| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 21, March 2023, pages 55-61
Aroma characterization and in vitro antihypertensive activity of Amazonian Camu-camu (Myrciaria dubia) fruit
Juliana María García-Chacóna, *, Diana Paola Forerob, Devin G. Petersonb, Coralia Osorioa, *
aDepartamento de Química, Universidad Nacional de Colombia, AA 14490, Bogotá, Colombia
bDepartment of Food Science and Technology, Parker Food Science & Technology Building, The Ohio State University, 2015 Fyffe Rd., The Ohio State University, Columbus, Ohio 43210, United States
*Corresponding author: Coralia Osorio and Juliana García-Chacón, Universidad Nacional de Colombia, AA 14490 Bogotá, Colombia. Tel: +57-6013165000, ext. 14472; E-mail: cosorior@unal.edu.coand jumgarciach@unal.edu.co
DOI: 10.31665/JFB.2023.18339
Received: March 5, 2023
Revised received & accepted: March 19, 2023
| Abstract | ▴Top |
The Amazonian fruit known as camu-camu (Myrciaria dubia) has recently attracted attention due to its sensory and biofunctional properties. A sensomics methodology was used to identify the odour-active volatiles of the whole fruit (pulp and peel) by using solvent-assisted flavour evaporation (SAFE), analysis by gas chromatography-mass spectrometry (GC-MS), and gas chromatography-olfactometry (GC-O), and sensory evaluation (aroma extract dilution analysis (AEDA)) techniques. Four odour-active volatile compounds were reported at concentrations above the odour-activity values: isoamyl acetate, α-pinene, limonene, and β-caryophyllene. Recombination aroma testing further verified that the compounds contributed to the fruity, herbal, citrus, and woody notes of the M. dubia fruit. Furthermore, the fruit in vitro ACE-I inhibition activity was 39.48 ± 12.09 % (at 50 μg/mL, using lisinopril as positive control), showing a potential use as a functional food.
Keywords: Odour-active volatiles; GC-O; SAFE; Antihypertensive activity; Terpenes
| 1. Introduction | ▴Top |
Camu-camu (Myrciaria dubia) is a Myrtaceae berry from the Amazon Rainforest. Consumption of the fruit has been related to health-promoting benefits due to the presence of bioactive compounds such as carotenoids, polyphenols (anthocyanins, flavones, and flavonols), and malic acid, as well as its high vitamin C content (Neves et al., 2015; Cunha-Santos et al., 2019). Specific biological activities of the fruit associated with the prevention of metabolic syndrome diseases include antihypertensive and antihyperglycemic activities by the inhibition of angiotensin-converting enzyme-I (ACE-I) and α-amylase and α-glucosidase enzymes (responsible for the hydrolysis of polysaccharides in the human intestine) have been respectively reported (Fidelis et al., 2018; García-Chacón et al., 2022). In fact, as part of traditional medicine, Amazonian indigenous communities consumed camu-camu fruit for the treatment of some diseases such as diabetes, influenza, hypercholesterolemia, inflammation, migraine, and cancer, in addition to using the fruit as a phytomedicine to counter secondary effects from cardiovascular diseases (CVDs) (Castro et al., 2020).
Hypertension is a medical condition suffered by 1.28 billion adults worldwide and is the primary cause of premature death from CVDs (WHO, 2023). In most cases, high blood pressure is treated with calcium channel blockers or by inhibiting the ACE-I enzyme (Angiotensin-converting-I enzyme), which prevents the conversion of angiotensin I to angiotensin II, reducing the high blood pressure and preventing renal insufficiency, along with cardiovascular and brain risks. Some common antihypertensive medications employed to reduce high blood pressure have caused side effects in patients, including effects on the immune system functions, dry cough, and angioedema (Felkle et al., 2022). Recent in vitro studies reported that camu-camu seed coat extracts, obtained with water, ethyl alcohol, and propanone, inhibited the ACE-I enzyme by 28–40% (Fidelis et al., 2018); however, neither the pulp nor the peel of the fruit of M. dubia has been investigated for antihypertensive activity.
