| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 20, December 2022, pages 80-89
Pharmacological bioactivity of enzymatically bio-transformed ginsenosides
Wei-Sheng Lina, #, Dhriti Choudharya, #, Yi-Chen Loa, Min-Hsiung Pana, b, c, *
aInstitute of Food Science and Technology, National Taiwan University, Taipei 10617, Taiwan
bDepartment of Medical Research, China Medical University Hospital, China Medical University, Taichung 40402, Taiwan
cDepartment of Health and Nutrition Biotechnology, Asia University, Taichung 41354, Taiwan
*Corresponding author: Min-Hsiung Pan, Institute of Food Science and Technology, National Taiwan University, No. 1, Section 4, Roosevelt Road, Taipei 10617, Taiwan. Tel: +886-2-33664133; Fax: +886-2-33661771; E-mail: mhpan@ntu.edu.tw
DOI: 10.31665/JFB.2022.18331
Received: December 2, 2022
Revised received & accepted: December 29, 2022
| Abstract | ▴Top |
Many ginsenosides have shown positive effects, including anti-cancer potential and anti-inflammatory effects. Of note, protopanaxadiol (PPD) and protopanaxatriol (PPT) are not easily absorbed by the body through the digestive tract due to their hydrophilicity. From this point of view, the cytotoxic potencies of the hydrolysates of PPD and PPT on CRC are much stronger than their source compounds. Moreover, several minor ginsenosides that are absent naturally but have high disease ameliorative efficacy can be obtained from major ginsenoside by enzymatic hydrolysis. Therefore, the first aim of this study was to determine the effectiveness of the biotransformation of ginsenosides via enzymatic hydrolysis to improve their bioactivity. Second, the anti-inflammatory and anti-cancer effects of the raw and bio-transformed ginseng metabolites were determined in vitro. The results suggest that enzymes can effectively biotransform major ginsenosides (i.e., PPD and PPT) into minor ginsenosides (i.e., compound K) by hydrolyzing the β-glucosidic linkage. Moreover, the bio-transformed ginsenosides were effective in inhibiting the proliferation of HCT-116 cells and suppressing lipopolysaccharide-induced nitric oxide production in RAW 264.7 murine macrophages. Therefore, the enzymatic hydrolysis of ginsenosides can be employed to functionally produce hydrolysates with increased bioactivity.
Keywords: Bio-transformed; Cancer; Compound K; Ginsenosides; Inflammation
| 1. Introduction | ▴Top |
Cancer is a disease that can be characterized by the evasion of growth suppressors, activation of invasion and metastasis, avoidance of immune-mediated destruction, and deregulation of cellular anabolism and metabolism (Chen et al., 2016; Mármol et al., 2017). There is?a vital need to develop new compounds to target cancer cells. While progress has been made in surgical and oncological management, colorectal cancer (CRC) continues to be the third most prevalent type of cancer and is the third leading cause of cancer-related death (Søreide, 2007; Siegel et al., 2021). The risk of developing CRC increases with age, and over 90% of sporadic CRCs occur in individuals over the age of 50 years (Hagland et al., 2013). However, the preventive effect and relation to subsite location in the colon remains unclear (Boyle et al., 2011).
Ginseng has been used for preventive and therapeutic purposes for thousands of years (Pan et al., 2014). It exhibits anti-cancer, antidiabetic, cardiovascular, and neuroprotective activity. The bioactive constituents of ginseng are ginsenosides (ginseng saponins) and polysaccharides, although the pharmacological activities of all components have not been elucidated (Kim, 2009). Total ginsenoside extracts are chemical mixtures containing a group of triterpene glycosides with similar ingredients and structure; they exhibit anticancer, anti-inflammatory, and neuroprotective activities and promote blood circulation to treat cardiovascular diseases (Wan et al., 2009; Zhang et al., 2020). Most known ginsenosides are classified as members of the dammarane family. Dammarane ginsenosides possess a four-ring, steroid-like structure (Liu et al., 2021). Ginsenosides can be classified into two main functional groups based on their C-6 position: protopanaxadiols (PPDs) and protopanaxatriols (PPTs). In PPD, sugar groups attach to the C-3 position of the carbon skeleton, while in PPT, sugar groups attach to the C-6 position (Leung and Wong, 2010; Shahrajabian et al., 2019). According to existing research, PPD-derived ginsenosides exhibit more potent cytotoxic properties than PPT-derived ginsenosides (Ahuja et al., 2018).
However, biotransformation may be required for ginsenosides to become active in mammalian systems. Recent studies demonstrated that ginseng metabolites have greater pharmacological bioactivity than major ginsenosides (Bae et al., 2004). Upon oral consumption, ginsenosides are partially transformed into PPD and PPT through a series of de-glycosylation steps by acid hydrolysis and the activity of intestinal bacteria (An et al., 2015; Yue et al., 2007). All of the metabolites, including CK, PPD, and PPT, are nonpolar compared with their parental component ginsenosides, which can be easily absorbed in the gastrointestinal (GI) tract and exhibit biological activity (Shim et al., 2009). Conventional chemical approaches, such as heating, hydrolysis with a weak acid, and cleavage by alkali, have been investigated; however, microbial or enzymatic conversion methods are considered more favorable due to their prominent selectivity, moderate reaction conditions, and environmental compatibility (Kim and Kim, 2018; Yan et al., 2008).
Pre-clinical and clinical research has demonstrated that ginsenosides have cancer-preventing activity in various tumors, including gastric carcinoma, breast cancer, liver cancer, ovarian cancer, colon cancer, melanoma, and leukemia (Mao et al., 2014). Extensive pharmacological studies on ginseng and notoginseng suggested that PPD and PPT were the main anticancer components, and that the anticancer activities of PPD were more powerful than those of PPT (Lee et al., 2011; Shim et al., 2009). Studies indicate that compounds with less polar chemical structures possess higher cytotoxic activity against cancer cells; from this perspective, the cytotoxic potencies of the hydrolysates of PPD and PPT are much stronger than the original ginsenosides. CK, Rh2, Rg3, PPD, and PPT may be responsible for the enhanced anticancer activity of ginseng. Considering the mechanism of action of ginsenosides, they are expected to regulate inflammatory responses, primarily through the inhibition of the NF-κB signaling pathway. In lipopolysaccharide (LPS)-stimulated macrophages and microglial cells, ginsenosides suppress the production of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and inflammatory enzymes, such as iNOS and COX-2. According to in vitro studies, ginsenosides exert anti-inflammatory activities in a variety of animal models of inflammatory diseases (Kim et al., 2017).
