therapeutic challenges due to their multifaceted pathological mechanisms. These conditions are characterized by several interrelated factors, including oxidative stress, protein aggregation, neuroinflammation, and metabolic dysfunction, each contributing to the progressive loss of neuronal function and structure (Ahlstedt et al., 2024; Valleti et al., 2024). SeFs may be promising therapeutic agents due to their ability to simultaneously target multiple pathological pathways, offering a comprehensive approach to neuroprotection that addresses both central and systemic aspects of neurodegeneration.
4.2.1. Amelioration of oxidative stress and mitochondrial dysfunction
The brain’s exceptional vulnerability to oxidative damage stems from its remarkably high oxygen consumption, which accounts for approximately 20% of the body’s total oxygen utilization, despite comprising only 2% of body weight (Tyagi and Pugazhenthi, 2023). This high metabolic demand, coupled with relatively limited antioxidant defenses, makes neural tissue particularly susceptible to oxidative stress-induced damage. In this context, SeFs enhance cellular antioxidant defenses while simultaneously reducing oxidative damage.
Se-Rutin, synthesized through an innovative approach using sodium selenite as a precursor, demonstrates markedly enhanced protective properties compared to conventional rutin. When tested in PC12 neuronal cells, Se-Rutin exhibited a remarkable ability to prevent hydrogen peroxide-induced cellular damage through multiple mechanisms (Zhu et al., 2023). The compound not only directly neutralized reactive oxygen species but also triggered the upregulation of endogenous antioxidant defenses through the activation of the Nrf2 pathway, leading to increased expression of protective enzymes including heme oxygenase-1 (HO-1). This dual mechanism of action, combining direct antioxidant effects with the enhancement of cellular defensive systems, illustrates how the incorporation of selenium can significantly amplify the therapeutic potential of traditional flavonoids. The efficacy of SeFs in protecting against severe oxidative stress is particularly evident in models of cerebral ischemia-reperfusion injury, where oxidative damage plays a central role in tissue destruction. Studies using SeFs have demonstrated remarkable tissue preservation in critical brain regions, including the anterior and medial striatum and extensive cortical areas (Choi et al., 2015). The significant reduction in infarction volumes observed in treated animals suggests that these compounds can effectively maintain cellular redox homeostasis even under conditions of severe oxidative stress. This protection likely involves multiple mechanisms, including the preservation of mitochondrial function, maintenance of cellular energy metabolism, and prevention of oxidative damage to cellular macromolecules.
4.2.2. Inhibition of protein aggregation and misfolding
The accumulation of misfolded and aggregated proteins represents a fundamental pathological feature across numerous neurodegenerative disorders, with particular significance in conditions such as Alzheimer’s disease, Parkinson’s disease, and other proteinopathies (Moretto et al., 2022).
Recent investigations into selenium-modified epigallocatechin-3-gallate (EGCG) have revealed promising developments in preventing β-amyloid aggregation. The incorporation of EGCG into selenium nanoparticles has yielded a system that not only prevents the initial aggregation of Aβ peptides but also promotes the dissociation of existing amyloid fibrils (Zhang et al., 2014). Comparative studies of selenium-enriched green teas have provided valuable insights into the relationship between selenium content and neuroprotective efficacy. Analysis of different varieties, particularly the comparison between Ziyang Maojian (ZYMJ) and Enshi Yulu (ESYL), has revealed a strong correlation between selenium concentration and protection against Aβ1-42-induced neurotoxicity (Ye et al., 2022). The superior performance of ZYMJ, with its significantly higher selenium content (4.33 ± 0.18 μg/g compared to 0.58 ± 0.01 μg/g in ESYL), suggests that selenium enrichment can enhance the inherent neuroprotective properties of tea polyphenols through multiple mechanisms, including improved bioavailability and enhanced interaction with protein aggregates. In addition, the development of innovative delivery systems has further expanded the therapeutic potential of SeFs in addressing protein aggregation. The creation of selenium nanoparticles incorporating silybin within a polysaccharide matrix (SLY-XG-Se) represents an approach to targeting protein aggregation in neuronal cells (Saini et al., 2022). These nanoparticles demonstrate enhanced cellular penetration and sustained release characteristics, allowing them to maintain therapeutic concentrations in neuronal tissues over extended periods.