Camu-camu has also been used in food formulation and processing, increasing its commercialization and sensory acceptance through food products such as cookies, yogurt, infusions, and candies (Santos et al., 2022). The first attempt to study the volatile composition of camu-camu fruit was made by Franco and Shibamoto (2000) on the headspace using a Porapak Q trap and further elution with hexane. The major constituents of this extract were terpenes (98%): α-pinene, d-limonene, and β-caryophyllene. Later, Campelo et al. (2020) studied the changes induced by dielectric barrier discharge plasma processing on the volatile composition of camu-camu fruit. After the process, they obtained the volatile extract of camu-camu fruit by HS-SPME (Headspace-Solid Phase Microextraction) using a DVB/CAR/PDMS fiber. In addition, the most abundant constituents were terpenes (mono- and sesquiterpenes), followed by some monoterpenols. The most abundant volatile compounds extracted by HS-SPME were β-caryophyllene, α-pinene, d-limonene, α-terpineol, α-humulene, α-calacorene, and γ-cadinene. However, the most relevant volatile compounds in the aroma of this fruit have not been characterized. This can be done using the sensomics approach that combines the instrumental and sensorial (GC-O) analyses of a volatile-rich extract to locate the compounds that can interact with the odorant receptors using a bio-response (Granvogl & Schieberle, 2022).
The aim of this research was to determine the odour-active volatile compounds responsible for the distinctive aroma of camu-camu fruit, together with assessing the in vitro antihypertensive activity of the fruit.
| 2. Materials and methods | ▴Top |
2.1. Chemicals
Dichloromethane (DCM) and anhydrous sodium sulfate (Na2SO4) were purchased from Merck (Darmstadt, Germany). The following compounds were commercially obtained and used as reference odorants: n-alkane mix (C7-C30), linalool, α-pinene, limonene, β-caryophyllene, and isoamyl acetate standards were acquired from Sigma-Aldrich (St. Louis, MO, USA); and cis-3-hexenol from TCI (Tokyo, Japan). Likewise, food-grade propylene glycol (Interpharma de Colombia S.A.S, Bogotá, Colombia) was employed for preparing the reference solutions for the sensory analysis. For in vitro ACE-I analyses, deionized water was obtained at a resistivity = 25 MΩ·cm a 25 °C.
2.2. Fruit material
The fruits of M. dubia were purchased at the local markets of Florencia (Caquetá, Colombia) and carefully transported in freezing conditions the same day to Bogotá for their analyses. Fully ripe fruits (pH 2.43 ± 0.03, soluble solid content of 7.38 ± 0.18° Brix, and acidity content of 2.20 ± 0.85% citric acid) were selected, and the seeds were manually removed. All the extracts were obtained with the whole fruit (pulp and peel).
2.3. Determination of odor-active volatile compounds
2.3.1. Isolation of volatile extract
A puree from whole camu-camu (pulp and peel, 180 g) was obtained using a commercial stainless-steel blender. After adding 100 mL of DCM to the puree, anhydrous sodium sulfate (210 g) was gradually added with continuous stirring and cooling. Subsequently, the mixture was filtered through defatted cotton wool, and the combined organic phases were extracted by the Solvent Assisted Flavour Evaporation (SAFE) technique, following the methodology published by Engel et al. (1999). The organic phase was concentrated to 1 mL using a Vigreux column (50 × 1 cm). Finally, a concentrated and colorless extract exhibiting the characteristic M. dubia aroma was obtained.
2.3.2. Chromatographic analyses
The SAFE extract was submitted to GC-MS and GC-O analyses using a 7890B gas chromatograph (Agilent Technologies Inc. Wilmington, DE, USA) coupled to an Agilent 5977A mass spectrometer, operated in electron impact mode (70 eV) with automatic injection and source temperatures of 250 °C, running in 2 GHz enhanced dynamic range mode. The spectral data were collected in profile at 10 Hz scan speed, and mass spectra were acquired in a mass range between 40 and 300 u and processed in ChemStation software (Rev. E01) with NIST library (version 2.2, NIST/EPA/NIH, 2014). Two capillary columns were used, a DB-WAX polar column (60 m × 0.25 mm id, 0.25 μm) and a DB-5 semipolar column (60 m × 0.25 mm id, 0.32 μm). The samples were injected in split mode (1:10), using Helium (2.0 mL/min) as the carrier. The DB-WAX column oven was programmed from 40 °C for 2 min to 180 °C, at 6 °C/min, then at 12 °C/min to 230 °C, and finally held at 230 °C for 7 minutes. Likewise, the DB-5 column oven was programmed from 60 °C for 6 min followed by a heating rate of 6 °C/min up to 180 °C for 15 min, and finally, the column was kept at 300 °C for 1 min. For GC-O analyses, the end of the capillary was connected to the sniffing port (Eltherm GmbH, Burbach, Germany), dividing the effluent into two equal parts. The same chromatographic conditions of the GC-MS analyses were used for the GC-O analyses, followed by an olfactory record of the odor-active volatile compounds.
