| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 20, December 2022, pages 2-10
Implications of type 1 and type 2 taste receptors on obesity-induced inflammation
Gar Yee Koh, Yu Wang*
Department of Food Science and Human Nutrition, Citrus Research and Education Center, University of Florida, 700 Experiment Station Rd, Lake Alfred, FL 33850, USA
*Corresponding author: Yu Wang, Citrus Research & Education Center, Institute of Food and Agricultural Sciences, University of Florida, 700 Experiment Station Road, Lake Alfred, Florida 33850, United States. Tel: 863-956-8673; E-mail: yu.wang@ufl.edu
DOI: 10.31665/JFB.2022.18323
Received: November 30, 2022
Revised received & accepted: December 27, 2022
| Abstract | ▴Top |
Obesity is characterized by chronic low-grade inflammation that could lead to the other health complications, such as cardiovascular disease, diabetes, and various cancers. Nutrient intake and dietary preferences are often modulated by taste receptors in the taste buds. Emerging evidence has shown that taste perception is altered during the development of obesity. It is demonstrated that suppression of taste receptor or taste signaling molecules can potentiate inflammatory response, whereas progressive inflammation has shown to attenuate the expression of taste receptors in vivo, which could be suggestive of an interplay between taste signaling and inflammation. This review summarizes the interactions between types 1 and 2 taste receptors and inflammation, as well as the impact of obesity on taste signaling. Taken together, taste receptors might play a crucial role in regulating the inflammatory response during obesity and hence may serve as a potential therapeutic target to prevent the progression of obesity.
Keywords: Obesity; Taste receptors; Inflammation; Signaling
| 1. Introduction | ▴Top |
Obesity and overweight are affecting one-third of the global population. It is generally recognized that obesity is characterized by chronic low-grade inflammation (Dandona et al., 2003; Kolb et al., 2016; Saktiel and Olefsky, 2017). Indeed, inflammation is a known underlying contributor to many chronic diseases, including colorectal cancer, cardiovascular disease, and diabetes. It has been reported that taste preference is altered in obese individuals (De Jonghe et al., 2005; Hajnal et al., 2005; Kaufman et al., 2020; Scruggs et al., 1994; Umabiki et al., 2010; Yamada et al., 1999), and thus may alter their perception to taste and eating behavior. Taste buds are known to be expressed in the tongue epithelium to detect the five basic tastes. However, recent evidence has suggested that, other than in the oral cavity, some taste related G protein-coupled receptors (GPCRs) and taste signal transduction molecules, are found in extraoral locations, such as pancreas, adipocyte, gastrointestinal tract (GI), and respiratory tract (Laffitte et al., 2014; Patel et al., 2018; Wang et al., 2020). These types of taste GPCRs may exert functions that is beyond taste detection to regulate the metabolism of the host (Gilca et al., 2017; Patel et al., 2018; Wang et al., 2020). The implications of taste GPCRs in inflammation has been supported by the evidence that type 2 taste receptors (T2Rs) in the upper airway epithelium can detect bitter components and regulate the respiratory innate immunity (Douglas and Cohen., 2017; Grassin-Delyle et al., 2019; Lee and Cohen, 2015; Sharma et al., 2017). This has prompted us to investigate the potential role of taste GPCRs in obesity and its impact on obesity-induced inflammation, a condition that dictate the morbidity and mortality rate among the obese.