In this study, we aimed to investigate the effectiveness of biotransformation of ginsenosides via enzymatic hydrolysis to improve their bioactivity. Furthermore, the anti-inflammatory and anti-cancer effects of the raw and bio-transformed ginseng metabolites were determined in vitro.
| 2. Materials and methods | ▴Top |
2.1. Sample preparation
Dry powdered ginseng (ginseng roots [GRs] and ginseng leaves and stems [GLs]) was extracted by a rotary evaporator using aqueous methanol (Echo Chemical Co. Ltd., Taipei, Taiwan) according to a method described previously (Corbit et al., 2005). Then, 150 g of sample was dissolved by agitation in 500 mL of 80% methanol (v/v) at 40 °C for 1 hour and sonicated for complete dissolution. The temperatures of the evaporator and condenser of the rotary evaporator were maintained at 40 °C and 4 °C, respectively. After complete evaporation of the methanol, the slurry was freeze dried overnight, and the total yield was calculated.
2.2. Organisms and culture conditions
Aspergillus niger 31130 (ATCC 16888) was maintained on malt extract agar plates and slants. Two hundred-milliliter aliquots of liquid medium contained in Erlenmeyer flasks (250 mL) were inoculated with 107?108 (hemocytometer for spore counting) viable conidial spores obtained from 7-day-old A. niger grown on malt extract agar. The culture was incubated at 25 °C for 14 days on an orbital shaker at 150 rpm and was harvested by filtration through a filter cloth. The filtrate was pooled and used as the crude β-glucosidase preparation (Narasimha et al., 2016).
2.3. β-glucosidase enzyme preparation from A. niger
β-glucosidase was precipitated by adding 90% (w/v) ammonium sulfate to the culture filtrate and kept overnight at 4 °C on magnetic stirrer (Narasimha et al., 2016). The precipitate was collected by centrifugation at 10,000 ×g for 40 min at 4 °C. The precipitate was then re-dissolved in 50 mM Tris-HCl buffer (pH 8) and dialyzed in a dialysis bag overnight against the same buffer at 4 °C. During the time interval, the buffer was changed frequently until no traces of ammonium were found in the buffer (Narasimha et al., 2016). The resultant solution in the dialysis bag was collected and centrifuged using a Vivaspin at 10,000 ×g for 45 min at 4 °C. The protein was collected and diluted using the same buffer.
2.4. Protein concentration assay
The Bradford protein assay is used to measure the concentration of total protein in a sample. The principle of this assay is that the binding of protein molecules to Coomassie dye under acidic conditions results in a color change from brown to blue at an absorbance of 595 nm. In a 96-well plate, 5 μl protein with 200 μL Bradford reagent was added in triplicate. The enzyme-linked immunosorbent assay (ELISA) plate reader was maintained at 30 °C and read at an absorbance of 595 nm. The protein concentration was calculated using the bovine serum albumin (BSA) standard curve of absorbance versus micrograms of protein.
2.5. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
β-glucosidase from different strains of A. niger has a molecular weight within a range of 68?220 kDa determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Narasimha et al., 2016). Electrophoresis was performed to determine the molecular weight of the protein from A. niger using 10% polyacrylamide gel in a mini system. The denatured protein and a molecular marker were electrophoresed at 50 V for 2?3 hours. Developed gels were stained with Coomassie blue R-250.
2.6. Enzyme assay
Enzyme activity was evaluated in assay mixtures containing p-nitrophenyl β-D-glucopyranoside (PNPG) at 0.5 mM in 0.1 M sodium acetate buffer (pH 4.5) and aliquots of diluted enzyme. After incubating for 24 hours at 37 °C, the reaction was terminated by adding 100 μL of 0.5 M Na2CO3, and the p-nitrophenol produced was analyzed by an ELISA reader at 405 nm. All assays were performed in triplicate. One unit of β-glucosidase activity was defined as the amount of enzyme required to release one micromole of p-nitrophenol per minute under assay conditions (Narasimha et al., 2016).
2.7. Enzymatic bioconversion of major ginsenosides to minor ginsenosides
For the preparation of minor ginsenosides, the substrates GR and GL in 0.1 M and pH 0.5 acetate buffer were reacted with same volume of crude enzyme from the A. niger strain at 37 °C for 0, 1, 2, 4, 6, 8, and 12 hours. The reaction was terminated at every time point by the addition of 0.5 M sodium carbonate (Na2CO3) (2 × volume of reaction solution), centrifuged, and the supernatant was concentrated to a solid form using a freeze drier. The solid form of the substrate was then dissolved in the solvent required for the specific experiment and adjusted to the required concentration.
2.8. LC-MS analysis
The liquid chromatography-mass spectrometry (LC-MS) system is a Q-Exactive Mass Spectrometer with Electrospray Ionization Interface (ESI) (DINOEX Ultimate 3000 LC system). The separation was performed on an RP18 column (specifications: 1.7 μm, 2.1 mm ID × 100 mm) (ACQUITY UPLC® BEH Shield) with a guard column. For LC-MS analysis, a 5-μl sample was injected into the column and eluted at 30 °C with a constant flow rate of 0.4 mL/min. Formic acid (0.1%) in H2O (eluent A) and formic acid (0.1%) in acetonitrile (eluent B) were used. The gradient used for elution is shown in Table 1. Detection was performed in Parallel Reaction Monitoring (PRM) mode. Ginsenoside standards and raw and enzymatically bio-transformed ginseng extracts were dissolved in methanol (80% v/v). All solutions were filtered through Millex 0.2 μm nylon membrane syringe filters (Millipore Co., Bedford, MA) before use.
 Click to view | Table 1. Eluent gradient used for LC-MS analysis |
2.9. Cell culture
HCT-116 cells were grown in Roswell Park Memorial Institute 1640 (RPMI) containing 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin in a humid atmosphere containing 5% CO2 at 37 °C.
RAW 264.7 murine macrophages were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated FBS and a 1% solution of penicillin and streptomycin under the conditions of a 5% CO2 atmosphere at 37 °C.
2.10. Cell proliferation (MTT) test
The methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay is a colorimetric test that is commonly used to measure metabolic activity to evaluate the cell proliferation rate. In the test, yellow MTT is reduced by mitochondrial reductase to purple crystal formazan ([E,Z]-5-[4,5-dimethylthaizol-2-yl]-1,3-diphenylformazon). A 0.2% MTT solution was prepared by dissolving 0.1 g of MTT powder in 50 mL of phosphate-buffered saline (1 × PBS). 100 μL of HCT-116 cell suspension (2.5 × 105 cells/mL) was added to each well of the 96-well plates. After 12 hours of incubation, the serum containing RPMI medium was replaced with fresh RPMI medium. The ginsenosides (GR0, GR8, GL0, and GL8) at final concentrations of 100, 200, and 400 μg/mL were added to the wells and incubated for an additional 24 and 48 hours. After 24 and 48 hours of incubation, HCT-116 cells were centrifuged at 2,000 rpm for 10 min, and the supernatant was removed after centrifugation. Then, 100 μL of PBS was added to the wells to wash off the cells, and the sample was centrifuged at 2,000 rpm for 10 min. The supernatant was removed after centrifugation. A mixture of 0.2% MTT reagent and RPMI (vol = 1:9) was prepared, and 100 μL of the mixture was added to the cells and incubated for 1 hour away from the light. After incubation, the HCT-116 cells were centrifuged at 3,500 rpm for 10 min, and the supernatant was removed after centrifugation. Purple crystals were dissolved in 100 μL of DMSO. The light absorbance (OD570) of the purple solution was measured using an ELISA reader.