2.3.3. Aroma extract dilution analysis (AEDA)
A series of successive dilutions (2n) of the SAFE extract was prepared, and then, each solution was analyzed by GC-O on a DB-Wax column (Grosch, 1994; Schieberle, 1995). Three trained panelists from the Flavour Research and Education Center (The Ohio State University (Columbus, OH, USA) identified the odour-active zones. Each compound flavour dilution factor (FD) was established as the maximum dilution at which the panelists perceived an olfactory note in the sniffing port.
2.3.4. Identification and quantitation of odour-active volatile compounds
The mass spectra of the compounds found to be significant contributors to M. dubia aroma were preliminarily compared with the NIST 2014 spectral library (Gaithersburg, MD). Then, the odour-active compounds were identified by comparison of their retention indexes (RI), mass spectra, and odour notes with those exhibited by standard solutions of the volatile compounds in DCM (30 μg/mL). A mixture of alkanes (C7-C30) was used to calculate the retention indexes.
The internal standard (IS) method was used to perform the quantitative analyses of odour-active volatiles IOFI, (2011). Linalool at a concentration of 250 µg/mL was used as the internal standard (IS). The analyses were done in triplicate by constructing calibration curves varying the nominal concentrations of each analyte (IS:analyte from 1:5 to 5:1), assuming the slope as the response factor.
These analyses were performed using an Agilent 7890B GC (Agilent Technologies, Santa Clara, CA) equipped with a DB-5 column coupled to an Agilent 7010B Triple Quadrupole Mass Spectrometer (Agilent Technologies, Santa Clara, CA). The same chromatographic conditions described above were used. The MSD conditions were as follows: capillary transfer line and source temperature were set at 250 °C, mass range 30–350 u, and it was operated in electron ionization (EI) mode at 70 eV. Data were collected using multiple reaction monitoring mode (MRM). The MRM methods were optimized by injection of pure standards. Previously, each standard was analyzed in full scan mode (30–350 m/z) to identify precursor ions. Then, selected precursor ions were fragmented with different collision energies (2, 5, 10, 15, 20, 30, and 40 eV). Product ions were selected based on the abundance and selectivity of the transition from precursor to product ion for each standard (Table 1). For linalool, the transition parameters were 71.0→43.1 u at a collision energy of 5 eV. Finally, the concentrations for each odor-active compound were calculated, and the odor activity values (OAV) were determined as the relationship between the concentration and the threshold value in water taken from the literature. The quantitative data were employed to prepare the recombinated solution of the fruit aroma profile.
 Click to view | Table 1. GC-MS data acquisition parameters for quantitation (MRM technique) of odour-active volatiles in camu-camu (Myrciaria dubia) fruit |
2.3.5. Sensory analysis
The sensory evaluation was performed by an 11-judge trained panel (six women and five men, ranging in age from 21 to 50 years old) recruited from Disaromas S.A. (Bogotá, Colombia). During several sessions, sensory experiments were performed at 20 ± 1 °C in a sensory room with single cabins. The judges were trained in orthonasal odor perception of descriptors, as well as managing intensity scales through reference solutions with the following aroma attributes: fruity (isoamyl acetate), green ((Z)-3-hexen-1-ol), herbal pine-tree-like (α-pinene), citrus (limonene), and woody tree-bark-like (β-caryophyllene). For that purpose, a stock solution of each odorant in propylene glycol (10 mg/mL) was employed for preparing aqueous reference solutions, each at different concentrations between 10 and 90 times above the odor threshold of the respective odorant.