| 2. Nutrient sensing via G-protein coupled-receptors | ▴Top |
The sense of taste has allowed mammals to evaluate food quality and influence our ability to metabolize food. Taste perception begins when chemicals in food interact with the taste buds of the oral cavity to distinguish five basic tastes – sweet, bitter, umami, sour, and salty. Sweetness and umami are innately attractive and usually perceived as pleasure and energy-rich foods. Bitter and sour taste could signal as toxic and spoiled foods, while saltiness is important for electrolyte balance. It is known that each of the distinct taste qualities are mediated by specialized taste receptors. For example, salty and sour are driven by ion channels, while the taste of sweet, umami, and bitter are usually mediated by specific GPCRs. Type 1 taste receptors (T1Rs) is the first identified taste receptor of the GPCR family. There are 3 different T1R receptor subunits – TAS1R1 (T1R1), TAS1R2 (T1R2), and TAS1R3 (T1R3), of which they often function as heterooligomers. Coupling of T1R2 and T1R3 is usually activated by sweet components, whereas T1R1 and T1R3 heterodimer detects glutamate and some L-amino acids, or the umami flavor (Julius and Nathans, 2012; Margolskee, 2002; Zhang et al., 2003). Another member of the GPCR superfamily includes the type 2 taste receptors (T2Rs), or commonly known as the bitter taste receptors. There is a total of 25 subtypes of T2Rs in human – some could response to a broad range of stimuli, while some only selectively activated by a few bitter agonists (Adler et al., 2000; Fisher et al., 2005; Meyerhof, 2005).
These taste GPCRs have seven transmembrane domains and belong to the family of Type II taste cells, which comprise approximately 30–40% of cells in the taste bud (SC 2016). While extracellular N-terminal interact with the ligands, the intracellular C-terminal is generally associated with a heterotrimetric G-protein. In the case with taste GPCRs, these would include the Gα-gustducin and the G-βγ heterodimer. The canonical pathway for sweet, umami, and bitter may involve different subset of type II taste cells; however, they do signal through a common downstream pathway to stimulate gustatory response (Zhang et al., 2013). Upon ligand binding, the G-βγ subunit will dissociate from the receptor and activate the cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Both IP3 and DAG are served assecondary messengers. IP3, specifically, induces the release of intracellular calcium to evoke the release of ATP ( Behrens et al., 2011; Kinnamon, 2012; Nelson et al., 2001). Unlike the G-βγ subunit, Gα-gustducin can act through the phosphodiesterases to lower intracellular cAMP. It is proposed that the reduced cAMP is needed to mitigate the phosphorylation of calcium signaling effectors and hence maximizing the taste stimulation response (Kan et al., 2008; Trubey et al., 2006).
| 3. Taste alternation in obesity | ▴Top |
Obesity is recognized as a public health crisis affecting about 42.4% of U.S. population (Prevention CfDCa, 2020). It is expected that, by 2030, over 573 million adults worldwide will become overweight or obese if the secular trends continue ( Kelly et al., 2008). Obesity is a complex and multifactorial disease that significantly increases the risk for other chronic diseases, including type 2 diabetes, cardiovascular disease, and cancers (Bardou et al., 2013; Kolb et al., 2016; Yu et al., 2020). Continuous effort has been made to evaluate the impact of eating behavior on the progression of obesity. Recently, taste loss was detected in mice fed a high-fat diet, in which taste cell proliferation and density of fungiform papillae, where taste buds are housed, was significantly reduced compared to mice fed a standard diet (Kaufman et al., 2020). This observation also translated to humans, of which the density of fungiform papillae was inversely related to adiposity in young adult men(Kaufman et al., 2020), which could suggest an alteration of chemosensory signaling during the development of obesity. Early studies utilizing genetically obese rodent models has recognized the preliminary impact of obesity on taste preference. It was shown in these studies that obese mice provoked an enhanced preference for sucrose or other sweet-tasting chemicals such as fructose and saccharin (De Jonghe et al., 2005; Hajnal et al., 2005; Yamada et al., 1999). These changes may further influence appetite and eating behavior, as well as body weight regulation. Interestingly, when subjected to gastric bypass surgery, a common bariatric procedure for weight reduction, these obese rodents exhibited improved glucose tolerance and decreased preference for sucrose (Hajnal et al., 2010). In line with these preclinical studies, sweet preference was shown to be significantly greater among obese individuals (Mizuta et al., 2008); however, patients who underwent weight loss, either surgically or via energy restrictions, were associated with enhanced sweet taste acuity and lower recognition threshold for sucrose (Scruggs et al., 1994; Umabiki et al., 2010). It was demonstrated that disruption of both T1R2 and T1R3 mitigate the accumulation of fat mass in mice fed an obesogenic diet (Simon et al., 2014; Smith et al., 2016). While the metabolic phenotype could be driven by the absence of either of the receptor, the regulatory role of T1R and T2R in adipogenesis remained to be elucidated.
