| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 21, March 2023, pages 21-27
Extracts of plant-based yogurts inhibit recombinant human angiotensin converting enzyme 2 (rhACE2) activity
Yu Hasegawa, Waritsara Khongkomolsakul, Bradley W. Bolling*
Department of Food Science, University of Wisconsin-Madison, 1605 Linden Dr. Madison, WI 53706
*Corresponding author: Bradley W. Bolling, Department of Food Science, University of Wisconsin-Madison, 1605 Linden Dr., Madison, WI 53706, USA. Tel: +1-608-890-0212; Fax: +1-608-262-6872; E-mail: bwbolling@wisc.edu
DOI: 10.31665/JFB.2023.18335
Received: February 9, 2023
Revised received & accepted: March 25, 2023
| Abstract | ▴Top |
Little is known about how diet and nutraceuticals modulate Angiotensin-converting enzyme 2 (ACE2). Plant-based yogurts (PBYs) are potential sources of functional components and are made from a variety of plant-milks with varying cultures and protein sources. This study assessed the recombinant human (rh)ACE2 inhibitory activity of PBYs available in the United States. Extracts from 21 almond, cashew, coconut, oat and soy PBYs were screened for rhACE2 inhibitory activity. Among these samples, 9 PBYs inhibited recombinant human (rh)ACE2 by 50% at less than 50 mg/mL (dry weight (dw) PBY basis). Extracts of soy, almond, and oat PBYs were among the most active inhibitors, from 4–11 mg/mL IC50 (dw PBY). Among plant milks, soy milk extracts were more active rhACE2 inhibitors than oat or almond milks. Isoflavones contributed to activity, as purified isoflavones inhibited rhACE2. Therefore, rhACE2 inhibition by PBY varies considerably between products. These results suggest that there are a variety of nutraceuticals in PBYs that inhibit ACE2, and their bioactivity depends on the method of manufacturing and ingredient selection.
Keywords: Plant-based yogurt; rhACE2; Isoflavone; Soy; Nuts; Almond
| 1. Introduction | ▴Top |
Plant-based yogurts (PBYs) have emerged as a growing category of plant-based foods. A wide range of formulations are used to produce PBYs, which include varying plant milks, stabilizers, microbes, and/or supplemental plant proteins (Boeck et al., 2021). Coconut, oat, almond, cashew, and soy are common plant milks used for producing PBYs. These vegetable, grain, nut, and microbial ingredients are potential sources of bioactives; however, the health-promoting potential of PBYs has not been well-described.
The anti-hypertensive activity of plant bioactives has been previously described. For example, polyphenol and peptide angiotensin converting enzyme (ACE) inhibitors have been identified from vegetables and seed protein hydrolysates (Ijarotimi et al., 2018; Olarewaju et al., 2018; Stack et al., 2018). In contrast, less is known about the ability of dietary components to interact with recombinant human angiotensin converting enzyme 2 (rhACE2), a component of the renin-angiotensin system important to modulating blood pressure, inflammation, and the host receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Microbial metabolites, bioactive peptides, and plant phenolics such as quercetin, tannic acid, epigallocatechin gallate, and genistein could all be potential rhACE2 inhibitors (Al Shukor et al., 2013; Liu et al., 2020; Montenegro et al., 2009; Takahashi et al., 2015). Furthermore, polyphenols that inhibit rhACE2 activity can also inhibit SARS-CoV-2 spike proteins from interacting with ACE2 (Ohishi et al., 2022; Schmidt et al., 2022).
Given the emerging importance of ACE2 and the increasing consumption of PBYs, the objectives of this study were to screen extracts of PBYs for inhibition of rhACE2 activity and identify factors common between inhibitory PBYs. We identified soy-isoflavone containing products were among the most active PBYs within this product category.
| 2. Materials and methods | ▴Top |
2.1. Extraction and drying of plant-based yogurts (PBYs) and plant milks
Plant milks and PBYs made from coconut, oat, almond, cashew, or soy that were plain or vanilla-flavored were obtained from local supermarkets in Madison, WI, USA (Table 1). PBYs and plant milks were frozen, lyophilized, and then ground to a powder by pestle and mortar. Extracts were obtained by homogenizing 150 mg of dry powder with ice-cold extraction buffer consisting of 1.8 mL of ultrapure water (Barnstead Lab Water Products, Lake Balboa, CA, USA), 2.4 mL of methanol (Sigma-Aldrich, St. Louis, MO, USA), and 4.8 mL of chloroform (Fisher Scientific, Hampton, NH, USA). Samples were then centrifuged at 3,000 ×g at 0 °C for 15 min. The resulting supernatants were dried under nitrogen and stored at −80 °C until analysis of the extracts.