To prepare the fruit aroma recombined, appropriate amounts (10–200 μL) of the stock solutions of the odorants were mixed and made up to 1 L with water to yield the same concentrations as determined in M. dubia fruits. Fresh M. dubia puree (7 g) was prepared under the same conditions as the isolated volatile extract. The fruit and the recombined mixture were placed in different amber glass bottles (20 mL each). Thus, assessors were asked to ortho-nasally evaluate the intensity of the five reference solutions in both samples using each linear 3-point attribute scale (0 = not perceivable; 1 = weakly perceivable; 2 = moderately perceivable; and 3 = strongly perceivable, Lawless and Heymann, 2010). Also, they compared the overall similarity of the aroma profile between the two samples. References solutions were presented at concentrations corresponding to the final point of scale (3). All samples were evaluated in three replicate sessions, and results were plotted in a spider web diagram.
2.4. Antihypertensive activity
Whole camu-camu fruits (100 g) were frozen at −4 °C and lyophilized in a Beta 1-8 LDplus lyophilizer (CHRIST, Germany) for 48 h, with a main drying phase of 40 h at 1.0 mbar and - 20°C. The final drying phase lasted eight h, at 0.001 mbar and −76 °C. After freeze-drying, the seeds were manually extracted from the fruits; and the pulp and peel were homogenized using a grinder (A11 basic, IKA, Staufen, Germany), obtaining a fine powder. Camu-camu sample (peel and pulp) was prepared at 50 μg/mL, where 500 μg of fine powder was dissolved in 10 mL of deionized water, sonicated, and filtered using a PTFE syringe filter of 0.45 µm (Agilent technologies, USA).
The in vitro antihypertensive activity of camu-camu, was determined by the ACE-I enzyme inhibition assay following the protocol provided by the manufacturer (Dojindo Molecular Technologies Inc, 2023). Commercial lisinopril was used as a positive control prepared at 50 μg/mL in deionized water (ACE-I inhibition: 95.31 ± 5.88 %). The absorbance of all samples was measured in a 96-well plate multireader using a BertholdTech TriStar2S spectrophotometer (Berthold Technologies GmbH & Co, KG, Germany), and the data was processed using ICE software, Version 1.0.9.0. The inhibition percentage of the samples was calculated considering the following equation
2.5. Statistical analysis
Statistical analyses were performed using Statistica v12 (StatSoft) and InfoStat (Version 2020) software. All experiments were performed in triplicate by analysis of variance (ANOVA) and regression analysis, and average values were compared using Tukey’s test with a probability p ≤ 0.05. Data are presented as the mean and standard deviation of the three independent experiments.
| 3. Results and discussion | ▴Top |
3.1. Odor-active compounds of camu-camu fruit
The volatile profile of Myrciaria dubia fruit was characterized by the presence of monoterpenes (α/β-pinene, limonene, β-myrcene, o-cymene, 3-carene, α/γ-terpinene, and α-phellandrene), sesquiterpenes (β-caryophyllene, α-humulene, γ-elemene, and α-calacorene), monoterpenols (terpinen-4-ol and α-terpineol), aliphatic alcohols (isoamyl alcohol, butanol, and (Z)-3-hexen-1-ol), esters (isoamyl acetate, 2-ethyl furoate, ethyl benzoate, and ethyl butyrate), and the aldehyde nonanal. Most of these compounds were also reported by Franco and Shibamoto (2000); however, o-cymene, isoamyl acetate, and isoamyl alcohol were here identified as volatile constituents of M. dubia fruit. The use of solvent extraction with a more polar solvent (dichloromethane) rather than the retention in Porapak Q trap and hexane elution used by Franco and Shibamoto (2000), allowed here to isolate the isoamyl acetate that is one of the odour-active volatile compounds in M. dubia fruit.
By using the molecular sensory approach, this study successfully identified the odor-active volatiles in the aroma of M. dubia fruit. After analyzing the SAFE volatile extract using GC-MS/GC-O and AEDA analysis, only (Z)-3-hexen-1-ol, isoamyl acetate, α-pinene, limonene, and β-caryophyllene were identified as odour-active volatile compounds in this fruit (Table 2). The AEDA analysis (Figure 1) showed that (Z)-3-hexen-1-ol and β-caryophyllene were the volatile compounds with the highest FD compared to the other compounds in the extract.