3.1. Taste modulator in obesity: leptin
The mechanisms underlying obesity and taste alteration is not entirely clear. One possible mechanism could involve the action of leptin. Leptin is an anorexigenic hormone secreted from the adipose tissue to regulate food intake and energy expenditure. Mizuta et al (Mizuta et al., 2008) was one of the first groups to show an association between the polymorphisms of leptin and leptin receptor gene with sweet preferences and obesity among the residents in Japan. Indeed, endogenous (Niki et al., 2015) and exogenous administration of leptin (Kawai et al., 2000; Yoshida et al., 2015) has shown to suppress the peripheral taste response in wild type lean mice by activating the K+ channels in sweet-responsive taste cells (Yoshida et al., 2015). In contrast, disruption of leptin signaling, such that commonly observed in the db/db mice, showed no difference in sweet taste sensitivities, suggesting that greater preference for sweet taste in obese mice could be driven by leptin (Yoshida et al., 2015).
| 3.2. Taste modulator in obesity: gut peptides | ▴Top |
Recent studies have revealed an emerging role of T1Rs and T2Rs along GI tract that could be beyond the conscious assessment of taste quality (Carey and Lee, 2019; Depoortere, 2014; Gu et al., 2015; Kok et al., 2018; Smith et al., 2016). Some of the distinct cell types are found in both the human gastric mucosa and upper GI tract, which is structurally and functionally similar to the lingual taste sensory cells (Kokrashvili et al., 2009b; Mace et al., 2007; Widmayer et al., 2012). These types of chemosensory mechanisms in the gut are usually responsible for modulating metabolic processes and nutrient uptake (Jiang et al., 2007; Janssen et al., 2011; Margolskee et al., 2007). Nutrient sensing in the gut, like the taste buds, is regulated by specialized epithelial cells, mainly the enteroendocrine and tuft cells. The enteroendocrine cells are located within the brush border and thus have direct contact with digested nutrients. Unlike the enteroendocrine cells, the role of tuft cells in the gut chemosensory is not entirely clear. Tuft cells share similarities with chemosensory cells in the taste buds and express taste signaling components. Thus, it is postulated that tuft cells may work cooperatively with enteroendocrine cells and enterocytes to regulate GI sensing.
It has been widely reported that expressions of intestinal T1R2 and T1R3, as well as their transduction proteins are critical mediator in regulating the intestinal glucose absorption, particularly via secretion of gut hormones, such as GLP-1 (Jiang et al., 2007; Kokrashvili et al., 2009a; Mace et al., 2007; Margolskee et al., 2007). Specifically, both the α-gustducin knockout mice and T1R3 knockout mice failed to produce GLP-1 in the duodenal cells upon intestinal glucose injection (Jiang et al., 2007; Kokrashvili et al., 2009a). This is further supported by in vitro studies demonstrated that deficient in α-gustducin in the enteroendocrine cell line NCI-H716 compromised the secretion of GLP-1 when stimulated with glucose (Jiang et al., 2007). Taken together, these studies have established a significant role of taste signaling elements in the gut in modulating glucose homeostasis.
