| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 21, March 2023, pages 3-20
Uses of carotenoid-rich ingredients to design functional foods: a review
Shahida Anusha Siddiquia, b, *, Salome Dinic, Yasaman Esmaeilid, Sahar Roshanake, Ali Ali Redhaf, g, Sajad Ahmad Wanih, *
aTechnical University of Munich Campus Straubing for Biotechnology and Sustainability, Essigberg 3, 94315 Straubing, Germany
bGerman Institute of Food Technologies (DIL e.V.), Prof.-von-Klitzing-Strae 7, 49610, D, Quakenbrck, Germany
cDepartment of Food Science, University of Otago, Dunedin, New Zealand
dDepartment of Food Science and Technology, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran
eDepartment of Food Science and Technology, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran
fThe Department of Public Health and Sport Sciences, University of Exeter Medical School, Faculty of Health and Life Sciences, University of Exeter, Exeter, EX1 2LU, United Kingdom
gCentre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovation (QAAFI), The University of Queensland, Brisbane, QLD 4072, Australia
hDepartment of Food Technology, Islamic University of Science and Technology, Awantipora, India
*Corresponding author: Shahida Anusha Siddiqui, Technical University of Munich Campus Straubing for Biotechnology and Sustainability, Essigberg 3, 94315 Straubing, Germany. E-mail: S.Siddiqui@dil-ev.de; Sajad Ahmad Wani, Department of Food Technology, Islamic University of Science and Technology, Awantipora, India. E-mail: sajad04@outlook.com
DOI: 10.31665/JFB.2023.18334
Received: November 8, 2022
Revised received & accepted: December 26, 2022
| Abstract | Top |
Carotenoids are isoprenoids that are extensively dispersed in foods that have always been part of the human diet. Certain carotenoids can be transformed into retinoids with vitamin A activity, which is needed for humans. Additionally, they are far more flexible, since they may be found in foods not just as sources of vitamin A, and also as natural colors, antioxidants, and health-promoting substances. Functional foods provide health advantages in addition to basic nourishment. They can be found in a variety of forms, including whole, fortified, enriched, or enhanced meals. A flood of information about the health advantages of functional foods has been supplied by several epidemiological research. This review discusses the factor for healthy and sustainable usage of carotenoid-rich ingredients for the design of functional food products primarily intended for health promotion. Furthermore, data on sources, intakes, and variables influencing bioavailability are summarized.
Keywords: Carotenoids; Food production; Bakery products; Extruded snacks; Gluten-free products; Meat products
| 1. Introduction | Top |
Carotenoids are natural substances and are biosynthesized by photosynthetic organisms (algae, plants, and cyanobacteria), fungi, and bacteria. Carotenoids are classified as xanthophylls or carotenes depending on their chemical makeup. Carotenes are made up of hydrogen and carbon atoms, whereas xanthophylls also include oxygen. Carotenoids play a part in photosynthetic activities in plants and provide pigments. Tissues with a yellow-red tint act as an attractant in many fruits and flowers (Kalac, 2012). Carotenoids cannot be biosynthesized by the great majority of animals, but they may be integrated into the diet and structurally changed afterwards. Certain invertebrates, including hemipterans (phylloxerids, adelgids , and alphids) and dipterans (gall midges), have been demonstrated to synthesize carotenoids from scratch (Rodriguez-Concepcion et al., 2018).
To execute their biological activities, carotenoids must be released from meals and made accessible for absorption by the human body; yet, when compared to other dietary phytonutrients, they are only 5 to 30% accessible from organic resources (Kopec and Failla, 2018; Bohn, 2018). As a result, new methods, such as digestion procedures, are guiding the development of innovative delivery systems and functional meals with improved carotenoid availability, as well as the development of technological techniques to increase carotenoid bioavailability from plant-food sources.
Carotenoids have been reported for a wide range of bioactive potentials including anti-cancer, anti-diabetic, anti-obesity, anti-inflammatory, and cardioprotective activities (van Chuyen and Eun, 2017).