4.2.3. Amelioration of neuroinflammation and gut dysbiosis
Chronic neuroinflammation represents a critical pathological mechanism in neurodegenerative disorders, characterized by persistent activation of inflammatory pathways and dysregulation of immune responses in the central nervous system (Azam et al., 2022). SeFs have demonstrated efficacy in modulating these inflammatory processes through sophisticated interactions with multiple cellular signaling pathways, offering comprehensive protection against inflammation-mediated neuronal damage.
Recent investigations into resveratrol-selenium nanoparticles (RSV-SeNPs) have revealed particularly promising results in addressing neuroinflammation through modulation of the Sirt1/miRNA-134/GSK3β signaling axis (Yang et al., 2023). This pathway represents a crucial regulatory mechanism controlling both inflammatory responses and cellular stress resistance. The incorporation of selenium into resveratrol nanoparticles appears to enhance the compound’s ability to activate Sirt1, a key cellular deacetylase that plays fundamental roles in regulating inflammatory responses and cellular metabolism. The activation of Sirt1 initiates a cascade of molecular events that ultimately lead to reduced expression of pro-inflammatory mediators and enhanced cellular stress resistance (Wu et al., 2023). The development of resveratrol-loaded selenium nanoparticles encapsulated in chitosan (Res@SeNPs@Res-CS-NPs) represents a significant advancement in targeting metabolic aspects of neurodegeneration. These sophisticated delivery systems demonstrate remarkable efficacy in restoring gut microbiota homeostasis, particularly through modulation of the crucial Firmicutes/Bacteroidetes ratio (Yang et al., 2023). This microbiota rebalancing has far-reaching effects, including improved metabolic function, reduced systemic inflammation, and enhanced cognitive performance.
4.3. Regulation of glucose and lipid metabolism
SeFs are emerging as promising therapeutic agents for metabolic disorders, demonstrating effectiveness across various stages of diabetes mellitus and its associated complications. Research has revealed multiple mechanisms through which these compounds influence glucose and lipid metabolism, from initial metabolic dysfunction to advanced complications.
At the fundamental level of glucose regulation, selenium nanoparticles synthesized with mulberry leaves and kudzu root extracts (MPE-SeNPs) have demonstrated significant metabolic effects. These nanoparticles, characterized by their primary components rutin and puerarin, exhibit comprehensive glucose-regulatory properties through several mechanisms: they enhance intestinal permeability and transepithelial transport, mitigate oxidative stress responses, improve pancreatic function, and augment glucose utilization in adipose tissue (Deng et al., 2019). Studies in both normal and diabetic rat models have confirmed these hypoglycemic effects, suggesting potential applications in early-stage metabolic regulation. As diabetes progresses, various complications can develop, with urinary system dysfunction representing an early manifestation (Saeedi et al., 2019). Diabetic ureter injury (DUI) presents with characteristic alterations in both the function and morphology of the urinary system. Research has shown that luteolin-loaded selenium nanoparticles (LT-SeNPs) offer a targeted approach to addressing this complication. The therapeutic mechanism involves the modulation of the Nrf2/ARE pathway, which subsequently inhibits NLRP3 inflammasome activation (Jing et al., 2024). This molecular pathway intervention suggests a novel preventive and therapeutic strategy for diabetes-related urological complications. In more advanced stages of diabetes, nephropathy emerges as a serious complication of prolonged hyperglycemia (Tatsumi et al., 2022). Studies utilizing streptozotocin (STZ)-induced diabetic nephropathy models have demonstrated the therapeutic potential of combining selenium nanoparticles (SeNPs) with rutin (Ru). This combination therapy operates through a sophisticated dual-pathway mechanism: it simultaneously upregulates the Nrf-2/HO-1 pathway while downregulating Jak-2/Stat3 signaling (Zaghloul et al., 2022). The treatment results in measurable improvements across multiple parameters, including reductions in fasting blood glucose levels, serum creatinine concentrations, and urea levels. The progression of research in this field indicates that SeFs may offer therapeutic benefits at various stages of metabolic disease development. Their ability to address both primary metabolic dysfunction and subsequent complications through multiple molecular mechanisms warrants further investigation.