Like T1Rs, the expression of T2Rs have also been identified along the GI tract to regulate the production of orexigenic and anorexigenic peptides, such as ghrelin, CCK, and GLP-1 (Dotson et al., 2008; Janssen et al., 2011; Kok et al., 2018). Bitter taste has evolved as a signal for harmful substances and hence facilitate a response to limit toxin ingestion and absorption. Mounting evidence has supported a role of extraoral T2Rs in various physiological processes, including obesity, hypertension, and cancer (Cancello et al., 2020; Choi., 2019; Cui et al., 2019; Singh et al., 2020). A cohort study has also demonstrated that genetic variation of T2R38 was associated with body weight changes (Choi., 2019). This is also supported by Latorre et al (Latorre et al., 2016) in which they have reported an upregulation of T2R38 in overweight and obese individuals. Yet the polymorphisms of T2R38 and body weight regulation remains controversial as others have reported no association between BMI and T2R38 polymorphisms, particularly in southern Italy (Gallo, Grossi, et al., 2016) and India (Deshaware and Singhal., 2017). In addition to body weight and adiposity, T2Rs were also heavily involved in maintaining glucose balance. It was revealed that disruption of a variant of T2R (i.e. T2R9) compromised glucose homeostasis and increased the risk for developing type 2 diabetes (Dotson et al., 2008). In the same study, Dotson and colleagues (Dotson et al., 2008) also confirmed the presence of T2R9 in the gut enteroendocrine L cells and stimulation with T2R9 ligand can elicit the secretion of GLP-1. In another study, chronic activation of T2R108 has shown to attenuate body weight gain via reduction of fat mass, along with an increased level of GLP-1 in diet-induced obese mice (Kok et al., 2018). These phenotypical changes were also accompanied by a significant improvement in glucose tolerance and insulin sensitivity, as well as suppression of several pro-inflammatory cytokines and chemokines, including TNF-α, IL-1b, IL-6, and MCP-1, all of which are usually induced during an obese state (Kok et al., 2018). T2R108 is one of the five T2Rs that are most abundant along the GI tract, including the enteroendocrine and tuft cells. Though the interaction between T2Rs and inflammation remained to be investigated, these data provide critical information on the possible involvement of T2Rs on GI inflammation induced by obesity.
| 4. Obesity and low-grade inflammation | ▴Top |
Several key inflammation markers have shown to consistently associated with obesity and obesity-related pathologies (Dandona et al., 2003; Kolb et al., 2016; Saktiel and Olefsky, 2017), indicating that the trigger of these immune responses could augment the prevalence of comorbidities in the obese. Hence, strategies to prevent obesity-mediated inflammation could be effective in managing the progression of secondary complications. The mechanisms underlying obesity and inflammation remain unclear. It is proposed that hypertrophic adipocytes in obese adults allows immune cells infiltration into the adipocytes, and that the subsequent recruitment of pro-inflammatory cytokines amplifies the signal that later contribute to systemic low-grade inflammation (Dandona et al., 2003; Kim et al., 2007; Xu et al., 2003). These signals are believed to be the “survival” signal to prevent uncontrolled expansion of adipocytes when caloric intake become excessive. However, overwhelmed production of these pro-inflammatory cytokines, such as TNF-a, IL-1b, and IL-6, may further disrupt the insulin receptor signaling in the peripheral tissues to promote ectopic lipid storage and hypertriglyceridemia, leading to a condition of what we commonly referred to as insulin resistance (Dandona et al., 2003).
Another possible mechanism that could lead to chronic low-grade inflammation in the obese could be due to the presence of lipopolysaccharides (LPS), an endotoxin commonly found on the outer membrane of gram-negative bacteria. Elevated plasma LPS is known as metabolic endotoxemia and it is associated with the onset of obesity and insulin resistance (Cani et al., 2007; Clemente-Postigo et al., 2019). LPS can cross through the intestinal mucosa through leaky tight junctions and enter the systemic circulation. Because LPS is a known ligand to toll-like receptor 4 (TLR4), it could subsequently activate a cascade of cellular signaling to induce inflammation and trigger innate immune response. Mice lacking the TLR4 were, however, protected from insulin resistance accompanied with reduced oxidative stress and chronic inflammation induced by a high-fat diet (Lin et al., 2020; Shi et al., 2006), suggesting that TLR4 plays a crucial role in regulating obesity-induced inflammation.
It has been demonstrated that gut dysbiosis, or imbalance of gut microbiota, was partially contributed to the pathophysiological regulation of endotoxemia (Cani et al., 2007). Cani et al demonstrated that in their study that mice fed a high-fat diet was associated with elevated plasma LPS and lower abundance of Bifidobacterium species, and that restoration of gut bifidobacterial content significantly improved the glucose tolerance and insulin sensitivity in these mice. The role of gut microbiota in obesity was established via the utilization of germ-free animal model. In these studies, germ-free mice were reported to be resistant to obesity, whereas colonization with gut microbiota derived from obese mice resulted in significantly increase in body fat in the germ-free mice (Bäckhed et al., 2004; Bäckhed et al., 2007; Wang et al., 2007). These findings have then shed some lights on how gut microbiota can manipulate energy balance of the host and their metabolic activities. Moreover, numerous other clinical and preclinical studies have shown that gut dysbiosis perturbs the homeostatic interactions between microbiota and gut health, which could subsequently lead to metabolic disorders such as inflammatory bowel disease and colorectal cancer (Cani and Jordan, 2018; Gagnière et al., 2016; Jangid et al., 2020; Lo Presti et al., 2019). However, it is still unclear on how gut microbiota interacts with the host system to regulate the inflammatory processes, specifically on obesity and obesity-associated complication.