Antioxidant and anti-inflammatory effects of carotenoids (Fiedor and Burda, 2014) can improve eye health, cognitive function (Bernstein et al., 2016; Feeney et al., 2013) and provitamin A activity (Conboy Stephenson et al., 2021). Carotenoids having an unsubstituted -ionone ring, carotenes (such as -carotene and -carotene), and the xanthophyll -cryptoxanthin all exhibit provitamin A activity. Vitamin A is needed for eyesight and is one of the most often deficient vitamins in the world, particularly in developing countries (Gurmu et al., 2014). Fucoxanthin is another carotenoid, a xanthophyll, that has a wide range of bioactivities. It is found mainly in brown seaweed (Mresse et al., 2020). Among its bioactivities, it has shown promising antidiabetic, anti-obesity (Maeda, 2013), antioxidant, anti-inflammatory, and anticancer activity (Mresse et al., 2020). It has been shown to be effective on many of the classical hallmarks of tumor cells. The main carotenoids that have remarkable applications is shown in Figure 1, including their main sources. The structure of key carotenoids is shown in Figure 2 (Fiedor and Burda, 2014).
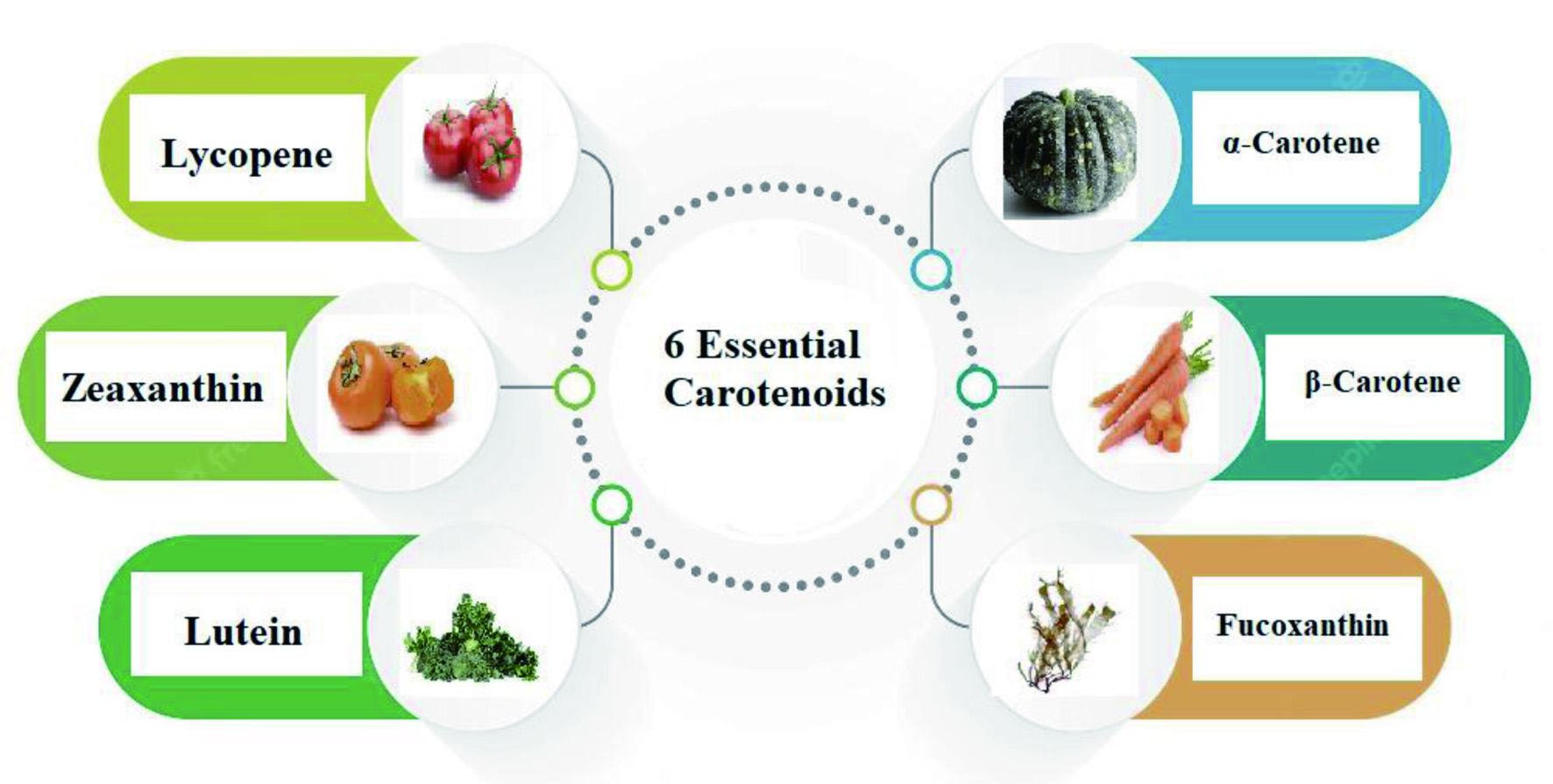 Click for large image |
Figure 1. Essential carotenoids and sources. |
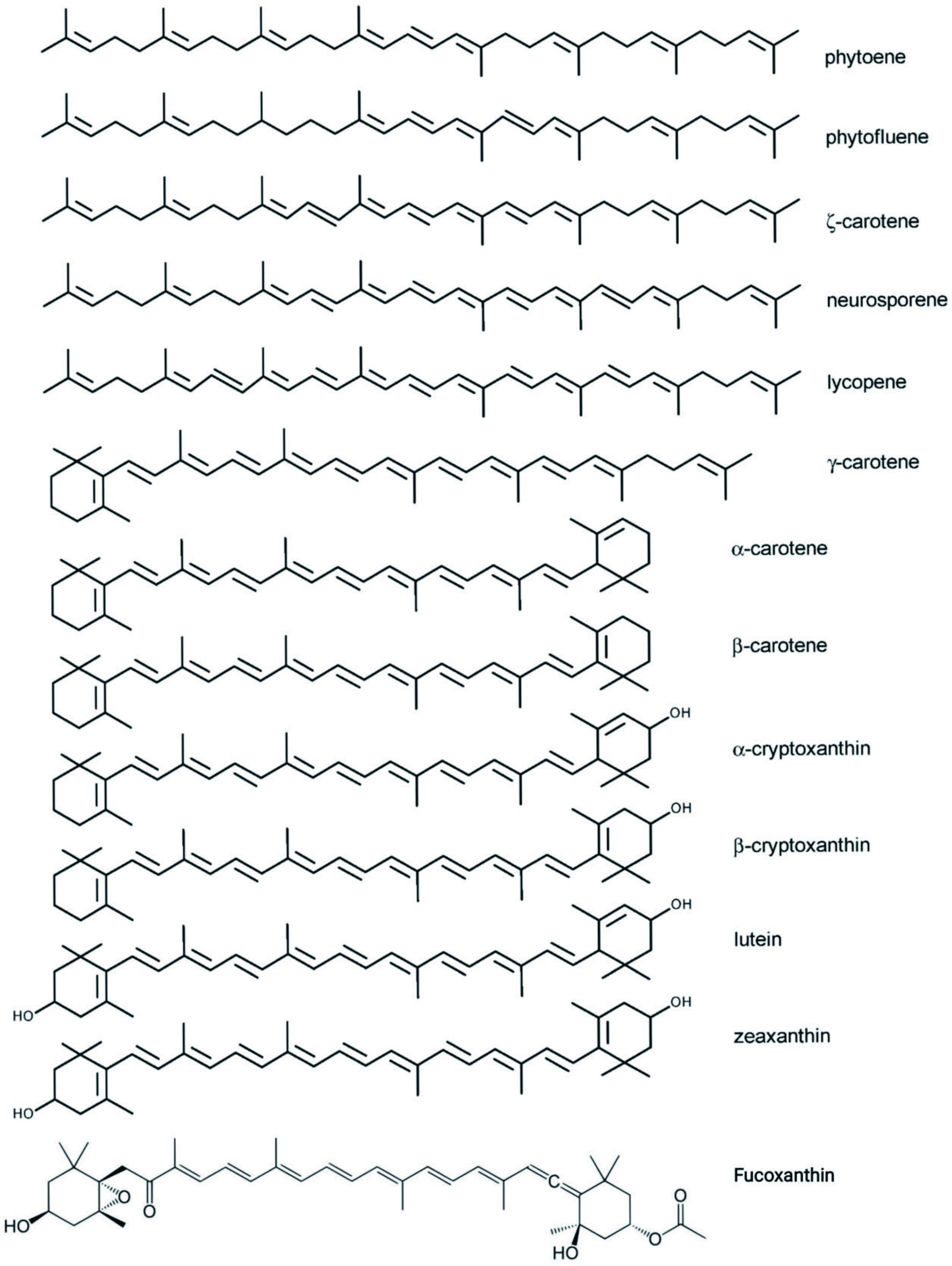 Click for large image |
Figure 2. Chemical structure of key carotenoids. |
It is also important to highlight that although higher plants are considered a major source of carotenoids, yet algae also have significant amounts of carotenoids (Shahidi and Brown, 1998). Blue-green algae, red algae, yellow algae, brown algae, and green algae are sources of carotenoid pigments. In addition, some animals such as crustaceans, molluscs, and fish contain a wide range of carotenoids. Salmon fish is abundant in astaxanthin which gives salmon fish the pink-red color (Helgeland et al., 2019). Among the wild salmonids, the highest amount of astaxanthin has been determined in Oncorhynchus species that ranged between 26 to 38 mg/kg flesh (Ambati et al., 2014).
In recent years, the rise of functional foods has prompted significant interest in alternative dietary sources of carotenoids. Although any universally recognized description exists, functional products are entire, enriched, improved, or upgraded foods that provide health benefits. Furthermore, rather than tablets or capsules, functional foods should be ingested as full meals (Conboy Stephenson, Ross, and Stanton 2021).
This review investigates the use of carotenoid-rich components in food product manufacturing, as well as why carotenoid is an important material for the creation of functional products and how it is being employed in modern food technology research.
| 2. Why choose carotenoids? | Top |
Choosing a diet rich in carotenoids has been linked to a decreased risk of developing some types of cancer risk, cardiovascular disease, age-related macular impairment, and eye disease development in epidemiological studies (Coutte et al., 2017; Sharoni et al., 2012; Leong et al., 2018). Clinical indications of the conjunctiva and corneal abnormalities, such as scarring, night blindness, corneal ulceration, keratomalacia, xerophthalmia and permanent blindness, occur when carotenoids are deficient (Sommer, 2008). Aside from the aforementioned, lack of provitamin A carotenoids causes eye impairment in humans as well as higher mortality owing to impaired innate and adaptive immunity (Stephensen, 2013). Carotenoids are interesting candidates for improvement and alteration because of their widespread use in the feed, food, cosmetic, and pharmaceutical sectors. In recent decades, molecular biology and biotechnology improvements have led to a better understanding of carotenoid synthesis in microorganisms and higher plants (Potrykus, 2003).
| 3. Processing of carotenoid-rich ingredients | Top |
When exposed to various circumstances (e.g., light, heat, oxygen), carotenoids degrade, resulting in a loss of benefits, change in physical properties (Figure 3) and prohibition of usage in food products (Mendes-Silva et al., 2020). Their hydrophobicity makes it harder to incorporate them into water-based diets. Microencapsulation methods have been used for decades to keep carotenoid-rich extracts stable throughout ordinary food processing and storage conditions. Bioactive substances are typically enclosed to protect them from degrading elements such as enzymes, heat, oxygen, and light. A study by Gomez-Estaca et al. (2016) presented a one-of-a-kind study on the encapsulation of carotenoid-rich animal-derived materials. Regarding microbiological sources, Haematococcus pluvialis cells have been employed in several research studies to obtain astaxanthin-rich extracts, a xanthophyll carotenoid with high antioxidant activity (de Freitas Santos et al., 2021a). Because of its ease of use and scale-up, as well as its simple, rapid, and low-cost operation, spray drying is one of the earliest and most extensively used processes for dehydrating or microencapsulating food and bioactive compounds. It also has the ability to create powders from fluids, convert hydrophobic compounds into water-dispersible powders, and increase the durability of critical bioactive compounds (Bakry et al., 2016).