 Click to view | Table 1. The ingredients and cultures of plant-based yogurt (PBY) and the base milk products as identified on product labels |
2.2. Analysis of rhACE2 inhibition
The activity of rhACE2 was measured as previously described (Liu et al., 2020), using rhACE2 protein (R&D Systems, Minneapolis, MN) and fluorescent substrate (AnaSpec, San Jose, CA, USA). After subtraction of background fluorescence, the rate of rhACE2-inhibition was calculated by dividing the fluorescence value of ACE2 with extracts by the fluorescence of the ACE2 control without the sample, and multiplying by 100. The rhACE2 inhibition values were fitted by concentrations as a variable slope [inhibitor]-normalized response model in GraphPad Prism 7.0 to obtain IC50 values (concentration of PBY required to inhibit 50 % of ACE2 activity). IC50 values were calculated as dry weight equivalents of PBYs or plant milks from three independent experiments with triplicates. Samples with IC50 values of less than 50 mg/mL were defined to be active inhibitors of rhACE2 activity,
2.3. High-performance liquid chromatography (HPLC) analysis of isoflavones
Isoflavones were analyzed using methods adapted from prior reports (Griffith and Collison, 2001). Briefly, lyophilized powders were extracted for 2 h at ambient temperature with acetonitrile/water/DMSO with 1 mM formononetin as an internal standard (Indofine Chemical Company. A Dionex UltiMate 3000 HPLC equipped with an autosampler and diode array detector was used for analysis, using a Kinetix Evo C18 50 x 3 mm, 2.6 μm column and 1 μL injection volumes. The gradient consisted of 0.1% formic acid in ultrapure water and methanol (v/v) as mobile phases A and B, respectively. The gradient consisted of 10% B at 0.6 mL/min to 12% B at 1 min, 22% B at 3 min, 23% B at 4 min, 35% B at 5 min, then 50% B at 6 min and held at this condition to 8 min. The gradient returned to 10% B at 8.1 min and was held to 9.5 min to equilibrate the column. Isoflavones were detected at 260 nm and identified by comparison to soy isoflavone external standards (daidzin, glycitin, genistin, daidzein, glycitein, genistein, and 6″-O-acetylglycitin from Indofine Chemical Company, Inc. (Hillsborough, NJ, USA); 6″-O-malonyldaidzin, 6″-O-malonylglycitin, 6″-O-acetyldaidzin, 6″-O-acetylglycitin from Fujifilm Wako Pure Chemical Company (Osaka, Japan); and 6″-O-malonylgenistin from Santa Cruz Biotechnology (Santa Cruz, CA, USA)) and purified soy isoflavones (Fujiflavone P40; Fujicco, Hyogo, Japan). External standard curves of the isoflavones were used for quantitation after accounting for recovery of the internal standard.
2.4. Statistical analysis
To evaluate differences between means, we used one-way ANOVA and Tukey’s multiple comparison tests. For the isoflavone analysis, the resulting p-values were adjusted by the Benjamini-Hochberg method to control for false discovery rate. Principal component analysis and data visualization were performed using R (version 4.2.0). Adobe Illustrator (version 26.0.1) was also used to generate figures. Statistical significance was defined when p-values were less than 0.05.
| 3. Results and discussion | ▴Top |
3.1. Soymilk-based and soy-protein-supplemented PBYs inhibited rhACE2
Commercially-available PBYs from the US market (n = 21 samples) were lyophilized and extracted by the Folch method to obtain polar rhACE2 inhibitors (Table 1). Only 9 PBYs had IC50 values less than 50 mg/mL dry weight basis (dwb) (Figure 1a). The most active rhACE2 inhibitor was SOY1 (3.63 ± 0.17 mg/mL IC50), which was significantly different from other PBYs except for ALM1 (7.01 ± 0.35 mg/mL IC50) and two oat-based PBYs (OAT1, IC50 = 11.3 mg/mL and OAT5 IC50 = 11.4 mg/mL; Figure 1).
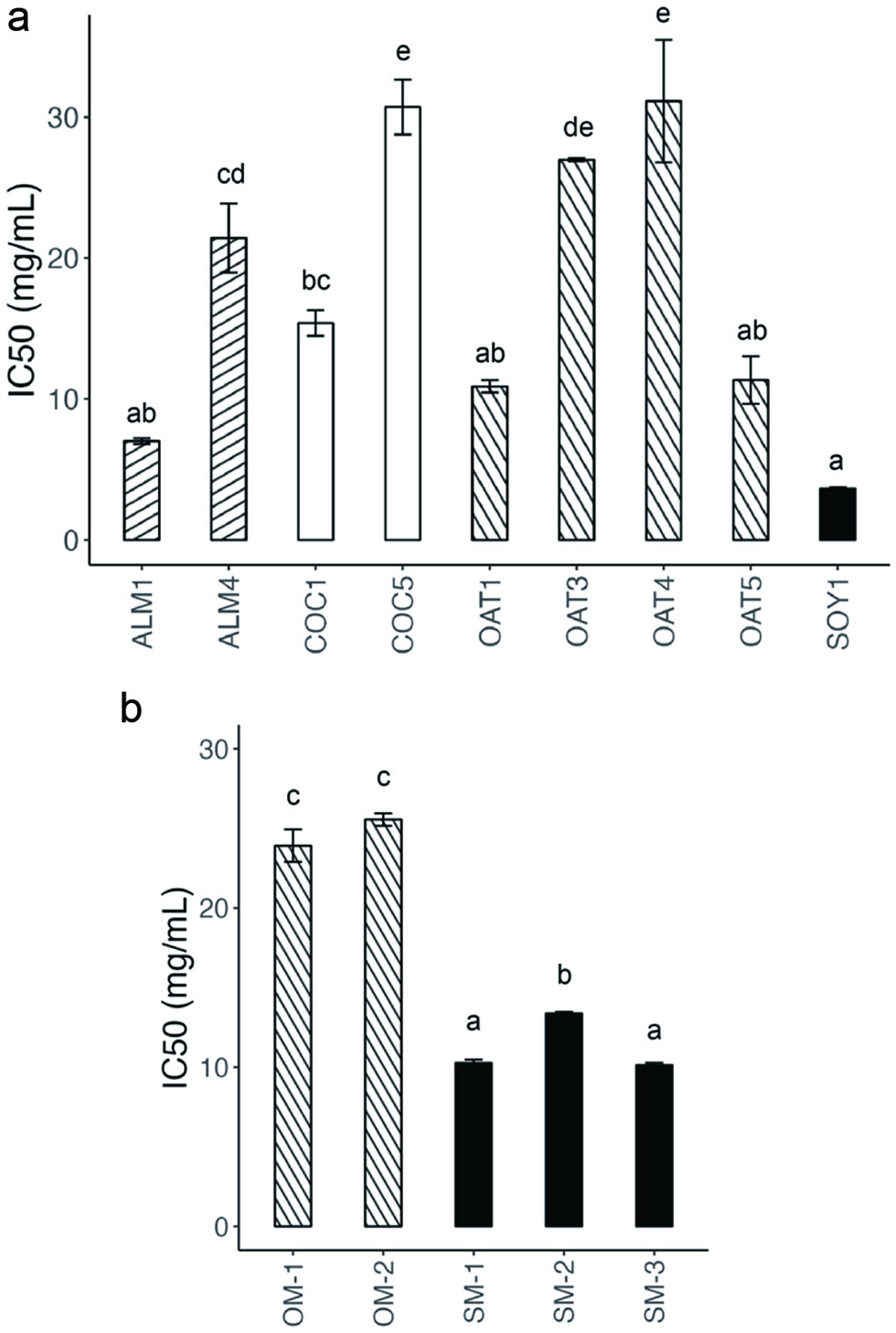 Click for large image | Figure 1. PBYs and plant milks that inhibited rhACE2. (a) PBYs and (b) plant milks with IC50 < 50 mg/mL dry weight basis (dwb). Different letters represent samples with significant difference by one-way ANOVA, followed by Tukey-Kramer HSD, p < 0.05. |
Other almond-based PBYs were less active than ALM1. In contrast to other almond-based PBYs, ALM1 contained soy protein isolate and fava bean protein (Table 1). OAT1 and OAT5 contained pea protein and fava bean protein, respectively (Table 1). However, these proteins were also present in OAT2 and OAT4, which were less active. Other factors such as the differences in the declared microbial components and production procedures between PBYs could contribute to differences in rhACE2 activity.
Next, we evaluated the rhACE2 inhibition of plant-based milks from the most-active PBYs. Commercial soy, almond, and oat milk beverages were lyophilized and extracted in the same manner as PBYs (Table 1). Among these beverages, soymilk products had the greatest rhACE2 inhibition (average IC50 = 10.3, 13.4, and 10.1 mg/mL), followed by oat milk (IC50 = 23.9, 25.6, and >50 mg/mL) (Figure 1b). Almond milk did not have strong rhACE2 inhibition (IC50 > 50 mg/mL). Thus, the rhACE2 inhibition by ALM1 was likely due to the addition of soy protein isolate or fava bean protein instead of the plant milk.
3.2. PBYs and plant milks varied in isoflavone profiles and bioactivity
Based on the analysis of PBYs and plant-based milks, soy bioactives appeared to contribute rhACE2 inhibition. Therefore, we hypothesized that isoflavones inhibit rhACE2 activity. A 40% purified soy isoflavone concentrate (Table 2) had an IC50 of 2.23 ± 0.47 mg/mL, suggesting that isoflavones do contribute to rhACE2 inhibition. In comparison, quercetin IC50 was 8.9 µg/mL under similar assay conditions (Liu et al., 2020). Pomegranate peel extract inhibited Spike/ACE2 binding by 50% at 0.4–0.6 mg/mL (Alsubhi et al., 2022). Among fruits and vegetables, hot water extracts of soy, tomato, cucumber, and asparagus inhibited >50% ACE2 activity at 0.1 mg/mL (Takahashi et al., 2015).
 Click to view | Table 2. Content of isoflavones in selected PBYs and soymilks as determined by HPLC analysis |
Next, we assessed the isoflavone content and profiles of soymilks available in the market (SM-1, SM-2, and SM-3), as well as two PBY products with the most potent rhACE2 inhibition (ALM1 and SOY1). Among these products, SOY1 had 368 mg/kg dwb of total isoflavones, significantly higher than other samples, which ranged from 25.7–64.8 mg/kg dwb of total isoflavones (p < 0.01) (Table 2). The isoflavone profiles were different between the isoflavone concentrate, soymilks and PBYs (Table 2). Soymilks were mainly genistein and daidzein, whereas SOY1 had relatively higher malonyl isoflavones. ALM1 had the greatest proportion of daidzein and glycitin relative to other products. The isoflavone concentrate had the highest concentrations of daidzin and glycetin. The increased content of malonyl isoflavones in SOY1 relative to other products indicate it had the lowest amount of thermal processing as heat-treatment of soy leads to isoflavone de-esterification (Xu et al., 2002).
PCA analysis was conducted to understand the relationship between the levels of isoflavone isoforms and IC50 values. The two major principal components accounted for 80.3 % of the total variance (51.5 % for PC1 and 28.8 % for PC2) (Figure 2). PC1 discriminated SOY1 from the three soymilk samples and ALM1. SOY1 was characterized by higher levels of 6 isoflavones (Group A: acetylglycitin, daidzin, genistin, malonyldaidzin, malonylgenistin, malonylglycitin). PC2 separated ALM1 from SOY1 and soymilk. The isoflavones on the positive side of PC2 included acetyldaidzin and acetylgenistein (Group B) and these were present only in SM-3 among the three soymilks. Also, the three soymilks were characterized by daidzin and genistin, which were abundant in these samples. ALM1 was distinctively abundant in daidzein, genistein, glycitein and glycitin (Group C), while glycitin was also abundant in SOY1. Interestingly, the IC50 values had an inverse relationship with isoforms in Group A, suggesting that Group A isoflavones may have a stronger contribution to rhACE2 inhibition than other isoflavones. Among the isoflavones studied in this study, genistein, which is readily converted from genistin once ingested, inhibited ACE activity both in vivo and in vitro (Montenegro et al., 2009). Although the mechanism of isoflavone ACE2 inhibition is not fully understood, previous studies illustrate the structure-function of flavonoid ACE2 inhibition (Al Shukor et al., 2013). For example, the activity of phenolic compounds is associated with the number of hydroxyl groups on the benzene ring and/or via interaction with the zinc ion in ACE active site (Al Shukor et al., 2013). Also, Liu et al., found that flavonoids with B-ring 3′,4′-hydroxylation inhibited rhACE2 activity in vitro (Liu et al., 2020).
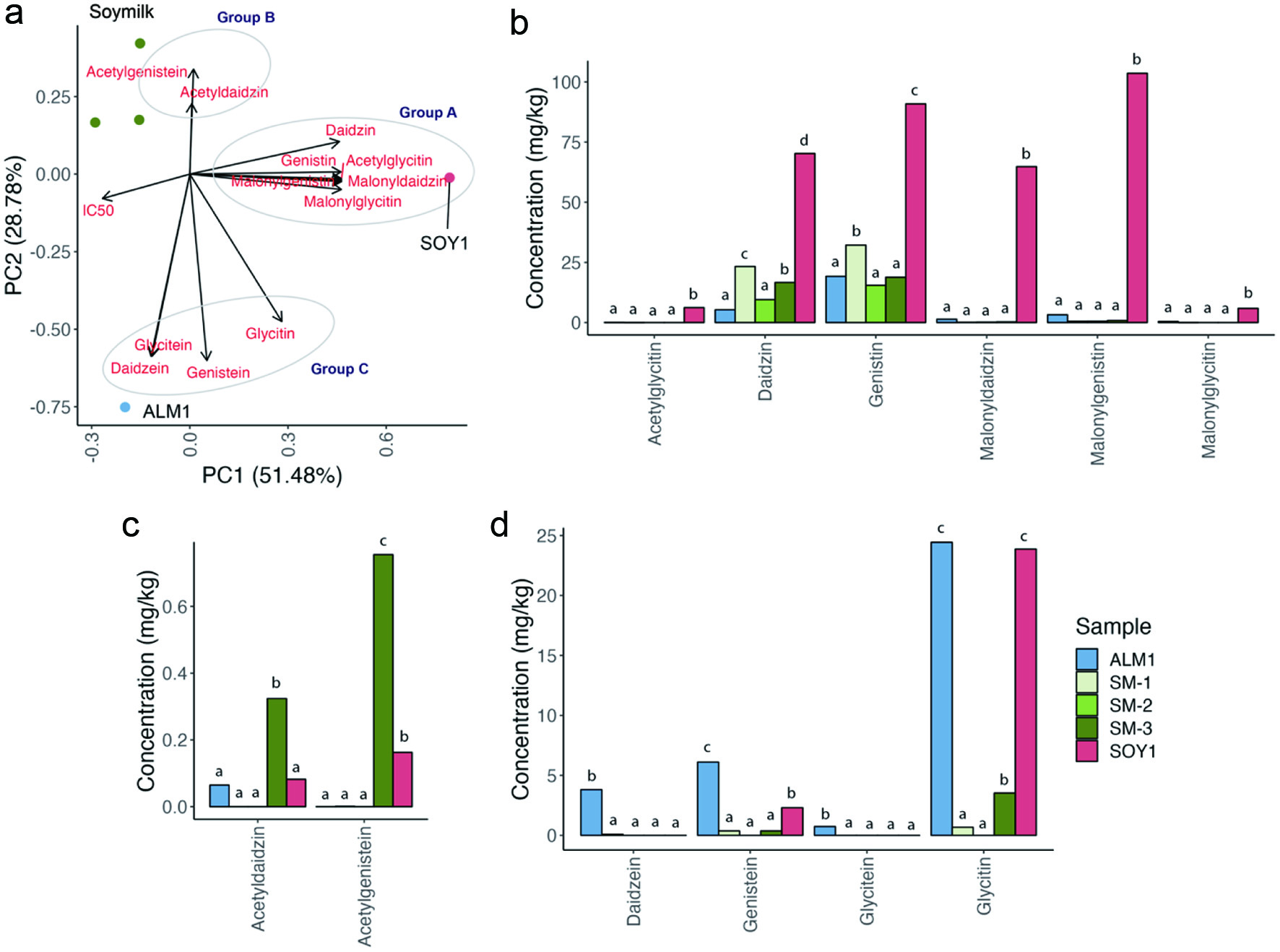 Click for large image | Figure 2. Assessment of isoflavone isoforms and their relationship with IC50. (a) PCA plot representing the relationship between isoflavones and IC50 (dwb) in two PBYs and three soymilks. Isoflavones were discriminated into three groups (Group A, B, and C). Bar graphs showing the levels of (b) Group A isoflavones (n = 6 isoflavones); (c) Group B isoflavones (n = 2 isoflavones); (d) Group C isoflavones (n = 4 isoflavones). Blue is for ALM1, green is for soymilks (light green for SM-1, green for SM-2, dark green for SM-3), and red is SOY1. Statistical analysis was by one-way ANOVA, followed by Tukey-Kramer HSD for each isoform. Bars bearing different letters are p < 0.05. |