| 5. Taste signaling and inflammation: implication in obesity | ▴Top |
The regulation of inflammatory response by T2Rs has been well established in the respiratory tract (Douglas and Cohen., 2017; Grassin-Delyle et al., 2019; Lee and Cohen, 2015; Sharma et al., 2017), highlighting the role of extra oral T1Rs and T2Rs in immune function. In this notion, taste signaling and/or taste GPCRs might have similar impact on obesity-induced low-grade inflammation. Obesity has been known as an emerging risk factor for gut-related diseases, including IBD and colorectal cancer (Adams et al., 2007; Park et al., 2012; Wunderlich et al., 2018), possibly driven by the sustainable mild inflammatory status during the progression of obesity. The role of taste signaling on gut inflammation was evidenced in a study by Feng et al (Feng et al., 2018) when α-gustducin knockout mice were shown to be more susceptible to colitis after administration of DSS compared to wild-type mice. In addition to enhanced immune cells infiltration in the colon of α-gustducin knockout mice, they also exhibited elevated expression of colonic pro-inflammatory cytokines (i.e. TNFα and IFN-γ) as well as a decreased expression of anti-inflammatory markers (i.e. IL-10, TGF-β, and IL13) in the colon (Feng et al., 2018). More importantly, expression of T2R38 bitter taste receptor have been identified in both resting and activated lymphocytes, which could further imply a direct regulatory role of T2R in immune function (Tran et al., 2018).
Several other studies have also described the inflammatory responses that are regulated by T2Rs. It was discovered that receptors for inflammatory cytokine are highly abundant in taste bud cells (Feng et al., 2014; Feng et al., 2012). Further, inflammation can modulate peripheral taste function and exacerbate the expression of pro-inflammatory cytokines, such as TNFα (Cohn et al., 2010; Feng et al., 2015) and interferon-mediated pathways (Wang et al., 2007). TNFα knockout mice, for example, have found to be less responsive to bitter compounds, suggesting that TNFα may be involved in taste sensory (Feng et al., 2015). It is likely that the hyporesponsive to bitter compound could be caused by the disruption of TNFα signaling rather than diminished structure of taste cells (Feng et al., 2015). This contrasts with what have observed in the study by Cohn et al (Cohn et al., 2010), where LPS-induced inflammation has shown to diminish the proliferation of taste progenitor cells as well as interfere with taste cell renewals. In line with this observation, Kaufman (Kaufman et al., 2018) also demonstrated that taste bud abundance and taste cell renewal were attenuated by low-grade inflammation resulting from obesity, whereas no changes were observed in the lean mice. In addition, high-fat diet induced obesity further potentiated the expression of TNFα in the taste buds, which was correlated with an increased adiposity in these mice (Kaufman et al., 2018). More recently, it was reported that expression of TLRs, including TLR4, was detected in the organoids derived from the mouse circumvallate stem cells (Feng et al., 2020). These organoids also encoded all five genes of the NF-kB family, a critical transcriptional factor in regulating inflammatory response. When stimulated with LPS, a ligand to TLR4, pro-inflammatory cytokines, such as TNFα and IL-6, were upregulated in the taste organoids (Feng et al., 2020). Clearly, taste cells exerted potential immunoregulatory role to alter taste preference, though the underlying mechanisms are still warranted.