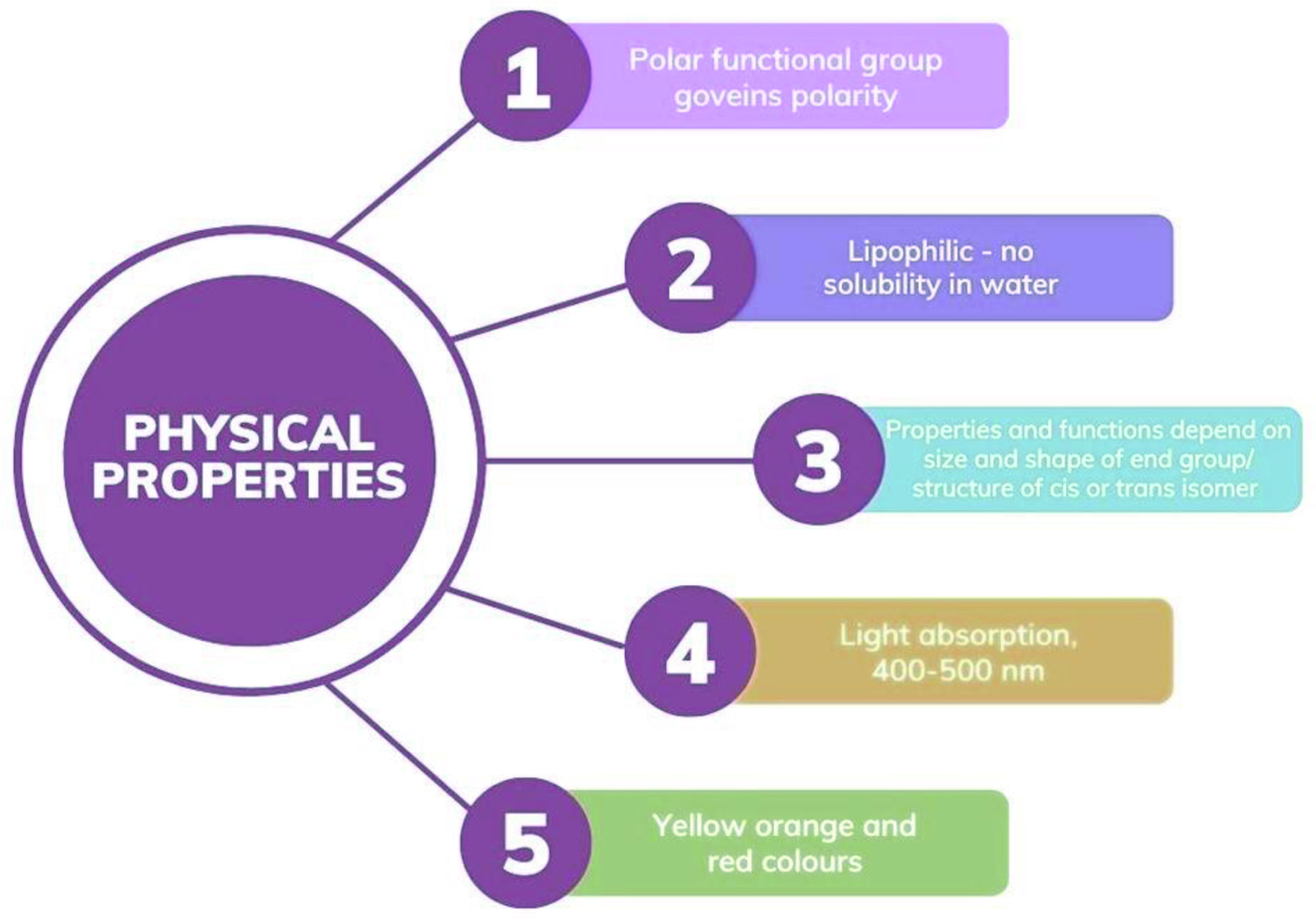 Click for large image |
Figure 3. Physical properties of carotenoids. |
It is important to underline that isomerization can also influence the bioavailability and functionality of carotenoids. For instance, E-isomers of -carotene have a higher bioavailability than the all-Z-isomer, yet the opposite is true for lycopene (Honda et al., 2018). Furthermore, different isomers of the same compound can show different bioactive potentials. Many of the Z-isomers of carotenoids have shown greater antioxidant activity than the all-E-isomers (Honda, 2021). In fact, they also have greater solubility than the all-E isomers in organic media and oils. Overall, it has been reported that the Z-isomerization of carotenoids can cause a significant change in the physicochemical properties (including solubility and crystallinity) of carotenoids, resulting in enhanced processing efficacy, bioavailability, and bioactivity (Honda, 2022).
Another research employed spray drying to reduce the hydrophobicity and susceptibility of tucum oil particles to deterioration. The study experimented different oil concentrations (100, 200, and 300 g/kg) and temperatures for drying (120 and 180 C). Because the carotenoid content of granules dehydrated at 120 C was greater (52.9162.1 mg/kg) than that of granules dried at 180 C (43.7130.6 mg/kg), they were chosen for further study (de Freitas Santos et al., 2021b).
Red capsicum, an outstanding supply of carotenoids, has been investigated as a new source for the enzymatic liquefaction (EL) of aquatic carotenoid-rich extract (ACE). Utilizing different carbohydrates digestive enzymes: pectinase, cellulase and viscozyme L the effects of liquefaction and the ability to recover greater carotenoids in the aqueous extract were investigated. When viscozyme and pectinase were used instead of cellulase, the restoration of carotenoids and other bioactive components was significantly higher. Viscozyme at a dosage of 0.3 per cent at 60 degrees Celsius produced the greatest results. Valorization of capsicum concentrates via EL might be a promising technique for recovering important food components while decreasing processing waste and maintaining environmental sustainability through green processing (Nath et al., 2016).
| 4. Advancement in the carotenoid analysis | Top |
Colorimetric, thin-layer chromatography, capillary electrophoresis, paper, open-column, and fluorometric, high-performance liquid chromatography (HPLC), and spectrophotometric techniques were used to identify and quantify carotenoids in the food matrix. For characterization and recognition study, HPLC is commonly used in combination with nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (positive mode atmospheric pressure chemical ionization; APCI+ mode). HPLC is used for differentiating cis isomers from the trans isomers throughout the processing procedure for characterization and analysis of carotenoids in biological and nutritional samples (Gupta et al., 2015; Zhao et al., 2022).
Because there are over 700 different forms of carotenoids in animals, microbes, fungi, and plants, developing and preserving pure carotenoid levels is a big difficulty in measuring. Aside from that, the complexity of dietary components, along with carotenoids vulnerability to cis and trans isomerization and oxidation during analysis and storage, are major impediments to carotenoid assessment. Due to a shortage of inexpensive standards, many laboratories continue to struggle with carotenoid identification and quantification. As a result, improved carotenoid purification processes must be developed. Refined carotenoids are frequently used in the cosmetics sector, in addition to the food industry (Anunciato and da Rocha Filho, 2012).