 Click to view | Table 2. Odour-active volatiles identified in camu-camu (Myrciaria dubia) SAFE extract |
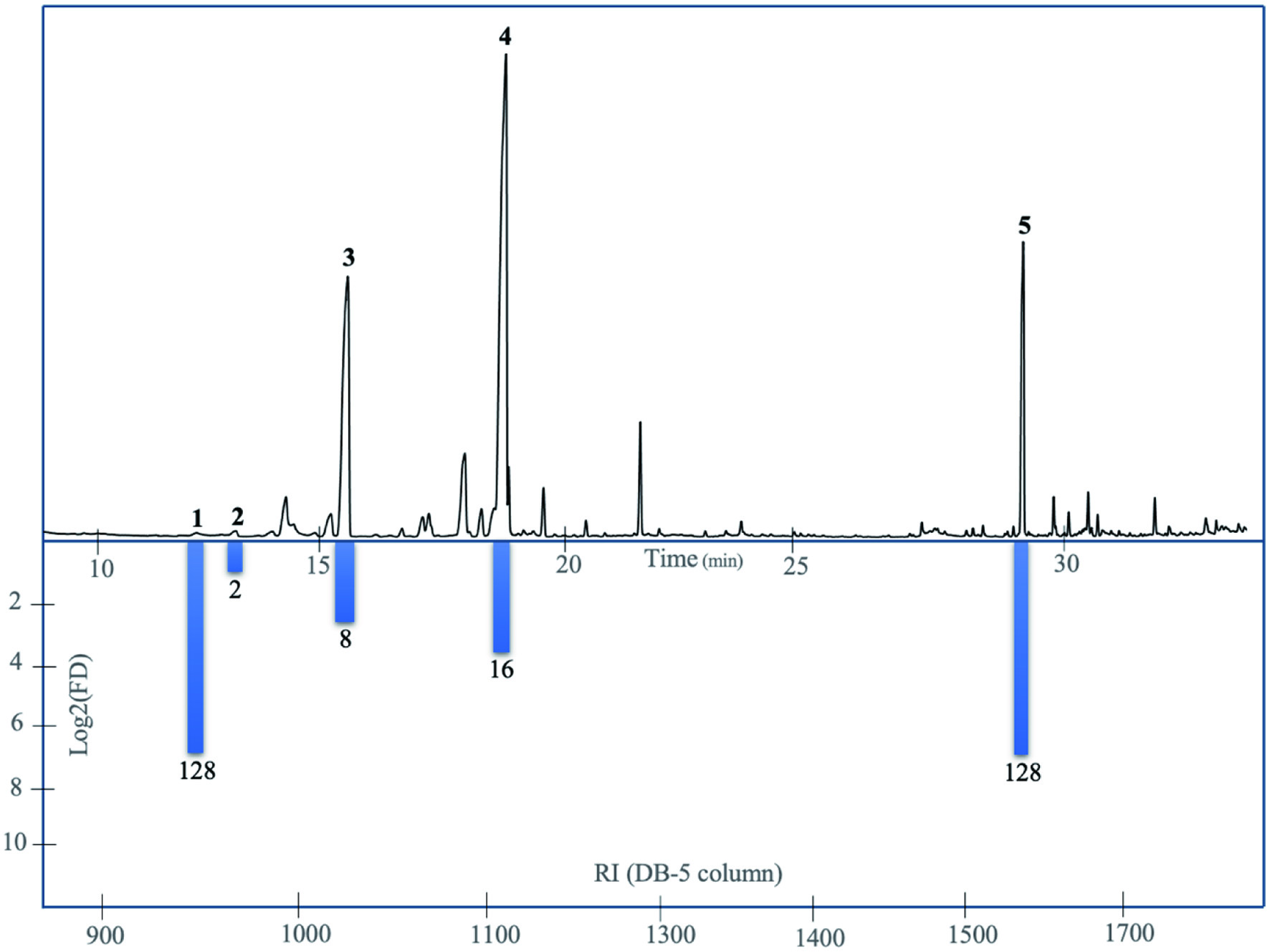 Click for large image | Figure 1. GC and AEDA analyses on DB-5 column of odour-active compounds from Myrciaria dubia fruit. Peak numbers correspond to the compound numbers in Table 2. |
The quantitation experiments were done by using the MRM technique with linalool as the internal standard. The MS parameters such as precursor and product ions are presented in Table 1. The OAVs were calculated for each compound, finding that a-pinene and limonene were the odour-active volatiles with the highest contribution to the aroma of this fruit. This finding agrees with the fact that terpenes are the main class of volatile compounds in different maturation stages of this fruit (Souza et al., 2021). It is noteworthy that a-pinene and limonene have also been reported as odour-active compounds in citrus fruit, such as clementine (Buettner et al. 2003) and orange (Hinterholzer and Schieberle, 1998).
An aroma recombination sample was prepared using the natural quantities found for each of the odorants listed in Table 2 and evaluated in comparison with the fruit puree (pulp and peel) by the sensory panel (Figure 2). Taking into account that the moisture content of fresh M. dubia fruit is above 90%, water was chosen as an appropriate matrix for the recombination experiments, and also was considered an odorless material. In general, trained judges agreed with the odor similarity between the reconstituted mixture and fresh fruit, determining more juiciness in the fruit sample. As (Z)-3-hexen-1-ol presented OAV < 1, it was not included in the preparation of the reconstituted mixture. However, the green note was evaluated in the recombinant experiments because it was perceived by the judges in both, the mixture and the fruit. Previous sensory evaluations of camu-camu juice from pulp reported woody, citrus, herbal, spicy, and camphoraceous notes in the aroma description (Campelo et al., 2020). Nonetheless, the presence of the peel in all of the experiments here performed likely contributed to reported different odour descriptors. The peel was included for better use of the fruit and reduction of residues after processing. In general, the intensities of the citrus, herbal, and woody (attributed to β-caryophyllene, α-pinene, and limonene, respectively) odor attributes in the aroma recombination sample were similar to those of the fruit puree. In contrast, the fruity note was rated lower than the original sample. Actually, judges associated the fruity-citrus notes with lemon peel. Because the aroma recombination sample closely matched the fruit aroma profile, it can be assumed that the key aroma compounds in camu-camu were satisfactorily characterized.
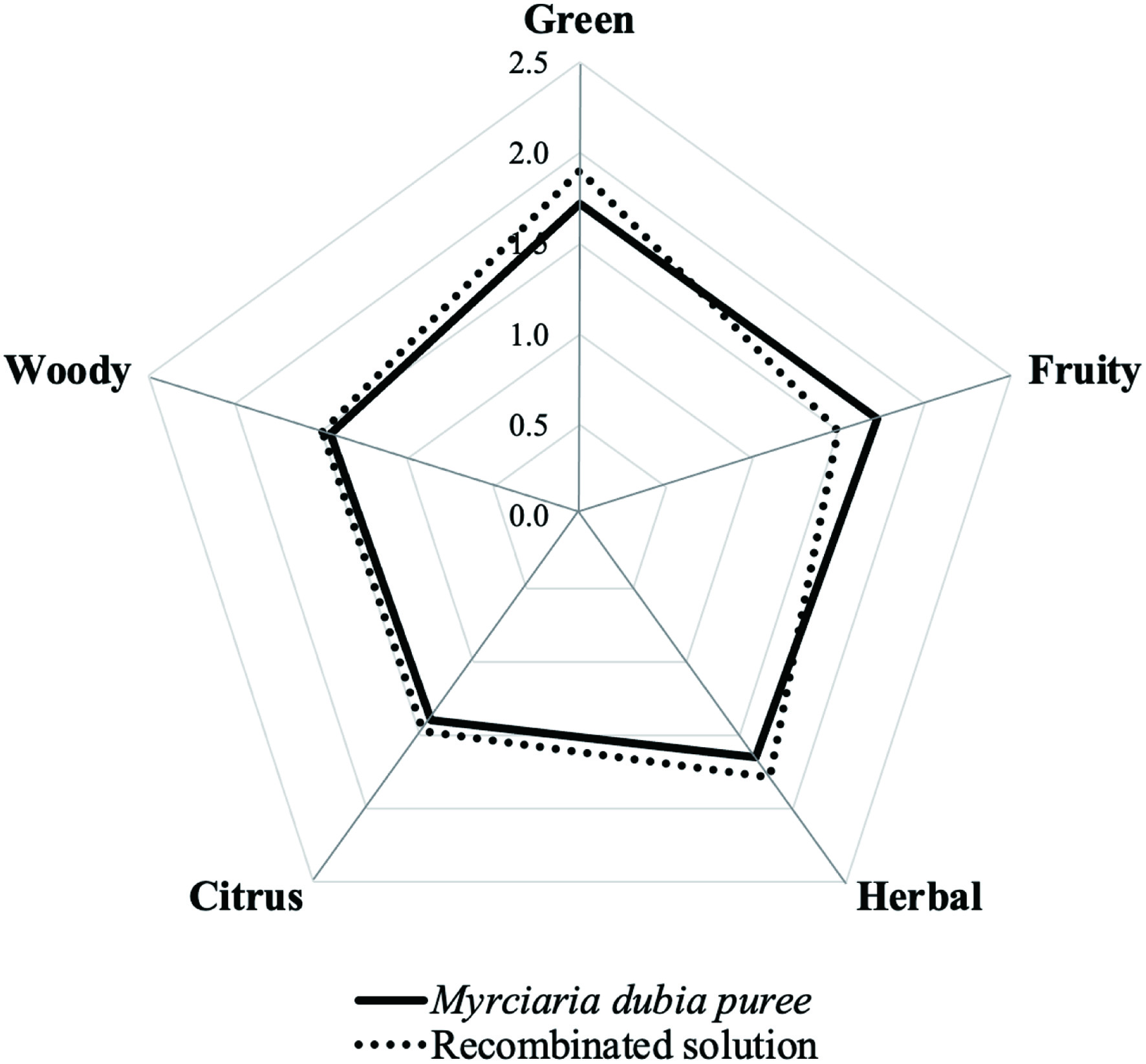 Click for large image | Figure 2. Comparative aroma sensory profiles of M. dubia fruit and aroma recombinated solution. |
The volatile profile of M. dubia was similar to that of other Myrtaceae fruits from the Amazon region, such as jaboticaba (Myrciaria jaboticaba), where terpenes (β-pinene and δ-cadinene) and alcohols (2-phenylethanol and linalool) were identified as the major volatile constituents (Plagemann et al., 2012). Guavaberry (Myrciaria floribunda) reported numerous sesquiterpenes with caryophyllene and γ-selinene being the most predominant volatile constituents (Mendoza García et al., 2021). In cagaita pulp (Eugenia dysenterica) α-pinene, (Z)-β-ocimene, and α-terpineol were the major constituents (Dos Santos Dias et al., 2021), whereas uvaiva pulp (Eugenia pyriformis) exhibited different terpenes in the volatile profile (Fernandes de Araújo et al., 2019). Likewise, the volatile profile of araza (Eugenia stipitata) consisted of β-ocimene as the major volatile compound (Fernandes de Araújo et al., 2021); and in pitanga fruit (Eugenia uniflora L.) the volatile composition in four ripening stages was predominantly composed by terpenoid compounds (Santos Silva et al., 2019). These findings suggest that the 1-deoxy-D-xylulose 5-phosphate (DXP) biogenetic pathway is highly activated from the production of terpenes (Souza et al., 2021). Similarly, various reports suggested that the formation of terpenes in Myrtaceae species is primarily attributed to metabolic pathways that begin with the geranyl and farnesyl cations, leading to monoterpenes and sesquiterpenes transformations (Silva da Costa et al., 2022). Terpenes are some of the most prevalent and structurally varied secondary metabolites in plants that enable interactions between plants and their surroundings, which are essential for all stages of their life cycle. Moreover, terpenes are also recognized for having biological and functional properties in food, pharmaceutical, cosmetic, and fragrance applications. In the food industry, terpenes have been used to add flavour and aroma to different food products, allowing the development of emerging food designs and trends. Additionally, terpenes are distinguished for exhibiting therapeutic and bioactive applications since they present anticancer, antibacterial, antihyperglycemic, anti-inflammatory, antioxidant, immunomodulatory, and antiviral activity, providing health benefits for the prevention and treatment of some diseases (Brahmkshatriya and Brahmkshatriya, 2013).
3.2. Antihypertensive activity
The freeze-dried Amazonian camu-camu fruit was assessed under ACE-I enzyme activity inhibition assay, which is used to determine the in vitro antihypertensive activity. The ACE-I inhibition is one the most employed strategies to prevent and manage hypertension, since it could prevent or delay the onset of high blood pressure and reduce side effects even from non-communicable diseases (Felkle et al., 2022). In plant materials, the capacity to inhibit ACE-I enzyme is usually given by its chemical composition, mainly constituted by vitamins, minerals, and dietary polyphenols. In fact, phenolic compounds such as flavonoids, and ellagitannins in berries like strawberry (Fragaria × ananassa), blueberry (Vaccinium corymbosum), or raspberry (Rubus idaeus), and seems to have played an important role in the modulation of chronic hypertension (Yousefi et al., 2020).