The RAW 264.7 cells were seeded on 96?well plates at a density of 1 × 106 cells/well for 12 hours before treatment with ginsenosides (GR0, GR8, GL0, and GL8) with final concentrations of 100, 200 and 400 μg/mL for 24 hours both with and without LPS (100 ng/mL). Absorbance was measured at 570 nm to ensure that the selected sample concentration did not exhibit cytotoxicity before further analysis.
The survival rates of HCT-116 and RAW 264.7 cells were calculated using the following formula:
2.11. Nitric oxide measurement by nitrile assay
The RAW 264.7 cell line is an indicator of the inflammatory response, which can be measured in terms of nitrite concentrations using a nitrite assay. After seeding for 12 hours, the cells were treated with samples at final concentrations of 100, 200, 400 μg/mL and LPS at a concentration of 100 ng/mL. After a treatment period of 24 hours, 100 μL medium was mixed with an equivalent volume of Griess reagent to produce a pinkish azo compound in the presence of nitrite. Absorbance was read with an ELISA plate reader at 550 nm, and nitrite concentrations were calculated using a standard curve produced with known concentrations of NaNO3.
2.12. Statistical analysis
Data are presented as mean ± SD for three independently performed trials. Statistical differences between groups were evaluated by one-way ANOVA followed by Student’s t-test. p < 0.05 was defined as a statistically significant difference between each group.
| 3. Results and discussions | ▴Top |
3.1. Simplified extraction of ginsenosides from ginseng powder
Table 2 shows the total yield (average of independently conducted experiments) of ginseng extract after freeze drying. The extraction yield of GR was highest (44.52%) followed by GL (33.26%), which was in line with other findings that indicate that the ginsenoside content is best found in roots compared with the other parts of the plant. Furthermore, to evaluate the composition of the ginsenosides present in the extract samples, they were injected in LC-MS for quantitative analysis (Figure 1).
 Click to view | Table 2. Summary of ginseng powder samples by methanol extraction |
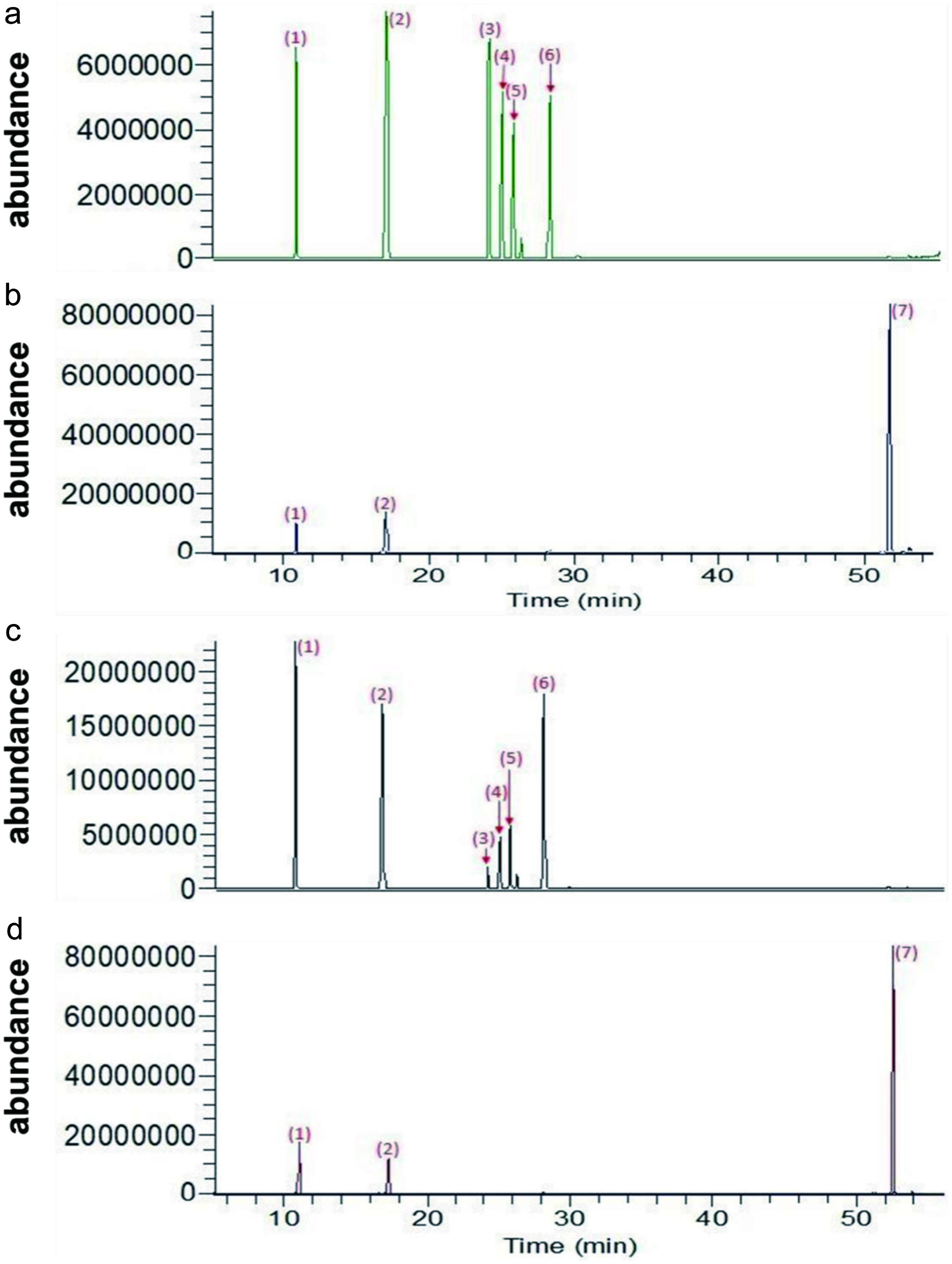 Click for large image | Figure 1. LC-MS chromatograph of ginsenoside samples (a) GR0, (b) GR8, (c) GL0, and (d) GL8. Chromatographic separation was performed on an RP18 (2.1 mm ID × 100 mm, 1.7 μm). Peaks: 1, Re; 2, digoxin (as internal standard); 3, Rb1; 4, Rc; 5, Rb2; 6, Rd; 7, CK. |
3.2. Enzyme preparation from A. niger
Among biological sources, the fungus A. niger is regarded as a good β-glucosidase producer. Both intracellular and extracellular enzymes have been purified and characterized (Yan et al., 1998). β-glucosidases constitute a heterogenous group of enzymes that catalyze the hydrolysis of alkyl and aryl β-glucosidase, disaccharides, and oligosaccharides. β-glucosidases have a high degree of variability and diverse physiological roles, including glucoside glycolipid catabolism in human tissues, cell wall pigmentation, and defense against pathogens in plants. In our study, the physiochemical properties of β-glucosidase were defined.
The fungal isolate A. niger was grown on agar plates (Figure 2A) and slants (Figure 2B) on a simple medium containing malt extract to facilitate the production of β-glucosidase. The fungus was further cultured in malt extract for 14 days at 25 °C (Figure 2C). The collected crude enzyme liquid was purified by adding 90% (w/v) powdered ammonium sulfate ([NH4]2SO4) at 4 °C. The resulting precipitate was collected by centrifugation at 10,000 ×g for 40 min followed by dialysis against Tris-HCl (50 mM, pH 8.0) overnight at 4 °C. A summary of the production process for β-glucosidase is presented in Table 3. The fraction was derived by adding 90% (NH4)2SO4 to the crude fraction containing the bulk of the total enzyme activity (11.72 U). The specific activity of the enzyme collected was 78.13 U/mg of protein (Table 3) under the standard assay conditions described in the Materials and Methods section.
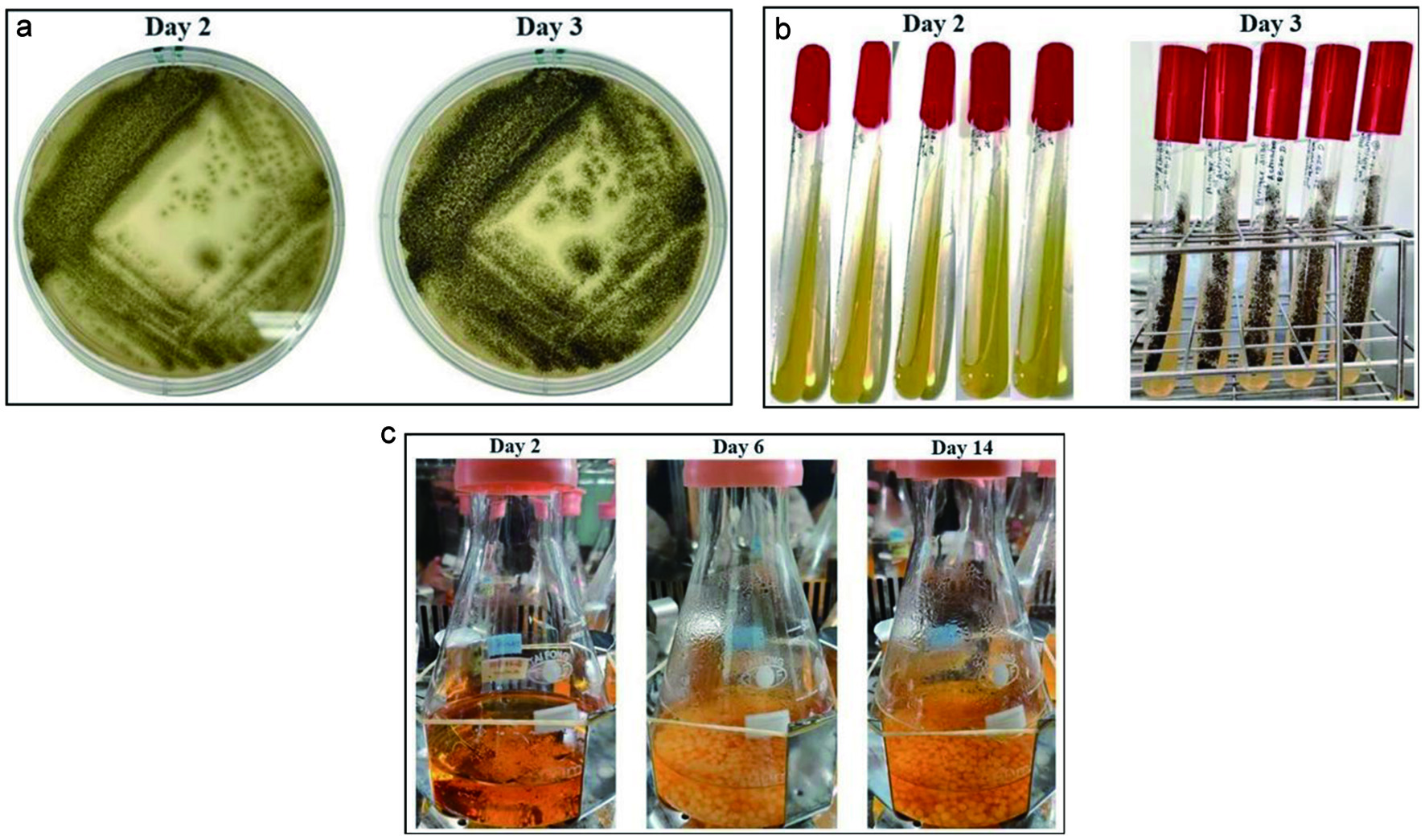 Click for large image | Figure 2. Culture of A. niger for the production of β-glucosidase enzyme. (a) First activation of A. niger (50 μL) from stock solution, (b) second activation, and (c) spore inoculation in malt extract. |
 Click to view | Table 3. Summary of enzyme production from A. niger |
3.3. Determination of the molecular mass of the enzyme extract from A. niger
The collected crude enzyme liquid was purified using (NH4)2SO4. The active protein fraction obtained after precipitation was analyzed by SDS-PAGE. β-glucosidase from different strains of A. niger has a molecular weight of 68–220 kDa determined via SDS-PAGE (Narasimha et al., 2016). Figure 3 shows multiple close migrating bands in lanes 1?8. The difference in the molecular weight of β-glucosidases in different organisms could be due to the presence of multiple forms of the enzyme and glycosylation of the enzyme. However, the multiple bands likely represent different sub-units of β-glucosidase and proteins from other similar enzymes that were denatured by SDS and other denaturants, as our protein is a crude extract. To identify the exact protein profile, the sample can be further analyzed via native PAGE to obtain its peptide mass fingerprints.
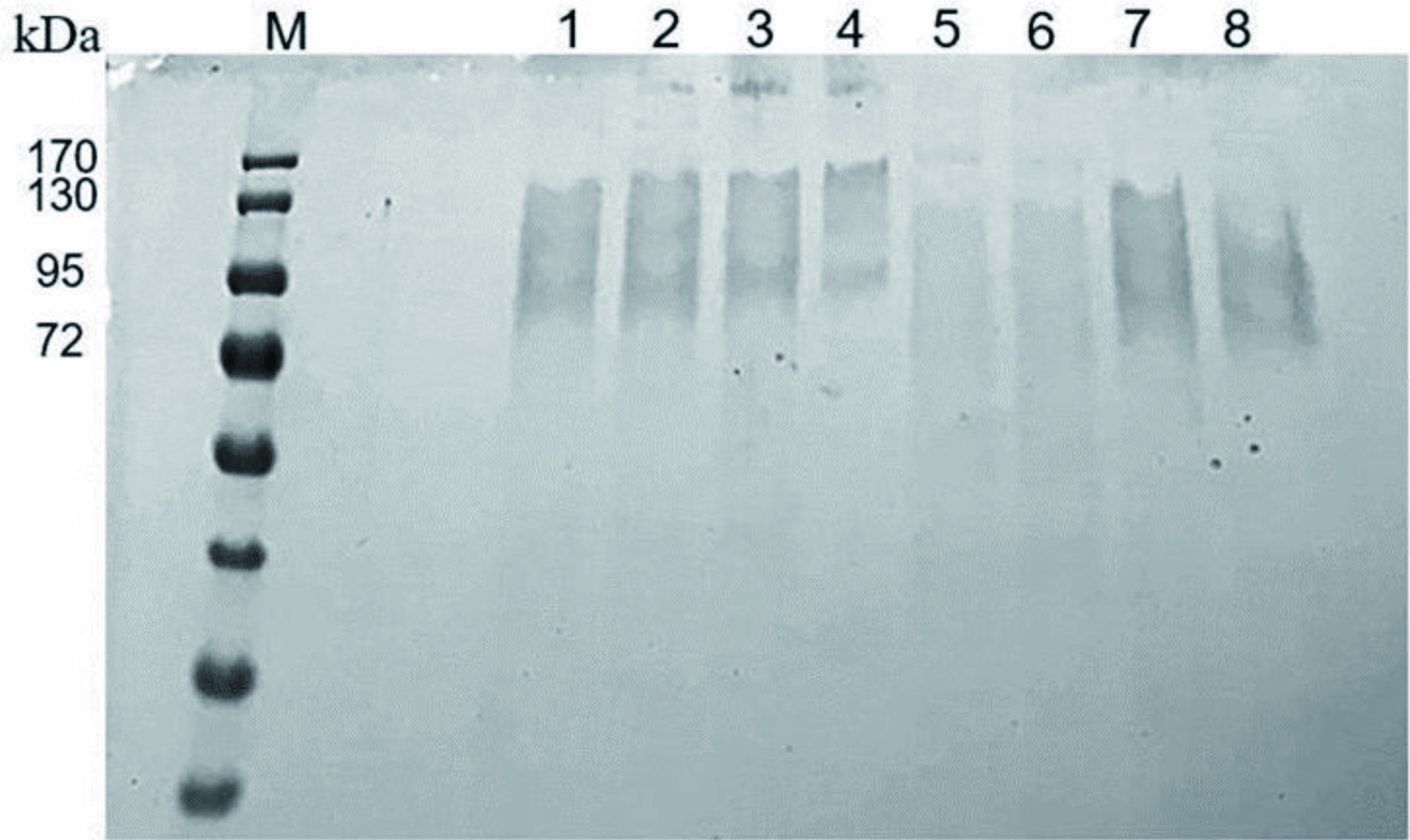 Click for large image | Figure 3. SDS-PAGE analysis of protein extract from A. niger on 10% separating gel. Lane assignment: lane M, protein marker; lanes 1?8, different batches of proteins produced by A. niger. |
3.4. Effect of temperature and incubation time on β-glucosidase enzyme activity
Enzyme activity can be influenced by a variety of factors, including temperature, pH, and concentration. Enzymes work best within specific time, temperature, and pH ranges, and sub-optimal conditions can cause an enzyme to lose its ability to bind to its substrate. High temperatures generally accelerate a reaction, and low temperatures slow down a reaction; however, extremely high temperatures can cause an enzyme to become denatured and stop functioning. The longer the enzyme is incubated with the substrate, the greater the amount of the product; however, the rate of formation of the product is not a simple linear function of incubation time. With time, the protein becomes denatured and loses its catalytic activity. In this case, the optimal temperature of the β-glucosidase enzyme was determined to be 37 °C (Figure 4A). The enzyme activity decreased with increasing temperature. In addition, enzyme activity increased with increasing time until 8 hours and then declined rapidly, resulting in the death phase of the enzyme cycle (Figure 4B). Therefore, the enzymatic bioconversion of ginsenosides by β-glucosidase was performed at 37 °C for 0, 1, 2, 4, 6, 8, and 12 hours.
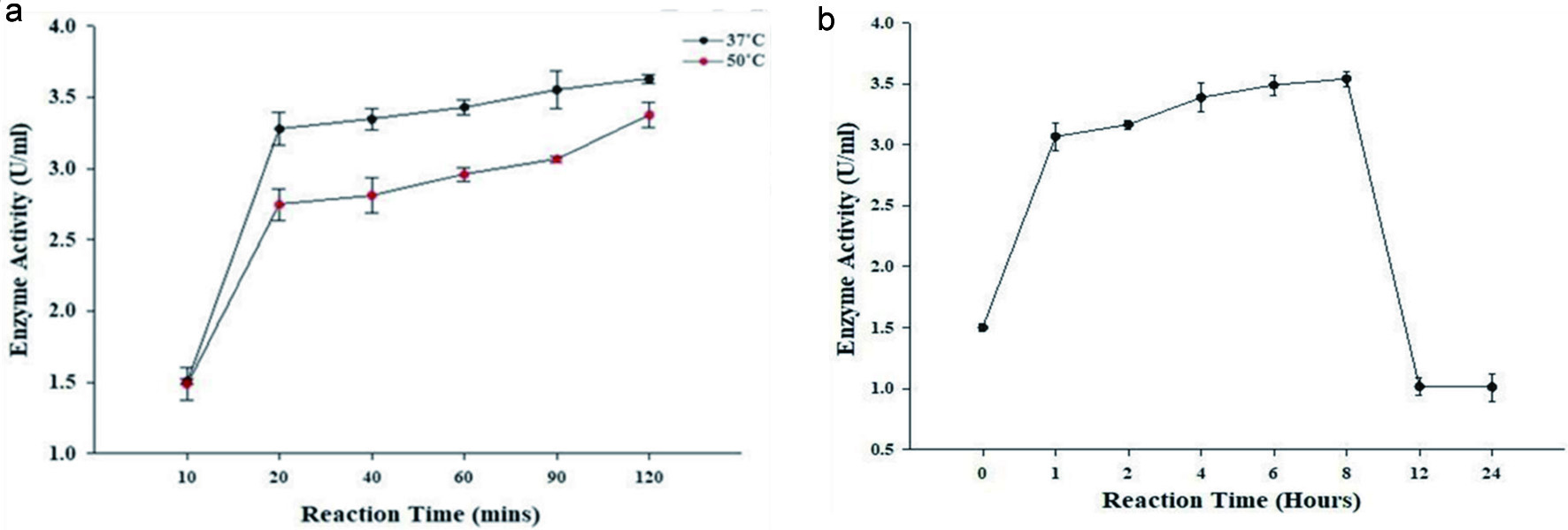 Click for large image | Figure 4. Effect of (a) temperature and (b) time at 37 °C on the enzymatic activity of β-glucosidase from A. niger. |
3.5. LC-MS analysis for the identification of ginsenosides
The exact composition of the enzyme reaction products was examined by LC-MS, and the presence of several ginsenosides was confirmed by LC-MS analysis. Comparison of the presence of ginsenosides before (GR0 and GL0) and after (GR8 and GL8) enzymatic bioconversion is shown in Figure 1. According to the chromatograph, ginsenosides present in the raw ginseng extract GR0 and GL0 before bioconversion (Figure 1A, C) included Rb1, Rb2, Rc, Rd, and Re; however, CK was not detected. The post-bioconversion chromatograph (Figure 1B, D) of GR8 and GL8 only showed peaks 1 and 7, which represented Re and CK, respectively, suggesting that most of the major ginsenosides (Rb1, Rb2, Rc, and Rd) were transformed into minor ginsenosides (i.e., CK) and other by-products by the β-glucosidase enzyme during the bioconversion process. β-glucosidase can remove glucose residues from the non-reducing end of a β-glucoside by catalyzing the hydrolysis of glycosidic bonds (Singh et al., 2016). Ginsenoside Rb1, which is the main PPD-type ginsenoside, is mainly de-glycosylated via the Rb1→Rd→F2→CK pathway (Bae et al., 2000). Rb1 has two C-20 glucoses and two C-3 glucoses; it loses one C-20 glucose to form Rd, Rd loses one C-3 glucose to generate F2, and F2 loses the other C-3 glucose to form CK. However, not all the Rb1 is converted to CK; there are other by-product ginsenosides formed along the way. Therefore, the enzyme can hydrolyze the 3-C position (3-O-) and 20-C position (20-O-) multiglycoside of PPD-type ginsenosides.
The β-glucosidase produced by A. niger first hydrolyzed the 20-O-β-D-(1→6)-glucopyranoside of Rb1 to Rd, then hydrolyzed the 3-O-β-D-(1→2)-glucopyranoside of Rd to F2 and further to CK. However, the enzyme first hydrolyzed the 3-O-β-D-(1→2)-glucopyranoside of Rb2 to C-O, hydrolyzed the -O-β-D- glucopyranoside of C-O to C-Y, and further hydrolyzed the 20-O-β-D-(1→6)-arabinopyranoside (arap) of C-Y to CK. In addition, the enzyme hydrolyzed the 3-O-β- D-(1→2)-glucopyranoside of Rc to C-Mc1, hydrolyzed the 3-O-β-D-(1→6)- glucopyranoside of C-Mc1 to C-Mc, and further hydrolyzed 20-O-α-L-(1→6)- arabinofuranoside (araf) of C-Mc to CK. The retention time for the ginsenosides is shown in Table 4, and the retention time for CK was 51.2 mins. Table 5 shows the quantity of ginsenosides present in GR and GL before and after bioconversion. According to Table 4, CK was not detected initially, and 26.35 ± 1.28 and 26.70 ± 1.24 of CK appeared after bioconversion in GR8 and GL8, respectively. In addition to CK, Rd (0.91 ± 0.05) was also detected in GR8 but was absent in GL8 (Liu et al., 2015).
 Click to view | Table 4. General data for target ginsenosides |
 Click to view | Table 5. Content of the ginsenosides |
3.6. Ginsenosides exhibit anti-CRC activity via HCT-116 cell viability
Tumor metastasis is one of the most important causes of death in cancer patients (Seo and Kim, 2011). Patients are at an increased risk of CRC because of chronic damage to the colon and rectum. Therefore, we focused on investigating the effect of ginsenosides on colon cancer cells. To investigate the growth inhibitory effects of ginsenosides, the human CRC cell line HCT-116 was treated with different concentrations (100, 200, and 400 μg/mL) of ginsenosides GR0, GR8, GL0, and GL8 for the indicated time (24 and 48 hours). Cell viability was assessed by an MTT assay. Collectively, the cells treated for 24 hours (Figure 5A) exhibited more potent inhibitory effects than the cells treated for 48 hours (Figure 5B). We employed 100, 200, and 400 μg/mL as low, medium, and high doses of ginsenosides, respectively. All the tested concentrations of ginsenosides were confirmed to be non-toxic (Figure 5). As compared with the initial raw ginsenoside groups (GR0 and GL0), the ginsenosides after bioconversion (GR8 and GL8) exhibited stronger inhibitory effects, suggesting that the less polar minor ginsenosides had more potent anti-cancer properties than the major ginsenosides. However, the obvious inductive effect was best observed in GR8 at a concentration of 400 μg/mL for 24 hours of incubation compared with GL8, indicating that GR offers better anti-cancer activity than GLs.