Utilization of the TLR4 knockout (KO) model has further supported that TLR4 is a key mediator in regulating food preference and post-ingestion metabolic changes as these TLR4 null mice exhibited reduced preference for sweet and lipids (Camandola and Mattson, 2017), suggesting that TLR4 activation is required to promote intake of obesogenic foods. However, Zhu et al (Zhu et al., 2014) has reported a different observation, where they have demonstrated that chronic activation of TLR4 via administration of exogenous LPS could inhibit or delay sweet taste response in mice (Zhu et al., 2014). The conflicted data reported by these studies is especially interesting. It should be noted that in the study by Camandola and Mattson (2017), high-fat diet was employed, whereas with Zhu et al (Zhu et al., 2014), standard chow diet was used prior to performing the two-bottle preference test. As discussed earlier, activation of TLR4 is a key step to trigger innate immune response during obesity. Hence, it is very likely that adiposity may have impacted the differential responses observed in these studies. Because inflammation status was not assessed in these studies, whether the change in taste preference is related to low-grade inflammation resulting from obesity and what is the role of these inflammation-associated molecules in taste signaling remained to be investigated. Instead, these studies have sparked an interesting crosstalk between innate immunity and chemosensory signaling.
5.1. GLP-1: an anti-inflammatory agent
While studies have suggested a potential interaction between taste signaling and inflammation, the role of taste receptors in mediating the changes of taste preference or taste perception in obesity is not entirely clear. One possible mechanism could be related to GLP-1 secretion that is regulated by taste GPCRs. GLP-1 is generally released upon stimulation of ingested nutrients. In humans, GLP-1 has a critical role in maintaining glucose homeostasis. Even though the receptor for GLP-1 was first identified in the gut and pancreatic β-cells (Mortensen et al., 2000; Niki et al., 2015), it has now been acknowledged that GLP-1 receptors are also expressed in the adipose tissues, central nervous system, and taste cells (Ejarque et al., 2019; Feng et al., 2008; Muscogiuri et al., 2017; Shi et al., 2015; Zhao et al., 2019 ) with functions that are beyond glycemic control. For example, GLP-1 receptor in taste cells has shown to alter taste sensitive to sweet and umami (Feng et al., 2008; Martin et al., 2009; Simon et al., 2014), while the receptor in the immune cells can directly regulate the production of inflammatory cytokines (Tran et al., 2018). In obese mice receiving exenatide, a GLP-1 agonist, they also tend to have lower body weight and enhanced sensitivity to sweet taste, compared to those that received vehicle treatment (Zhang et al., 2013). In fact, GLP-1 analogs have also been used as a common anti-inflammatory drug to alleviate complications resulting from type 2 diabetes (Jung et al., 2015; Shi et al., 2015), gut inflammation and inflammatory bowel disease (Anbahagan et al., 2017; Hogan et al., 2014), as well as cardiovascular diseases (Liu and Kong, 2020; Nauck et al., 2017).Hence, activation of taste GPCRs to produce GLP-1 could be a protective mechanism to suppress inflammatory responses.
5.2. Crosstalk between gut microbiota and taste signaling
Gut microbiota is an emerging factor contributing to metabolic disorders. The GI tract has a dense population of gut microbial with at least 1000 different species of known bacteria. The composition of gut microbiota can be manipulated by diet and other external factors to alter energy harvest capacity of the host. Interestingly, it has been shown that in germ-free mice, expression of T1R3 in the intestinal epithelium, but not in the lingual epithelium, was significantly higher compared to conventional mice, accompanied by an increased preference for sucrose solution at high concentration (Swartz et al., 2012). Furthermore, Latorre et al also reported a preliminary observation by which an antibiotic treatment has suppressed the elevation of T2R38 in the gut. The concept that gut microbiota may attribute a role in taste receptor-mediated inflammation is relatively new. However, it is well documented that in the respiratory tract, T2R38 can mediate the innate immune defense mechanism upon activation by a quorum-sensing molecule secreted by gram-negative bacteria (Carey et al., 2017; Gaida et al., 2016; Lee and Cohen, 2015; Maurer et al., 2015). Hence, it is possible that bacteria or bacteria metabolites have the similar ability to modulate inflammation in the gut or other targeted tissues. Because high-fat diet is known to alter gut microbial community (Cani et al., 2007; Netto et al., 2018), this could subsequently influence the expression of taste GPCRs and their downstream signaling, thus altering innate immunity and metabolism of the host. Though more work must be done, this new information may shed some light on the possible interaction between gut microbiota and taste GPCRs, as we as their implications on obesity-induced inflammation.
Gut microbiota can also impact inflammation via the production of short chain fatty acids (SCFAs). Receptors for short-chain fatty acids (FFARs) have been identified in the intestinal L-cells to possibly regulate the homeostasis glucose and lipid metabolism (Kaji et al., 2014). FFARs are also belong the family of GPCRs. Though it has been a debatable topic on whether fat can be classified as the sixth basic taste, FFARs are found in taste cells located in the oral cavity to regulate orosensory response to fats (Godinot et al., 2013). It is uncertain whether FFARs in the oral cavity and in the gut share similar functions; yet, intestinal FFARs have shown to be a critical factor in facilitating the crosstalk between gut microbiota and host metabolism upon binding to SCFAs. SCFAs are by-products of microbial fermentation of dietary fiber. Some of the common examples are butyrate, propionate, and acetate. Not only do these SCFAs serve as an energy source for colonocytes, others, such as acetate and propionate, are critical in hepatic gluconeogenesis (Ji et al., 2019; Yoshida et al., 2019). Studies have also demonstrated that SCFAs can modulate the secretion of gut peptide hormones, including GLP-1 and PYY (Christiansen et al., 2018; Psichas et al., 2015). In addition, SCFA-mediated FFAR activation has shown to protect intestinal integrity by restoring the expression of tight junction proteins (Feng et al., 2018; Zheng et al., 2017). Mice that are deficient in FFARs can further augment the inflammatory status in mouse model of colitis, colon cancer, and obesity (Kimura et al., 2013; Pan et al., 2018; Sina et al., 2009; Smith et al., 2013), when compared to the wild type mice. On the other hand, stimulation of FFARs with SCFA or intake of dietary fiber can attenuate the secretion of selected pro-inflammatory cytokines (Hu et al., 2018; Wenzel et al., 2020; Xu et al., 2008), indicating that FFARs could be a critical mediator underlies the protective effect of SCFAs.
| 6. Conclusions | ▴Top |
Obesity is a growing epidemic that is affecting about 42% of the U.S. population. Characterized by inflammation, obesity increases the risk for developing secondary conditions, such as cancer and cardiovascular disease. It has been proposed that taste can directly regulate food intake and hence may play a significant role in body weight regulation. Moreover, mounting evidence has suggested that taste perception is altered in obesity and that T1R, T2R, or associated transduction signaling molecules are involved in regulating innate immunity. It is hypothesized that inflammation status could be dependent on taste signaling, which could also serve as a negative feedback signal to alter nutrient intake in order to maintain energy homeostasis (Figure 1). Studies have suggested that diet can directly modulate the composition of gut microbiota to manipulate host immunity and metabolism. While there is limited information into the interaction between gut microbial community and taste GPCR on obesity-associated inflammation, it is well established that bacteria are capable to interact with selected taste receptors in the respiratory tract to regulate airway immunity. Therefore, it is very likely that gut bacteria might play a similar role in mediating obesity-induced inflammation via taste GPCRs. Additionally, GLP-1 secretion upon activation of T1Rs and T2Rs may serve as a protective mechanism to suppress inflammatory response during the progression of obesity. Simultaneously, GLP-1 and other gut peptides may work in conjunction with leptin to regulate taste signaling and hence directly influence the appetite and food intake of the host, either by altering their taste perception or diminishing the structure of taste cells. It is undoubtedly that more work is still needed to confirm the regulatory role of taste signaling in inflammation, particularly under the obese state. Further understanding on the involvement of T1Rs and T2Rs in inflammation could possibly explain the crosstalk between diet, eating behavior, and metabolic dysfunction in obesity. Hence, treatment or dietary interventions targeting the taste receptors could be a useful strategy to prevent the progression of obesity-induced inflammation as well as the associated health complications.