4.4. Antitumor effects
SeFs show antitumor potential in various cancer types through multiple molecular mechanisms, exerting their anticancer effects by modulating cellular pathways involved in apoptosis, cell cycle regulation, oxidative stress and metabolism.
Research in hepatocellular carcinoma (HCC) has revealed comprehensive therapeutic approaches using SeFs, with studies demonstrating efficacy through multiple interconnected mechanisms. In thioacetamide (TAA)-induced rat HCC models, the combination of selenium nanoparticles (SeNPs) with quercetin (QCT) demonstrates comprehensive therapeutic effects through multiple mechanisms: improving liver marker profiles, reducing serum AFP levels, and enhancing hepatic structure while minimizing necroinflammatory regions (Mohamed et al., 2022). At the molecular level, the treatment demonstrates sophisticated regulation of oxidative stress markers, modulating hepatic MDA, GSH, and GPx levels while preventing dysregulation of the oncogenic p53/β-catenin/cyclin D axis, which plays a crucial role in hepatocarcinogenesis. Complementing these findings, research on selenium-enriched oolong tea extract has revealed enhanced antiproliferative effects against HuH-7 hepatocellular carcinoma cells compared to regular oolong tea extract. This enhanced efficacy stems from the synergistic effect between organic selenium and tea polyphenols, the mechanism of which involves an increase in intracellular ROS production, leading to G2/M phase cell cycle arrest, along with a significant modulation of key regulatory proteins such as p53, Bax, and caspase-3 (Wang et al., 2021).
The investigation of breast cancer treatment strategies has yielded significant insights into the potential of SeFs, particularly through the development of advanced delivery systems and novel selenium derivatives. Research on selenium-apigenin nanoparticles (SeNPs-apigenin) has demonstrated sophisticated mechanisms of action in breast cancer treatment, showing concentration-dependent inhibition of MCF-7 cell proliferation and viability (Al-Otaibi et al., 2022). These nanoparticles operate through a precise molecular cascade, directly targeting key apoptotic pathway regulators including Bcl-2, Bax, and caspase-3, ultimately triggering the release of cytochrome C from mitochondria to cytosol. This process represents a carefully orchestrated sequence of events leading to programmed cell death. Further advancing the field, two selenium derivatives, SePQue and Selenium-chrysin (SeChry), have demonstrated remarkable effectiveness in suppressing MCF-7 cell colony formation through distinct but complementary mechanisms. Their action primarily involves TrxR inhibition, with SeChry showing superior activity and more pronounced effects on mitochondrial membrane potential, suggesting a more potent approach to breast cancer treatment through targeted disruption of cellular energy metabolism.
The research on selenium-flavonoid compounds in non-small cell lung cancer (NSCLC) has revealed sophisticated therapeutic mechanisms operating at multiple cellular levels. A significant advancement in this field has been the development of biomimetic selenium-baicalein nanoparticles (ACM-SSe-BE), which demonstrate exceptional anticancer efficacy through a complex interplay of cellular mechanisms. These nanoparticles function by orchestrating a series of cellular responses: they systematically reduce SOD activity while simultaneously increasing MDA content, ABTS activity, and ROS levels, creating a comprehensive assault on cancer cell survival mechanisms (Shi et al., 2024). The modulation of these oxidative stress parameters is accompanied by careful regulation of apoptosis-related proteins, establishing a multi-targeted approach to cancer cell death. In vivo studies using nude mice models have provided compelling evidence of ACM-SSe-BE’s superior antitumor efficacy compared to conventional treatment modalities, suggesting potential clinical applications. Furthermore, research on SeChry has uncovered its remarkable ability to modulate multiple metabolic pathways in NSCLC cells, including the intricate regulation of glycolysis, gluconeogenesis, tricarboxylic acid cycle, and amino acid metabolism (Mendes et al., 2024). This metabolic modulation leads to systematic disruption of cellular homeostasis, affecting key metabolites such as acetate, lactate, glucose, and amino acids, ultimately compromising cancer cell survival through multiple pathway interventions.