It is plausible that bioactives other than isoflavones contribute to rhACE2 inhibition by PBYs. Soy contains nicotianamine, a known rhACE2 inhibitor with similar potency to quercetin (Takahashi et al., 2015, Liu et al., 2020). Microbial hydrolysis of proteins could increase inhibitor bioactive peptides and increase phenolics (Çabuk et al., 2018). Furthermore, plant proteins may carry inhibitory polyphenols through the manufacturing process (Alonso et al., 1998). This suggests that fortification and manufacturing processes could further increase PBYs ACE2 inhibition. Ultimately, the efficacy of plant polyphenols and other bioactives will depend on the metabolism, bioavailability, and the interaction with the intestinal immune system (Pei et al., 2020).
| 4. Conclusion | ▴Top |
In conclusion, soy-containing PBYs were among the most active inhibitors of rhACE2 activity and isoflavones contributed partly to rhACE2 inhibition. This study revealed that rhACE2-inhibition by PBYs varied considerably within this product category, highlighting the importance of ingredient selection to deliver bioactives in the final product. Also, additional research studies are needed to confirm the impact of PBYs on human health. Nonetheless, considering that ACE is an essential component in the renin–angiotensin–aldosterone system (Erdös and Skidgel., 1987), PBYs may be good vehicles to deliver bioactives targeting this pathway for cardiovascular health.
Acknowledgments
Research funding for this work was from the Fritz Friday Chair of Vegetable Processing Research, College of Agricultural and Life Sciences, University of Wisconsin-Madison. The graphical abstract was created with BioRender.com. The authors express gratitude for the technical assistance of Matthew Dorris and Andrea Noll.
Conflict of interest
BWB and YH have received unrelated research funding from the University of Wisconsin Dairy Innovation Hub. BWB has received prior unrelated research funding from the National Dairy Council, Almond Board of California, and Kikkoman R&D USA; past consulting for Allergy Amulet, Almond Board of California, Healthy Imports Beverages, International Tree Nut Council; honoraria from National Dairy Council, and American Dairy Association North East. WK reports no conflicts of interest.
YH: conceptualization, formal analysis, writing- original draft, writing – review & editing; WK: conceptualization, formal analysis, investigation, writing – review & editing; BB: formal analysis, writing – original draft; writing - review & editing, supervision.
| References | ▴Top |