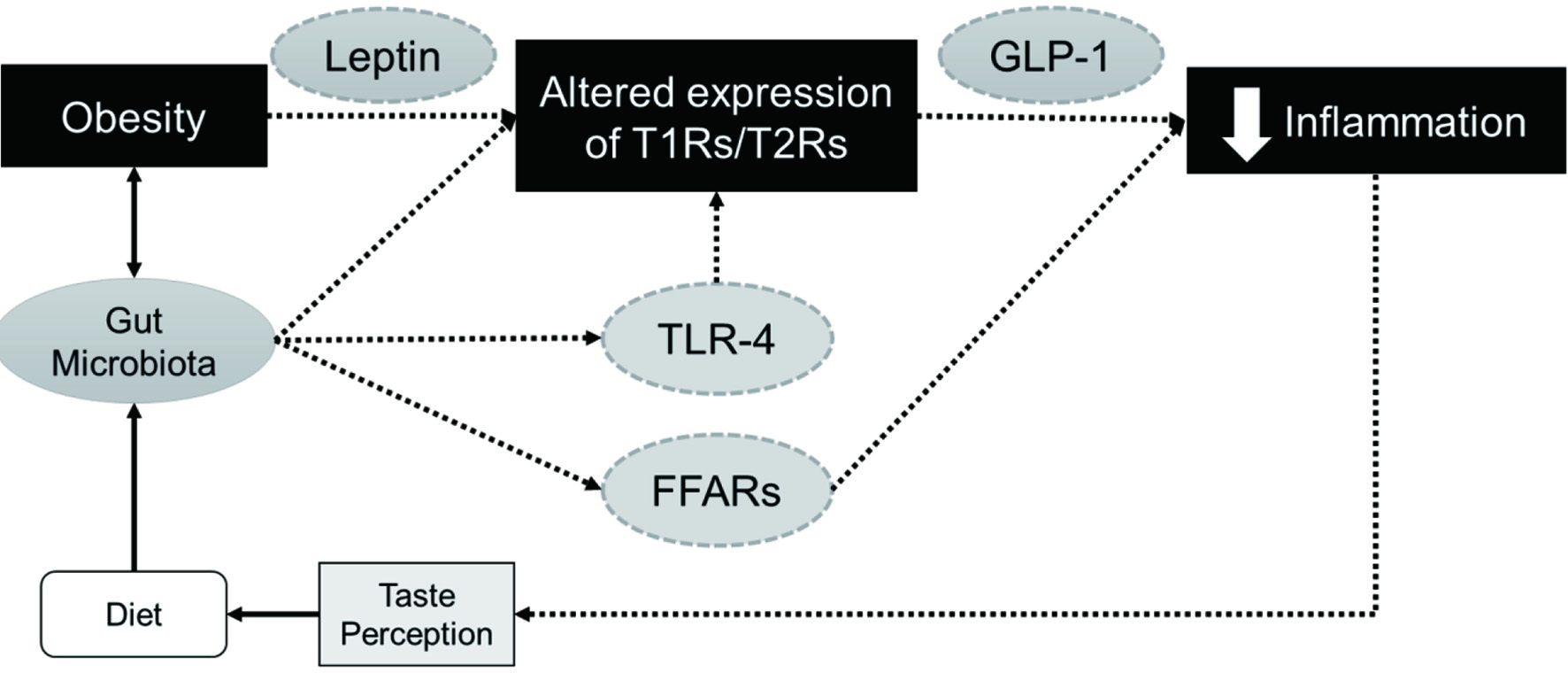 Click for large image | Figure 1. Potential regulatory role of T1Rs and T2Rs in obesity-induced inflammation. T1Rs and T2Rs can modulate the secretion of GLP-1, a potential anti-inflammatory agent, and work in conjunction with leptin or other gut peptides to regulate food intake and host metabolism. Obesity can also alter gut microbiome, which could impact the expression of taste receptors directly or via TLR4 attenuation to inhibit subsequent inflammatory responses. Another putative mechanism might involve the activation of FFARs upon binding to short chain fatty acids resulting from microbial fermentation of dietary fiber. |
This work was supported by funding from USDA-NIFA 2018-07925.
| References | ▴Top |