The ACE-I inhibition activity exhibited by the M. dubia fruit was 39.48 ± 12.09 % at the endogenous fruit concentration (50 μg/mL), with lisinopril used as the positive control. This is a promising result, considering that Fujita et al. (2015) did not report any enzymatic ACE-I inhibition for M. dubia pulp, even at a sample concentration 200 times higher (10 mg/mL) than the one employed in this assay. Furthermore, the jaboticaba (Myrciaria jaboticaba) seed extract inhibited the ACE-I enzyme (22.4 ± 0.2%), which was attributed mainly to the hydroxyl groups in the benzene ring of the phenolic compounds such as castalagin, procyanidin A2, and ellagic acid (Fidelis et al., 2021).
The polyphenols previously detected in M. dubia pulp powder obtained by spray-drying were the flavonol myricetin and its conjugates (3.05 ± 0.07 mg/100 g powder), ellagic acid derivatives (9.75 ± 0.10 mg/100 g powder), ellagitannins (16.10 ± 0.33 mg/100 g powder), and anthocyanins (19.63 ± 0.60 mg/100 g powder), being 2-fold concentration higher in the fresh peel than in the fresh pulp (Fracassetti et al., 2013). Moreover, the main phenolic compounds identified in M. dubia peels were myricetin-O-pentoside (4.91 ± 0.08 mg/g extract) and cyanidin-3-O-glucoside (4.68 ± 0.07 mg/g extract). Taking into account that M. dubia peel (33.4 ± 0.5 mg/g extract) exhibited a higher polyphenolic content that the pulp (4.32 ± 0.03 mg/g extract) (Conceição et al., 2020), the results here obtained potentially can be attributable to the polyphenols present in the M. dubia peel.
It has been reported that polyphenols block ACE-I enzyme by filling the bioactive sites. Specifically, flavan-3-ols and procyanidins have an inhibitory effect on the ACE-1 activity, which depends on the number of epicatechin units of procyanidin (Actis-Goretta et al., 2003). Phenolic compounds inhibit ACE-1 enzyme by generating chelates with the zinc atom within the active center, among other proposed mechanisms (Chen et al., 2021).
| 4. Conclusion | ▴Top |
The sensomics approach allowed the identification of the four volatile compounds that contributed to the aroma of the M. dubia fruit. Likewise, the sensory evaluation showed the relevance of terpenes (α-pinene, limonene, and β-caryophyllene) in the aroma of this fruit, exhibiting herbal, woody, and citrus odour notes. Moreover, the camu-camu fruit (pulp and peels) showed an in vitro antihypertensive activity by inhibiting the ACE-I enzyme activity. These results show that camu-camu fruit is a promising raw material for developing added-value products with bioactive properties based not only on vitamin C but also on the polyphenol content, allowing sustainable exploitation of this Amazonic fruit. With these results, the medium-term objective is to increase consumer awareness of this native and tropical Amazonian fruit and evaluate its potential role as raw material for functional foods.
Acknowledgments
J. G.-Ch thanks the scholarship from Ministerio de Ciencia, Tecnología e Innovacioń of Colombia (Minciencias): Programa de Becas de Excelencia Doctoral del Bicentenario, Corte-I, and Fulbright Visiting Scholar Program 2021. The authors thank Julie Peterson, Ella Lin, Megan Booth from FREC (The Ohio University State, Columbus, Ohio, USA); Andrea Caicedo, and Alirio Guevara from Disaromas S.A. (Bogota, Colombia) for the support in the sensory evaluation with trained panelists. The authors also thank Dr. Alberto Fajardo for carrying the fruits. The ANLA and Ministerio de Ambiente y Desarrollo Sostenible granted permission to collect samples and perform this research (Contrato Marco de Acceso de Recursos Genéticos y sus Productos Derivados No. 357 del 17 de noviembre de 2022 suscrito entre el Ministerio de Ambiente y Desarrollo Sostenible y la Universidad Nacional de Colombia).
Conflict of interest
The authors declare no conflict of interest. The founding sponsors were not involved in planning the study, data collecting, analysis, interpretation, manuscript writing, or the choice to publish the findings.
| References | ▴Top |