A single-step application of an organic solvent to extract carotenoids from the matrix is the most common approach for collecting them for further study. Even though diol and silica capsules have only shown good retention for lutein, a far more polar carotenoid, solid-phase extraction (SPE) is rarely acknowledged in carotenoid academic research, the most popular compounds are C30 and C18 (Ferreiro-Vera et al., 2011). SPE may also be used to improve the recovery efficiency of carotenoids extracted using organic solvents. The most widely used approach for detecting and characterizing diverse oxygen-functionalized carotenoids and carotenes, including ketone hydroxyl and epoxy groups, is air pressure chemical ionization (APCI)-tandem mass spectrometry (MS/MS) in positive ion mode (Rivera and Canela-Garayoa, 2012).
Mass spectrometry is a helpful method for detecting new chemicals; fragment signals may even separate pigments with similar structures. In structural elucidation, mass spectrometry (MS) is commonly combined with proton nuclear magnetic resonance (1H-NMR) data to produce absolute confirmatory results on highly similar isomers (Qiu et al., 2014). Rivera et al. (2014) compiled positive MS fragment information of specific carotenoids acquired using various ionization methods and composites in a recent study. This studys fragmentation pattern and intensity fragment ratios will be very valuable in evaluating MS data for carotenoid identification. Qiu et al. (2014) recently used C30-HPLC-APCI-MS in conjunction with UV/Vis spectroscopy, gel permeation chromatography (GPC), and NMR spectrometry to identify nine different isomers of canthaxanthin. Recently, accelerated solvent extraction (ASE) was utilized to extract carotenoids from orange carrots, and it was shown that the most crucial elements impacting ASE extraction are solvent properties and temperature. Cardenas-Toro et al. (2015) tried to compare the output, carotenoid characteristics, and financial sustainability of pressurized liquid extraction (PLE), percolation (LPSE-PE), and Soxhlet extraction (LPSE-SOX) of crushed palm fiber for the full recovery of carotenoid-rich concentrations to assess the techniques and industrial applicability (PPF). Compared to LPSE processes, the PLE approach is more efficient to extract carotenoids. When compared to PE, however, LPSE has the lowest production cost. A carotenoid separation process for lutein, -carotene, and -carotene from a fresh carrot extract was devised using a Englert et al, technique, and double co-current chromatography (CCC). Using dual CCC, 51 mg of -carotene, 32 mg of -carotene, and 4 mg of lutein were separated from 100.2 mg of pure carrot concentrate in a short amount of time and with high purity of 9599 per cent (Englert et al., 2015).
Saponification may result in the degradation or structural modification due to the origin of the carotenoid and the kind of food (Divya et al., 2012). Rasmussen et al. investigated if saponification of standard lutein may produce meso-zeaxanthin, a xanthophyll present in the macula, in addition to zeaxanthin. Peaks with zeaxanthin-like spectra, chiral normal-phase and meso-zeaxanthin retention lengths were obtained from pure lutein saponification (Rasmussen et al., 2012). Irakli et al. discovered significant -carotene and lutein recoveries following grain saponification, but lesser recoveries (ranging from 46.7 per cent for -carotene to 74.5 per cent for lutein) after extraction without saponification. In recent research on table olives, Amorim-Carrilho et al. (2014) reported limited -carotene recovery after saponification. A study investigated the concentration of -carotene in coriander before and after saponification and discovered that saponification destroyed 2030% of -carotene and 50% of another carotenoid (Divya et al., 2012). The number of studies that focused on analytical methods for detecting carotenoid compounds was summarized in Table 1.
 Click to view |
Table 1. Analytical techniques applied for the determination of carotenoids |
More recently, non-invasive analytical techniques started to be applied in determination of carotenoids. These techniques are commonly based on infrared spectroscopy that measures the vibrational spectrum of the sample of interest, without the need of applying any extraction methods. The infrared radiation passes through the samples and records the wavelengths that have been absorbed and the degree of absorption. A study by (Toledo-Martn et al., 2018) applied near infrared spectroscopy to determine the carotenoid content in blackberries. This method was suitable for screening purposes, but not quantification. On the other hand, (Ikeogu et al., 2017), was successful in developing a quantification model using a portable visible and near infrared spectrometer to quantify the carotenoid content of fresh cassava roots. This green technique has also shown promising prediction of provitamin A carotenoids content in maize (Rosales et al., 2022) as well as carotenoids in tomato products (Saad et al., 2021). Infrared spectroscopic methods could be further developed to support the food industry in determining the carotenoid content of the produced products. These methods do not require sample preparation, extraction processes, and use of solvents. In fact, they are relatively quick and cheap in comparison to chromatographic techniques that makes them good candidates as analytical techniques in the industry (Zare et al., 2019).
| 5. Summary of the role of carotenoids in human health | Top |
Carotenoids are intriguing natural substances that can be transformed into a variety of other molecules. Retinoids with vitamin A activity stand out among them, as they are critical for human health and development. Due to the fact carotenoids are colored, one of the most effective aesthetic attributes considered by people to assess food attractiveness. This advantage can be used to develop functional foods contributing to the human health. It is important to highlight that the presence of conjugated double bonds in the structure of carotenoids is responsible for the chromophore of carotenoids (Khoo et al., 2011). These bonds absorb light, and as the number of these double bonds increases, the absorbance of the red color wavelength increases too.
Carotenoids have a lot of evidence that they can assist enhance health and lower the risk of many illnesses. Because carotenoids include the provitamin A carotenoids (PAC) and serve as Vitamin A precursors, they stand out among the wide array of bioactive phytochemicals. Their function as pigments is also important for food marketability, aesthetics, and customer preferences. Carotenoids (and/or their derivatives) are good for ones health, according to an increasing body of data. Carotenoids, too, have been demonstrated in tests to offer cosmetic benefits in addition to their ability to protect the skin (Melndez-Martnez et al., 2019).
Vitamin A is recognized as the vitamin having the most diverse range of activities in humans (Alvarez et al., 2014). It is required for cell differentiation, reproduction, immunity, eyesight, epithelial tissue maintenance, proliferation, apoptosis, brain function, and/or embryonic growth and development, among other things. Vitamin A deficiency (VAD) (serum retinol values 0.7 mol/L) is a worldwide public health concern, especially in developing nations. VAD is divided into different categories, with 1020 g/dL (0.350.7 mol/L) circulation levels considered low and 10 g/dL (0.35 mol/L) circulation levels considered inadequate. VAD causes a wide range of clinical symptoms, including xerophthalmia (night blindness that develops into softening ulceration and corneal necrosis) and adverse effects on growth, foetal development, and susceptibility to severe infection. VAD is believed to account for a significant fraction of child mortality rates (about 12.5 million per year) in poor nations (Alvarez et al., 2014).
Numerous studies conducted over the last 40 years have discovered that carotenoids and their derivatives may be beneficial to health and reduce the risk of a variety of ailments. In the traditional carotenoid publications as well as more recent modifications of the original studies, carotenoids have been linked to possible improvements in immune function and illnesses such as ovarian, breast, cervical, prostate, and colorectum, cardiovascular problems, skeletal, epidermis, and eye concerns (Eggersdorfer and Wyss, 2018; Hayhoe et al., 2017; Van Hoang et al., 2018).