Studies on colorectal adenocarcinoma have revealed that HT-29 cells treated with selenium-flavonoid compounds initiate a complex cascade of cellular events culminating in programmed cell death (Chen et al., 2023). This process begins with precise modulation of apoptotic protein expression, characterized by a simultaneous upregulation of pro-apoptotic protein Bax and downregulation of anti-apoptotic protein Bcl-2. This carefully orchestrated balance in apoptotic protein regulation triggers sophisticated cellular responses that operate through dual pathways: the mitochondrial pathway and the Caspase-3-dependent pathway.
4.5. Impact on bone metabolism
Selenium-flavonoid compounds have demonstrated therapeutic potential in addressing skeletal system disorders, particularly through research on osteoporosis-related bone defects using ovariectomized (OVX) rat models. Studies examining the combination of silibinin and selenium (SSe) have revealed comprehensive effects on bone metabolism and regeneration through multiple interconnected pathways (Tao et al., 2022). When compared to control groups and selenium-only treatments, SSe therapy exhibits superior outcomes in cellular mineralization and osteogenic activity by reducing reactive oxygen species (ROS) levels while simultaneously upregulating key osteogenic proteins. These proteins include SIRT1 (Silent Information Regulator Type 1), which regulates bone mass through effects on both formation and resorption; SOD2 (Superoxide Dismutase 2), which provides protection against oxidative stress; RUNX-2 (Runt-related Transcription Factor 2), and OC (Osteocalcin), which are crucial markers of bone formation. In addition, the effect of SSe treatment on bone regeneration and mineralization processes was demonstrated by microcomputed tomography and histological analysis, and the synergistic effect between selenium and silybin enhanced bone regeneration through multiple mechanisms.
| 5. Conclusions and future perspectives | ▴Top |
The extensive research on SeFs have revealed their significant potential as therapeutic agents across multiple disease conditions, while simultaneously highlighting areas that warrant further investigation. The integration of selenium into flavonoid structures has consistently demonstrated enhanced biological activities compared to their parent compounds, suggesting a promising direction for drug development and therapeutic interventions.
Current research has established several key findings that form the foundation for future investigations. First, the molecular mechanisms underlying SeFs synthesis and metabolism have been elucidated, revealing sophisticated pathways that regulate their biological activities. The discovery of specific selenium accumulation patterns in different plant species has provided valuable insights into natural SeFs production. Second, detailed structural analyses have identified critical molecular features that contribute to their enhanced biological activities, particularly the role of selenium incorporation in modifying compound properties such as bioavailability and receptor interactions. Third, comprehensive studies across various disease models have demonstrated their therapeutic potential, especially in conditions characterized by oxidative stress, inflammation, and metabolic dysregulation. Despite current research progress, several key areas warrant further investigation. First, the isolation of well-defined plant-derived SeFs remain a significant challenge, with the structural complexity of these compounds making the separation process particularly demanding. The isolation process is further complicated by the susceptibility of selenium-containing compounds to undergo oxidation or other chemical modifications during separation, introducing additional complexity to maintaining structural integrity throughout the purification workflow. Second, the optimization of SeFs synthetic methodologies requires additional in-depth research, particularly in developing scalable processes that maintain cost-effectiveness while ensuring product quality. Finally, while SeFs have shown promising advances in addressing neurological injuries, metabolic disorders involving glucose and lipids, and tumors, their underlying mechanisms lack comprehensive investigation and demand more detailed exploration.
In conclusion, SeFs represent a promising class of compounds with significant therapeutic potential. While substantial progress has been made in understanding their properties and applications, continued research efforts are needed to fully realize their potential in clinical settings.
This work was supported by Guangdong-Macao Science and Technology Innovation Joint Research Special Fund (2023A0505020013), Macau Science and Technology Development Fund (0031/2022/AGJ), Research projects of the General Administration of Customs (2023HK135), Guangdong Provincial Observation and Research Station for Coupled Human and Natural Systems in Land-ocean Interaction Zone (2024B1212040003), Colleges and universities priority projects in Guangdong province (2022KCXTD036).
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |