| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 19, September 2022, pages 124-135
A review on fruits bioactive potential: an insight into phytochemical traits and their extraction methods
Anuradha Gautama, Sonia Moryaa, *, Arno Neumannb, Farid Menaac
aDepartment of Food Technology & Nutrition, School of Agriculture, Lovely Professional University, Phagwara- 144411, Punjab, India
bBET Bioscience Extraction Technologies Inc. Abbotsford, B.C. Canada
cDepartment of Nanomedicine and Advanced Technologies, California Innovations Corporation, San Diego, CA, USA
*Corresponding author: Sonia Morya, Department of Food Technology & Nutrition, School of Agriculture, Lovely Professional University, Phagwara- 144411, Punjab, India. E-mail: sonia.morya8911@gmail.com
DOI: 10.31665/JFB.2022.18318
Received: July 6, 2022
Revised received & accepted: September 30, 2022
| Abstract | ▴Top |
In recent years the uptake of fruits is increased due to bioactive compounds present in it. Given this, the purpose of the review is to give information about the extraction process of phytochemicals and the method of their determination. The systematic review comprised recent year papers to bring the knowledge about the bio actives and techniques of their extraction and determination. Most of the fruits contain bioactive compounds and provides various health benefits. Studies included in this paper indicate the infusion of bioactive compounds in fruits and could benefit in improving consumer health. The present review aims to analyze the different phytochemicals that are present in fruits and their mode of extraction and determination.
Keywords: Phytochemicals; Nutraceutical; Bioactives; Health benefits; Ultrasound-assisted extraction
| 1. Introduction | ▴Top |
Phytochemical is a word that has been derived from the Greek word phyto, which means plant. These are chemical compounds, produced mostly by plants and have biological functions (Mendoza and Silva, 2018). They defend plants against disease and injury while also improving their color, scent, and flavor, also protect from environmental threats such as pollution, stress, drought, UV exposure, and pathogenic attacks. A nutraceutical is a food or component of food that has medicinal and nutritional health benefits, such as disease prevention or treatment (Santini et al., 2018). Nutraceuticals, phytonutrients, and secondary metabolites are found in abundance in plants. The health benefits of nutraceuticals are well-known, which include lowering the risk of cancer and heart disease, preventing hypertension, high cholesterol, obesity, osteoporosis, diabetes, arthritis, muscular degeneration, cataracts, menopausal symptoms, insomnia, and memory loss (Dolkar et al., 2017). In case of phytochemicals carotenoids, polyphenols, and flavonoids are the prominent category. Based on the chemical structure, flavonoids are divided into classes including anthocyanins, flavones, flavanones, and isoflavones, as well as flavanols (Shahidi, and Ambigaipalan, 2015). Acid, Sugar, and polysaccharides are good sources of phytochemicals, which are secondary metabolites of plants with antioxidant activity and other qualities (Escobedo-Avellaneda et al., 2014). To preserve and control microbial spoilage in foods, phytochemicals such as flavonoids, polyphenols, anthocyanins, and carotenoids are traditionally been used (Negi, 2012). In vitro screening methods have been used to confirm the biological activity of isolated phytochemicals. However, for a large number of phytochemicals and their health effects, large-scale trials are necessary, a method that is both expensive and ineffective (Yoo et al., 2018). As a result, in silico methodologies have been presented to discover the potential health impacts of phytochemicals from a large number of possibilities, most of which are based on molecular or ethnopharmacological knowledge.
1.1. Classification and functional activities of phytochemicals
Fruits are the most readily available and inexpensive source of nutrients, such as carbs, proteins, vitamins, minerals, and amino acids, (Bumgarner et al., 2012). They varied in their composition, and eating a variety of colored fruits provides your body with a diverse range of nutrients and phytochemicals that are essential for overall health as given in Table 1. Polyphenolic components found in most fruits have antioxidant properties that can benefit human health. Other chemicals, such as xanthones, carotenoids, and saponins, provide health benefits in addition to polyphenols. There are a number of classifications put forward for the various family of phytochemicals as given in Figure 1 and Figure 2 (Dolkar et al., 2017).