Long-term supplementation with 50 mg of -carotene every other day for 18 years has also been shown to provide psychological beneficial results to an otherwise healthy population (n = 4,052 males, Physicians Health Study II) (Grodstein et al., 2007). The role of carotenoids and their variants in diabetes, obesity, and some other metabolic diseases is also becoming more widely recognized (Mullan et al., 2017; Leermakers et al., 2016). Recently, there has been a surge of motivation in studying more about the positive advantages of carotenoids through pregnancy and childhood (Leermakers et al., 2016). Furthermore, studies have been performed to assess the relationship between carotenoids and the risk of developing various disorders, such as respiratory disorders and Parkinsons disease (van Lent et al., 2016). The development of cardiovascular disease is linked to oxidative stress, inflammation, dyslipidemia, and thrombosis, and there are indications that carotenoids may assist with some of these aspects. Astaxanthin, a carotenoid produced by marine creatures, has been proven to lower low-density lipoprotein peroxidation as well as enhance blood lipids and blood flow capacity. Low-density lipoprotein (LDL) oxidation susceptibility is recognized to lead to atherosclerotic. 3.6 mg and above lowered the vulnerability of LDL to oxidation, as indicated by a 5.0, 26.2, 42.3, and 30.7 per cent, respectively, prolongation of the LDL oxidation lag phase in a 14-day investigation into alleged 24 healthy adults who took astaxanthin at doses of 1.8, 3.6, 14.4, and 21.6 mg/day (Eggersdorfer and Wyss, 2018).
Lycopene is a non-provitamin A carotenoid, with remarkable antioxidant activity. It has grabbed the attention of many researchers recently exploring its effectiveness in preventing prostate cancer. Based on the in vitro and in vivo evidence, lycopene has anti-cancer, anti-progressive and apoptotic activities (Mirahmadi et al., 2020). This compound has been effective in suppressing progression, proliferation, arresting the in-cell cycle, and inducting apoptosis of prostate cancer cells. Nevertheless, systematic reviews and meta-analyses of clinical trials have concluded that the current evidence does not prove the potential of lycopene in preventing prostate cancer. A recent systematic review and meta-analysis evaluating the evidence of the effect of lycopene supplementation on prostate-specific antigen (PSA) reported that no significant differences in PSA levels in individuals supplemented with lycopene or tomato extract containing lycopene (WMD = 0.12 ng/ml; 95% CI: 0.62, 0.38 ng/ml; P = 0.64) compared to individuals who did not receive any supplementation (Sharifi-Zahabi et al., 2022). Similarly, another systematic review and meta-analysis reported that lycopene did not influence PSA levels in men with non-metastatic prostate cancer (WMD = 0.60, 95% CI = 2.01, 0.81 g/L) (Sadeghian et al., 2021).
Many carotenoids included in a healthy diet concentrate in the skin, where they provide adequate protection against sunburn, ageing, and UV-induced damage. Carotenoids have a significant ability to scavenge reactive oxygen species such as peroxide radicals or singlet oxygen molecules due to their unique structure of ten or more conjugated double bonds. Several medical investigations have found that -carotene can help prevent sunburn, also known as erythema (Stahl and Sies, 2012). A summary of key nutritional and biological activities of carotenoids are shown in Figure 4 (Milani et al., 2017).
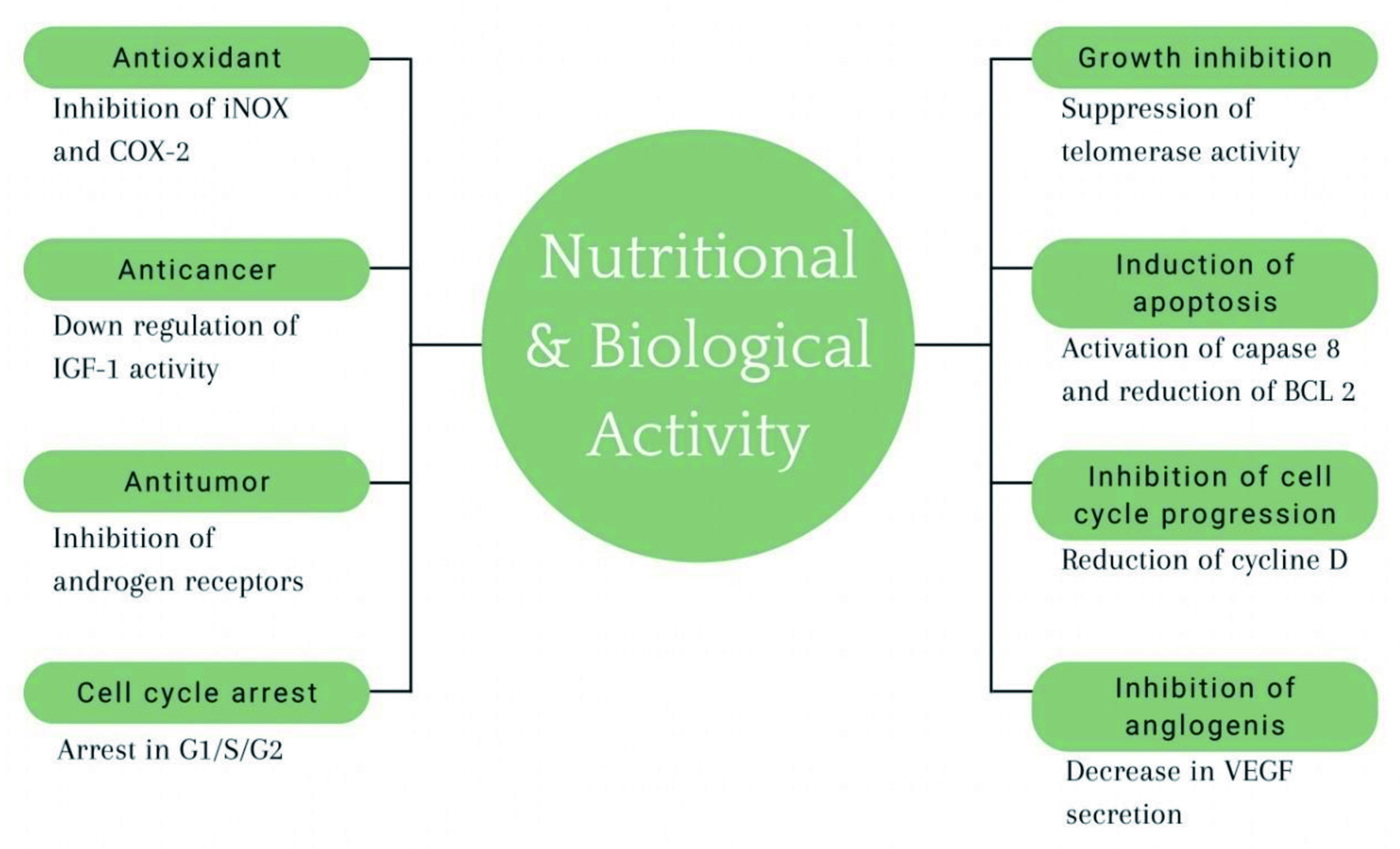 Click for large image |
Figure 4. Nutritional and biological activity of carotenoids. |
| 6. Absorption and metabolization of carotenoids | Top |
Carotenoids and other fat-soluble micronutrients track the destiny of lipids in the human upper gastrointestinal tract. Their digestion begins with their disintegration during the fat phase of the food. This phase is emulsified in the stomach and duodenum into lipid droplets. It was hypothesized that carotenoids cis-isomerization may occur during gastrointestinal digestion, however a human investigation ultimately disproved this theory. Recent in vitro digestion tests simulating duodenal circumstances shown that lycopene, -carotene, and lutein did not undergo any appreciable isomerization (Reboul, 2019). Absorption efficiency of marked -carotene is considerably diverse across clinical investigations, changing from 3 to 80%, but generally ranging from 10 to 30%. This may partially be related to the varied bioavailability of -carotene, but it may also represent its modest absorption and transport via the enterocyte. It should be highlighted that -carotene absorption capacity was generally tested after a single meal. However, the gut may retain -carotene from a first meal to release it during later postprandial stages in humans. -carotene absorption capacity may, consequently, be underestimated in certain experiments. Approximately 40 percent of ingested carotenoids are remain undigested. BCO1 (-carotene oxygenase 1), a cytosolic enzyme, may convert -carotene into retinal in a single step in the enterocyte. The generated retinal is then transformed into retinol and esterified into retinyl esters by the lecithin:retinol acyltransferase (LRAT) and presumably by the diacylglycerol acyltransferase 1 (DGAT1), which has an acyl-CoA:retinol acyltransferase activity (Amengual et al., 2013). BCO2 may also cleave both provitamin A and nonprovitamin carotenoids asymmetrically into apocarotenoids (Palczewski et al., 2014).
| 7. Techno-functional characteristics of carotenoids | Top |
Our food incorporates a diverse set of components with a variety of technological and functional qualities. These components interact with one another throughout the food processing process, from harvesting to consumption. The complexes formed by their interaction have qualities that differ significantly from the inherent properties of the individual ingredients in the food system. Many properties, such as water retention, gelation, viscosity, and emulsification, are altered by interactions between macromolecules, carbohydrates, proteins, and lipids, as well as micro-molecules. Many features of meals and food supplies, such as microstructure, particle size distribution (PSD), and mouthfeel, are impacted by many components and their interactions during the food manufacturing process. The connection of compounds used in nutrient content, as well as those found naturally in food, has a massive effect on the emulsification behavior, water retention characteristics, foaming and foam consistency, gelation solubility, water holding capacity (WHC), flavour characteristics, and viscosity of the food system (Bobade et al., 2021).
In vivo and in vitro research have revealed that the interplay of phytochemicals, particularly carotenoids and polyphenolic compounds, is linked to a lower risk of certain degenerative illnesses such as cancer and atherosclerosis. Carotenoid absorption in humans is mostly associated with a fruit and vegetable diet. In recent decades, the rise of functional ingredients has sparked substantial interest in alternative dietary factors of carotenoids. Functional foods are defined as full, fortified, enhanced, or upgraded foods that have beneficial health impacts on the human body. Furthermore, functional meals should be in the form of conventional foods rather than tablets and capsules (Conboy Stephenson et al., 2021; Dunham and Johnson, 2013).
A recent study (Mrquez-Cardozo et al., 2021) compared fresh pieces to thermally processed pumpkin (Cucurbita maxima) completely dried at various temperatures (55, 60, 65, and 70C). After determining the drying kinetics and powder quality parameters, the modified Page model offered the best match, with energy levels of 29.47 kJ mol1 and 16.06 kJ mol1 for drying fresh and thermal pre-treated sliced, respectively. Powders retained their techno-functional qualities; oil absorption capacity was 1.001.30 g/g, water content was 3.426.52 g/g, and. The solubility in cold water varied from 5.36 to 6.46 per cent. The DPPH and ABTS techniques revealed substantial differences in the physicochemical qualities and total carotenoids of powders generated from heat-treated pumpkin pulp, but not in the techno-functional characteristics and antioxidant capacity.
| 8. Uses of carotenoid-rich ingredients in the development of food products | Top |
Foods with therapeutic benefits for human health have been around for over 2,500 years in various cultures. Any chemical included in food products, animals, or crops that have a function on the human body of consumes it is referred to as a bioactive compound. Bioactive substances, in this context, are the ones that, when applied to foods, promote health. Such elements may be included in bioactive compounds found in natural sources, such as nutritious fiber; or in foods enriched with bioactive molecules, such as probiotics and antioxidants; or in foods fortified with bioactive substances, such as prebiotics. Proteins, probiotics, peptides, plant extracts, omega-3 phytonutrients and formalized lipids, natural fiber, mineral resources, vitamins, special antioxidants, carotenoids, and carbohydrates are the principal bioactive ingredients, all of which can be collected from a variety of sources and are provided to consumers in terms of supplements, personal care products, beverages and functional foods (Fernandes et al., 2019; Dacoreggio et al., 2021).
When developing a carotenoid-fortified food product, a range of factors must be addressed, including optimum carotenoid delivery and a tradeable food item with increased consumer value. Carotenoids limited bioavailability, solubility, and consistency are key hurdles for the food industry when producing a fortified food product. Carotenoid levels in plasma are commonly used to calculate systemic absorption of functional meals. In vivo bioavailability of carotenoid-rich foodstuffs influences their bioavailability and consequent bioactivity. Identifying the barriers and factors to limited carotenoid bioavailability is essential for developing a functional meal. A recent study presented a comprehensive review of carotenoid absorption and variables limiting bioavailability, such as dietary ingredients, carotenoid-nutrient interaction, and treatment techniques. Carotenoids uptake and availability can be significantly altered by dietary components. Dietary fats increase carotenoid absorption from low-fat fruits and vegetables. While producing a fortified product for maximum bioactivity in vivo, dietary fat absorption, type of lipid, food composition, and carotenoid composition should all be factored (Conboy Stephenson et al., 2021).
8.1. Carotenoids in bakery products
Bakery products, particularly bread, are among the top leading staple foods in most countries and cultures due to their easy preparation, affordability, diverse tastes, and relatively prolonged shelf life (Salehi, 2019). This product is a complex multi-component system consisting of carbohydrates (e.g., starch as the main form), plant proteins, lipids, fiber, B vitamins, and minerals (e.g., magnesium, phosphorus, and potassium). They are usually prepared from flour or meal derived from grain (Silow et al., 2016). Bread consumption patterns are quite different between countries as turkey had the first-highest bread consumption (app. 104 kg), followed Bulgaria (app. 95 kg), and the UK (app. 32 kg). Although the bread has a key role in meeting the daily nutritional needs of people, it is not considered a complete food source alone. Therefore, enriching flour and bread with fruits and vegetables rich in functional compounds, particularly carotenoids, increases the nutritional value of bread and ultimately improves peoples diet (Minaroviov et al., 2018).
Various studies have found that incorporating fruit wastes into bakery products, such as muffins, cakes, and cookies, increased their nutritional values and antioxidant activities, as summarized in Table 2. (Olawuyi and Lee (2019) studied muffins prepared by the combination of shiitake mushroom (SM) powder and carrot pomace (CP) powders (5, 10, and 15%) with rice flour. The incorporation of SM and CP increased total polyphenol, total carotenoid content (ranging from 34.68 to 66.96 g g1), and antioxidant properties in a dose-dependent manner. Moreover, the results showed a direct link between the MP and CP concentrations and the weight and hardness of enriched muffins. Based on the sensory ratings (e.g., taste), the enriched muffins had better consumer acceptability than the control muffins. Still, it changed color from cream to brown which is attributed to Maillards reactions. It is a chemical reaction between an amino acid and a reducing sugar that usually takes place in the presence of heat during the baking process. It also reduced the pasting viscosities, which may be due to their lower carbohydrate and the presence of proteins which caused dilution of starch content in the powder blends and influenced the integrity of the starch granules (Nath et al., 2016; Singh et al., 2014). Moreover, the addition of nettle in white flour could remarkably enhance the carotenoid and mineral contents and antioxidant properties (from 372 to 597 g GAE/GFW) of bread (Maietti et al., 2021). As such, Eletr et al. (Eletr et al., 2017) reported an enhanced 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of lycopene-added cookies and cakes with an increase in the concentration of lycopene up to 5% without considerable negative effects on sensory parameters of both groups. Reis et al. (Reis et al., 2020) studied chemical attributes and consumer acceptance of bread and cake containing different classes of carotenoids from orange passion fruit peel (OP). The increase of drying temperature in OP up to 80 C resulted in an increase of total carotenoids by 23%. At this temperature, OP flour had good water holding (7.89 0.14 g/g) and oil holding capacities (2.96 0.04 g/g). Also, the lowest proteins, carbohydrates, Na and total fat values and a higher level of fiber were achieved when adding yellow passion fruit peel flour at 12.22%. The fortified flour provided bread and cakes with more than 80% acceptance rate and greater antioxidant capacity (2.64 0.01 g/100 mL).
 Click to view |
Table 2. Incorporation of carotenoids in development of bakery products |
Although the wide range of synthetic colorants known as tartrazine has been widely used in bakery products, carotenoids considered a safe, natural pigment are finding their place in this industry. The total carotenoids extracted from mandarin epicarp (containing 140.70 2.66 mg -carotene/100 g) by ultrasound-assisted extraction with close to zero E values is a suitable alternative to tartrazine in the production of cakes and bread (Ordez-Santos et al., 2021). The bread enriched with orange-fleshed sweet potato (OFSP) containing a significant amount of -carotene could improve the shelf-life compared to the wheat bread by controlling the mould growth and lowering water quality and moisture content (Wanjuu et al., 2018). In a study, dough formation and oven baking influence the muffin and bread enriched with hairless canary seed and corn. Due to enzyme degradation and oxidation, higher total carotenoids were found in dough formation in both bakery products (Abdel-Aal, 2008).
The sensitivity of carotenoids to heating during baking is challenging. The effective strategies to overcome this issue were (Xu et al., 2022), adding egg yolks enriched with zeaxanthin and lutein in the muffin formulation and butter. Still, the incorporation of butter influenced the bioaccessibility of carotenoids. Another study (Nogueira et al., 2018) reported a maximum of 25% -carotene loss during storage in the bread containing yellow sweet potato flour, which might control moisture content by designing a suitable packaging.
8.2. Carotenoids in extruded snack products
Extruded snack products are popular ready-to-eat foods among consumers and redesigning this type of food to enhance their nutritional characteristics, such as micro or macronutrients, phytochemical ingredients, antioxidants, and vitamins has been developed recently in food industries (Pensamiento-Nio et al., 2018). Carotenoids, as a bioactive component, which confer numerous health benefits, can be incorporated into snacks to improve not only their nutritional value but also their sensory characteristics (McClements, 2019). Carotenoids are abundant in agricultural wastes like tomato by-products (Wang et al., 2022); therefore, those components can be exploited to fortify snacks. In this regard, recently, tomato pomace powder at varying concentrations (5, 10, 15, and 20% (w/w)) was used to formulate extruded snacks. It was reported that when the tomato pomace powder was increased in products, the bioactive compounds and their bioaccessibility were increased (Yagci et al., 2022). Beside the carotenoid-rich additives like tomato pomace powder, carotenoid-rich flours can use for enriching the extruded snacks. A study (Kolniak-Ostek et al., 2017) used amaranth, Jerusalem artichoke, and pumpkin in the formulation of extruded snacks and reported that the highest -carotene and lutein belonged to the products made of pumpkin flour. In another study, (Ruiz-Armenta et al., 2018) evaluated the optimization of snack production added with bagasse of naranjilla (Citrus mitis B.) fruit as the optimizations of a natural source of carotenoids. The by-products (bagasse) of the naranjita juice industry are a good source of bioactive compounds (including flavonoids, carotenoids, and dietary fibers). In this study, the influence of moisture content (MC) (21.1032.89%), extrusion temperature (ET) (89.8140.2 C), and dehydrated naranjita bagasse content (DNB) (1.1211.88%) were evaluated. their results showed that the significant impact on the response variables expansion index and penetration force was extrusion temperature. In contrast, the greatest effect on total carotenoids and sensory acceptability was represented by DNB and the optimum extrusion process conditions evaluated with the numerical method were MC = 23%, ET = 125 C, and DNB = 8.03% (Ruiz-Armenta et al., 2018). The influence of physical characterizations (moisture of the feed (1317%, wb) and temperature of the barrel (120160 C)) of maize grits on the carotenoid profile of expanded snacks was evaluated by Romero Rodrguez et al. (2021) and they reported that carotenoids were affected differently. The positive values for total carotenoids (except at 17%120 C), zeaxanthin (just at 15%120/140 C), -cryptoxanthin, and -carotene represent a higher amount of these components after extrusion, which indicates that the process is favorable for these carotenoids extractability. In addition, they suggested a worthy process, from nutritional and technological aspects, to extrude at the processing conditions ranging between 120132 C barrel temperature and 13.213.7% feed moisture.
Extrusion process conditions, such as temperature, feed moisture content, shear rate, and screw speed, can impact the carotenoid content of the yielded snacks; consequently, these parameters should be taken into account. Thermomechanical stress exerted on extruded snacks enriched by carotenoids during the process could degrade carotenoids (Pinho et al., 2021). Different carotenoids show varying degrees of sensitivity to the process. For instance, (Cueto et al., 2017) elucidated that 60% of lutein content declined, whereas zeaxanthin decrement was 40% in extruded snacks fortified by carotenoids. As these bioactive compounds are vulnerable to the high shear conditions and temperatures of the extrusion process, protecting them from degradation using strategies such as encapsulation can be an ideal solution. In a study, encapsulation of proanthocyanidin-rich cinnamon extract via a complex conservation technique increased the carotenoid content in extruded snacks (Favaro-Trindade et al., 2020). Igual et al. (2021) evaluated the impact of rosehip (Rosa canina) co-product powder in the extrusion process and recorded that by increasing this powder, swelling power, water absorption, and hygroscopicity were increased. Furthermore, the expansion value, water solubility, porosity, and image factors (perimeter and area) of the extrudates decreased. The main carotenoids of the extrudates were lycopene, lutein, carotene, and zea-esters. While catechin, procyanidin dimmer 1, procyanidin dimmer 2, di-gallic acid, and isorhamnetin-glucuronide were the major flavonoids components. Strong Pearson correlations were observed between carotenoids, vitamin C, total flavonoids, antioxidant activity, and total folate (Igual et al., 2021).
8.3. Gluten-free products
Celiac/coeliac disease is a lack of dietary gluten tolerance that is present in wheat, barley, and rye during the lifespan (Lindfors et al., 2019; Lebwohl and Rubio-Tapia, 2021). Therefore, the gluten-free foods production for individuals who have celiac disease is necessary. The consumption of gluten-free foods has increased so far as it was valued at $4.3 billion in 2019 and is predicted to have reached $7.5 billion in 2027 (Kajzer and Diowksz, 2021). On the other hand, since gluten, as a functional protein play the main role in the structure and texture of foods, it is challenging to substitute gluten with other ingredients to compensate for the lack of this protein. Starches and flour of different botanical sources, such as corn, rice, millet, sorghum, pseudo-cereals (quinoa, buckwheat, and amaranth), teff, potatoes, tapioca, legumes, oilseeds, nuts, fruits, and vegetables can be incorporated into foods to modify the structure and texture of foods in absence of gluten (mdov and Rysov, 2022; Witczak et al., 2016). Generally, a mixture of these flour is applied to enhance the quality of gluten-free products. Recently, innovative ingredients, including cladode flour (CF) and cactus mucilage (CM) were utilized for gluten-free snack making. It was reported that snacks yielded by CF did not only display great technological properties but also had an enhanced carotenoid content (Dick et al., 2020).
In another study, microalgae were exploited as a functional composition to raise the nutritional characteristics of gluten-free bread. The addition of microalga improved the protein content, omega-3 content, and lutein content in the fatty acids (Diprat et al., 2020). Further, it was proved that adding 5% of freeze-dried purple and red potatoes to gluten-free bread could increase the amount of phenolic and carotenoid contents of the product (Gumul et al., 2017). The number of studies that focused on gluten-free products was summarized in Table 3.