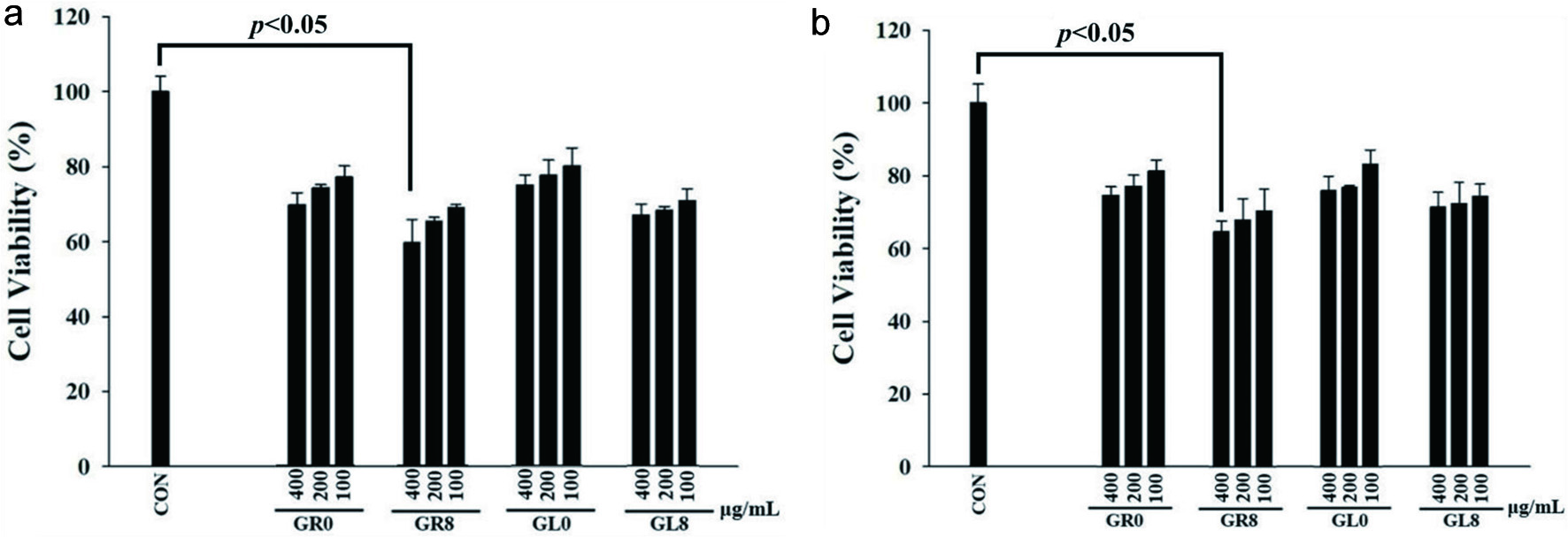 Click for large image | Figure 5. HCT-116 cell viability after treatment with GR0, GR8, GL0, and GL8 at concentrations of 100, 200, and 400 μg/mL for (a) 24 hours and (b) 48 hours. Cell survival was determined by MTT assay and was calculated as a ratio of the control. Results were statistically analyzed using Student’s t-test. Data are presented as mean ± SD from triplicate wells and three independent experiments. |
3.7. Effect of ginsenosides on RAW 264.7 murine macrophages
Chronic inflammatory disorders are often associated with an increased risk of developing cancer. Several inflammatory mediators, including nitric oxide (NO) and pro-inflammatory cytokines, are crucially involved in the initiation of inflammatory diseases and are characterized by activation of different signaling pathways, including the NF-κB/IKK pathway (Ahn et al., 2015; Lasry et al., 2016). The evaluation of cell viability using the MTT assay verified that the anti-inflammatory effects of enzymatically bio-transformed ginsenosides were not due to cytotoxicity. The tested ginsenosides (GR0, GR8, GL0, and GL8) exhibited effects on cell viability at all concentrations (Figure 6). As the concentration of the treatment increased, the cell proliferation decreased for GR0 and GL8; however, 400 μg/mL of GL8 had a significantly increased effect compared with other samples and concentrations. Based on these results, the rest of the experiments were performed at concentration of 400 μg/mL.
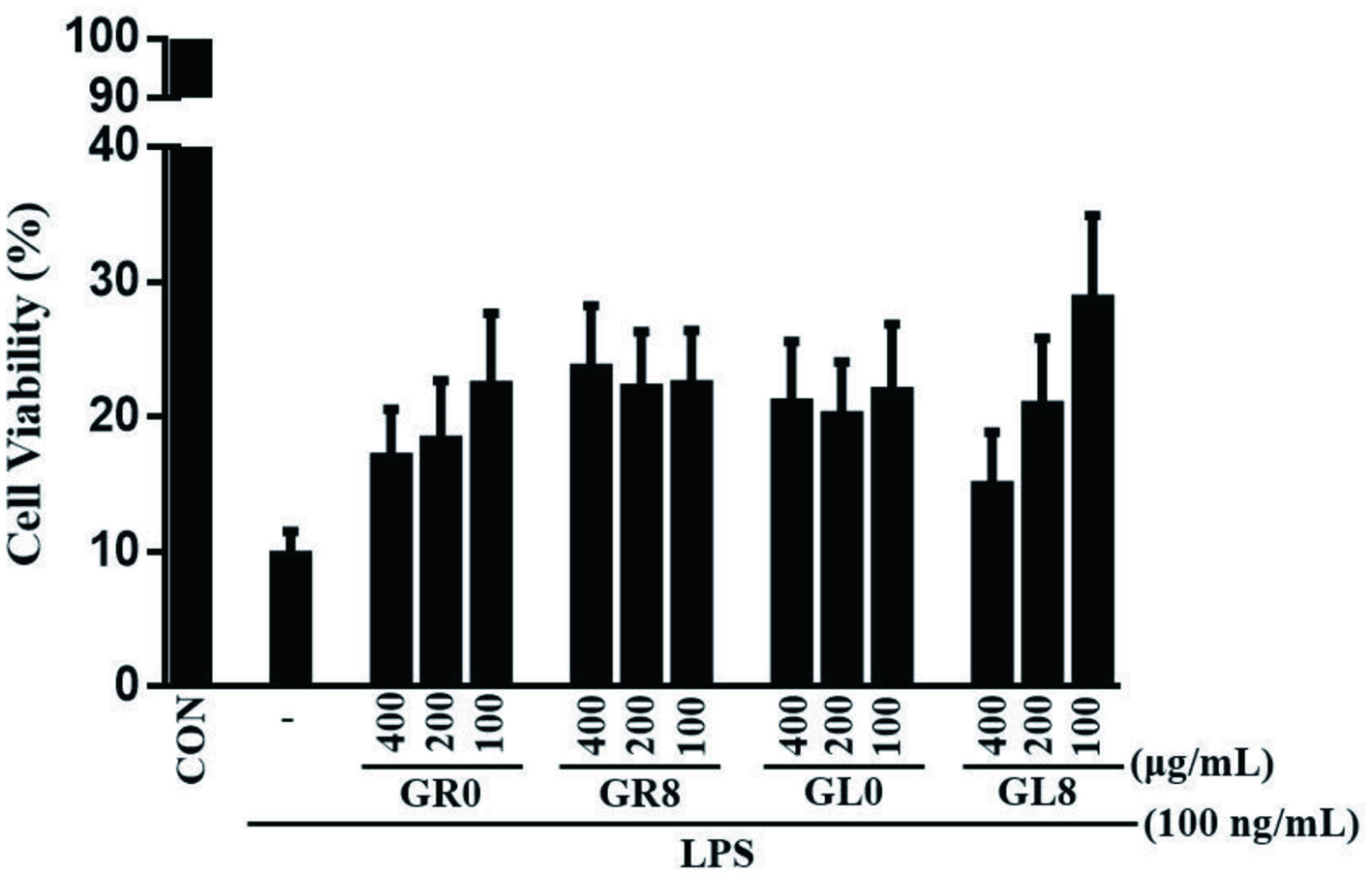 Click for large image | Figure 6. RAW 264.7 murine macrophage cell viability after treatment with GR0, GR8, GL0, and GL8 at concentrations of 100, 200, and 400 μg/mL for 24 hours. Cell survival was determined by MTT assay and was calculated as a ratio of the control. Data are presented as mean ± SD from triplicate wells and three independent experiments. |
3.8. Inhibition of LPS-induced NO production by ginsenosides
NO is one of the most important pro-inflammatory mediators. It is continuously produced as a protective mechanism that helps cells combat foreign invasion (Saba et al., 2018). Therefore, inhibition of the over-production of this agent is an attractive target in drug development for inflammatory diseases. Upon infection, pro- inflammatory molecules, cytokines, and anti-inflammatory factors are released (Martín, 2012). It is the balance between the pro- and anti-inflammatory agents that eliminates the invading pathogen and protects the body from the damage caused by inflammation (Saba et al., 2018).
To investigate the anti-inflammatory effects of ginsenosides, we evaluated whether ginsenosides could modulate NO synthesis in LPS-stimulated cultures of RAW 264.7 murine macrophages cells. Treatment of RAW 264.7 cells with LPS induced NO production; in contrast, the production of LPS-stimulated cells was considerably decreased by the ginsenosides (Figure 7). In our study, we found that bio-transformed ginsenosides sharply attenuated NO levels. Both GR8 and GL8 exhibited strong inhibition of LPS-induced NO production compared with GR0 and GL0; however, our data strongly suggested that GR8 produced a more significant reduction in LPS-induced NO production at 400 μg/mL than GL8.
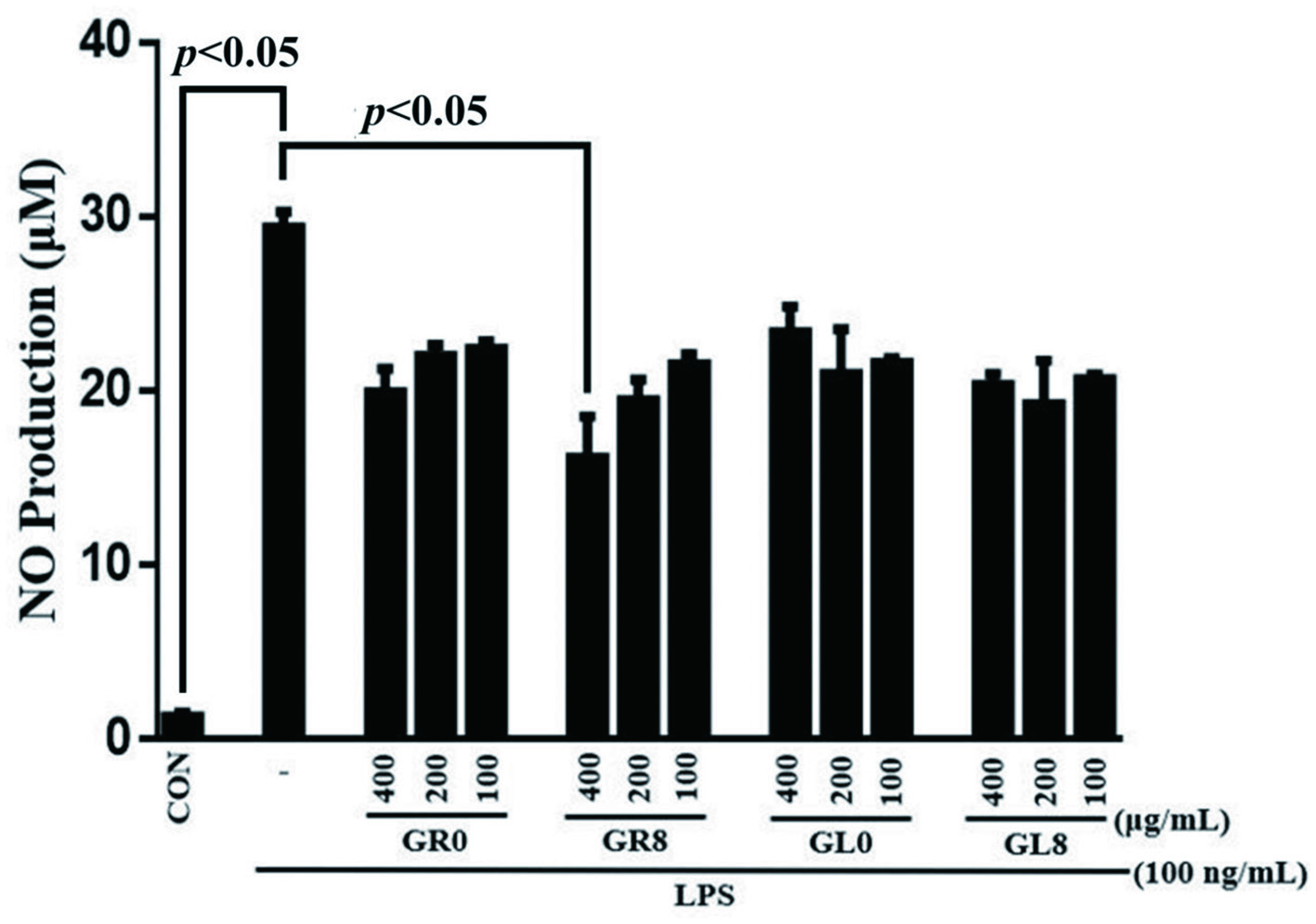 Click for large image | Figure 7. Effect of RAW 264.7 murine macrophages on nitric oxide production after treatment with GR0, GR8, GL0, and GL8 at concentrations of 100, 200, and 400 μg/mL for 24 hours. Results were statistically analyzed using Student’s t-test. Data are presented as mean ± SD from triplicate wells and three independent experiments. |
| 4. Conclusion | ▴Top |
The present study compared both raw and transformed samples of different parts of the ginseng plant through the simultaneous quantification of ginsenosides using LC-MS and the analysis of the bioactivity of ginseng. Our findings indicated high yields after simplified extraction of ginseng and suggested that GRs possess better ginsenoside quality and quantity than GLs. The properties of the enzyme from A. niger were characterized. Under optimized conditions, the enzyme from A. niger completely converted major ginsenosides present in raw ginseng extract to minor ginsenosides. The basic enzymatic hydrolysis pathway of the ginsenosides was Rb1→Rd→F2→CK, suggesting that the enzyme produced from A. niger hydrolyzed both the β-(1→2)-glucosidic and the C-3 β-(1→6) glucosidic linkage at C-20. After enzymatic bioconversion, the minor ginsenosides reduced the survival of HCT-116 cells. In addition, the enzymatic bioconversion produced anti-inflammatory effects on the suppression of LPS-induced NO production in RAW 264.7 murine macrophages.
These authors contributed equally.
This work was supported by the Ministry of Science and Technology, Taiwan [109-2320-B-002 -012 -MY3 and 110-2320-B-002 -019 -MY3].
Conflict of interest
The authors declare that they have no conflicts of interest.
| References | ▴Top |