 Click to view |
Table 3. Incorporation of carotenoids in development of gluten-free products |
8.4. Carotenoids in meat products
Carotenoids are considered tetraterpene pigments with orange, red, yellow, and purple colors, which are formed in photosynthetic bacteria, plants, algae, some types of archaea and fungi, and animals (Mori, 2020). Hence, natural resources with carotenoids can be used in meat product ingredients to improve their color during storage or process. The by-products of tomato manufacturing industries, such as peels and seeds, contain a high number of active components including carotenoids (e.g., lycopene, phytoene, -carotene, lutein, and phytofluene) (Alahakoon et al., 2015). In a study, tomato pomace extract, as a natural source of -carotene, was spread on the lamb meat surface to improve the shelf-life due to its antioxidant properties. The redness in lamb meat covered with tomato pomace extract was, therefore, higher in comparison to the control sample after 7 days of storage at 2 C (Andres et al., 2017). Also, the tomato by-products are used in other meat products, such as patties and burgers, dry-fermented sausage, frankfurter and cooked sausages, minced meat, Luncheon roll, and Mortadella to decrease lipid oxidation and improve sensory properties (Domnguez et al., 2020). In addition, adding tomato extracts into meat product formulations has shown a reduction increases the nitrite contents involved in the curing reactions when added together with nitrite and thus reducing the residual nitrite percentage (Alahakoon et al., 2015).
Moreover, the attendance of carotenoids in meat products can inhibit color alteration induced by some conservation methods like irradiation and modified atmosphere (Caldern-Oliver and Lpez-Hernndez, 2022). A study recorded that using fiber microparticles (2.0% w/w level) evaluated from pulp and peel of Japanese plum by-product, which contained coextracted -carotene, - and -tocopherols, and lutein as well as polyphenols (quercetin derivatives, cyanidins, and pentameric proanthocyanidins) reduced in 50% the formation of thiobarbituric acid reactive substances in raw patties during storage at 4.0 C for 10 days (Basanta et al., 2018). Similar results were reported by Another study investigated the influence of berry leaves and their inhibitory activity on lipid peroxidation of broiler chickens thigh meat. They also recorded that by increasing the doses of vitamin E, zeaxanthin, lutein, and -carotene, as well as polyphenols, total antioxidant capacity and inhibiting meat lipid peroxidation was increased (Varzaru et al., 2020) .
However, if some specific carotenoids, will be utilized in high dosage, they lost their beneficial impact and instead act as pro-oxidants (Bolognesi and Garcia, 2018). Accordingly, finding a proper portion of carotenoids in food formulations is crucial. Recently, papaya epicarp flour, which includes various carotenoids such as -cryptoxanthin, zeaxanthin, -carotene, -carotene, and lycopene, was incorporated into the beef burger to produce a nitrite-reduced burger. The optimal color-maximization in the beef burger was obtained when the nitrite/flour ratio was 150 mg/kg nitrite/40 mg/kg flour, and the maximum values for lycopene, -cryptoxanthin, and zeaxanthin in the sample were 0.251, 0.451, and 0.447 mg/100 g, respectively (Velasco-Arango et al., 2021). It is worth noting that carotenoids that are intrinsically present in some meats can play a protective role. In this regard, the protein-bound carotenoid present in salmon may preserve unsaturated lipids from tyrosine (Radnz et al., 2021). In addition to fortifying foods with natural sources of carotenoids, there is another strategy to increase the carotenoids in meat. In this technique, animals are fed by plants enriched with carotenoids, and consequently, desired characteristics appear in their meat. For instance, it is claimed that a high carotenoid diet of chicken possessed better bioavailability of provitamin A carotenoids and higher yellowness scores in their meat (Nabi et al., 2020). In Table 4, studies investigating the effect of adding citrus by-products as carotenoids source to meat products are summarized.
 Click to view |
Table 4. Incorporation of citrus by-products in meat and/or meat products |
8.5. Incorporation of carotenoids in other products
In addition to the abovementioned food categories, carotenoid sources were used to products promote the functional properties of other food products, like pasta, dessert, and sauces. For example, ripe and unrip gac fruit (Momordica cochinchinensis Spreng) enrichment (515% w/w) of wheat flour pasta increased antioxidant activities (%) and undigested starch (%) in the past. The replacement of gac powder (GP) in the flour could increase the cooking loss but reduce swelling and water absorption. The enriched flour with the 10% powder significantly enhanced the pastas hardness, adhesiveness, and cohesiveness. The modified pasta with unripe GP at 5% GP had the highest scores in all tested sensory attributes (Chusak et al., 2020). A comparative study Porto Dalla Costa et al. (2016) confirmed higher total carotenoids and antioxidant capacity of pasta enriched by 20% waste than that produced with 30% synthetic -carotene. No carotenoid loss was observed after cooking at a moderate temperature. In another study, stinging nettle (3.5% w/w) enrichment of egg pasta (20% w/w) and semolina improved the lutein and -carotene contents by 44 and 60% compared to non-enriched pasta. The cooking process caused about 20% carotenoid loss and the maximum bioaccessibility of both carotenoids lasted for two h of colonic fermentation (Marchetti et al. 2018). The addition of 15% tomato peel in wheat enhanced the lycopene and -carotene to 12.94 and 7.93 mg/100 g, respectively. But the quality of enriched pasta was not acceptable when it comes to adhesive, hardness, cooking loss, and sensory paymasters. Using hydrocolloids in this formula was not compensated for these adverse effects (Padalino et al., 2017). The impact of S. cumini pulp (from 0 to 40%) on pastas sensorial, phytochemical, nutritional, and functional attributes were assessed. This formulation doubled and increased the antioxidant activities and -carotene up to more than five times, respectively, compared to the non-enriched samples. The most acceptable formula belonged to the pasta with 30% pulp due to the highest nutritional compounds and the most acceptable color, taste, and texture (Panghal et al., 2018). A study by Waqas et al. 2017 used 15% carotenoids extracted from tomato peel containing 690 mg/100g carotenoids for wheat flour spaghetti and found 55% enhanced antioxidant capacity and 102 higher score accessibility in compression to no tomato-added spaghetti. In another investigation (Agustini et al., 2017), 9% S. platensis paste added to the noodle improved -carotene (from 0.06 0.007 to 1.51 0.305 mg/100 gr), protein (from 8.88 0.39% to 28.603 0.33%), and moisture contents with acceptable sensory. A study by Nour (2021) demonstrated the inhibition of peroxide values and lipid peroxidation by 64% and 45%, respectively, in a functional property developed by fortifying soymilk mayonnaise with sea buckthorn juice containing 14.66 mg/100 g total carotenoids. Adding sources of carotenoids to snacks can boost the nutritional values of these products for children. In a study another study (Hashim et al., 2021), Manilkara zapota was used in the formulation of pastel at different concentrations from 1020%, and the results were demonstrated. In Table 5, studies investigating the effect of adding carotenoids to a variety of products are summarized.
 Click to view |
Table 5. The effects of carotenoid-rich sources to a variety of other products |
| 9. Conclusions | Top |
Carotenoids as tetraterpene pigments can be derived from various plant, alga, and bacteria species. They offer a wide variety of functional effects, namely anti-inflammatory, anti-cancer, and antioxidant activities and protect our body from degenerative disorders. The most common methods applied for carotenoids characterization and quantification in food matrix are thin-layer chromatography, colorimetric, HPLC, and spectrophotometric, among which HPLC is predominant. The main obstacle to carotenoid uses in different types of food products includes poor water solubility, poor stability under different pH ranges, sensitivity to oxidation, high extraction cost, and low bioaccessibility. Encapsulation and nano delivery systems are effective strategies for such issues, but more research is still needed to confirm their efficiency and safety. The incorporation of carotenoids into diverse food products, namely bakery, snacks, gluten-free, meat, and dairy products, can not only improve their nutritional values like antioxidant activities but also improve their physicochemical behaviors such as color, flavor, texture, and crispness.
This research received no funding.
Conflict of interest
The authors declare no conflict of interest.
| References | Top |