 Click to view | Table 1. Fruits phytochemicals and their functional activities |
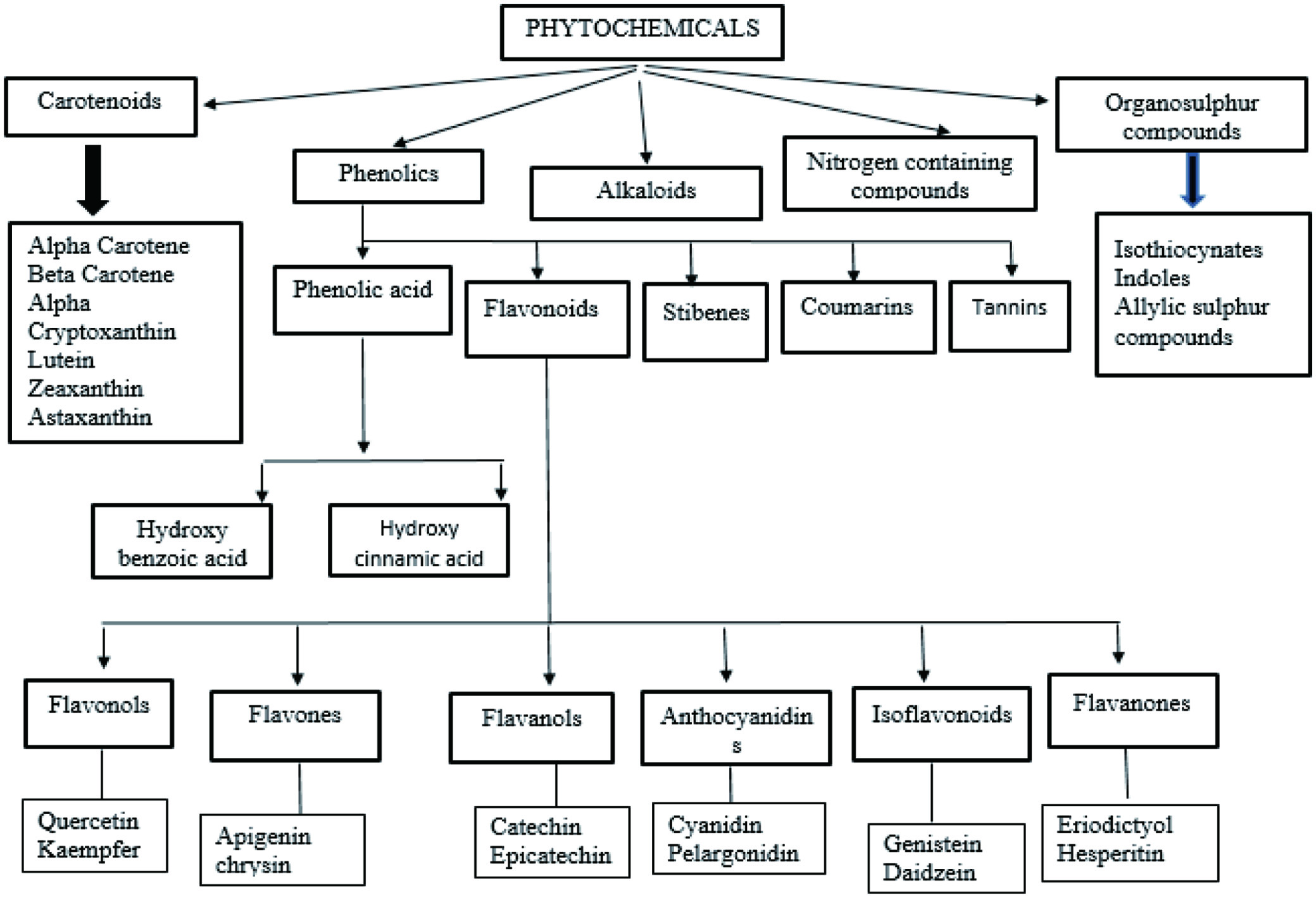 Click for large image | Figure 1. Classification of dietary phytochemicals. |
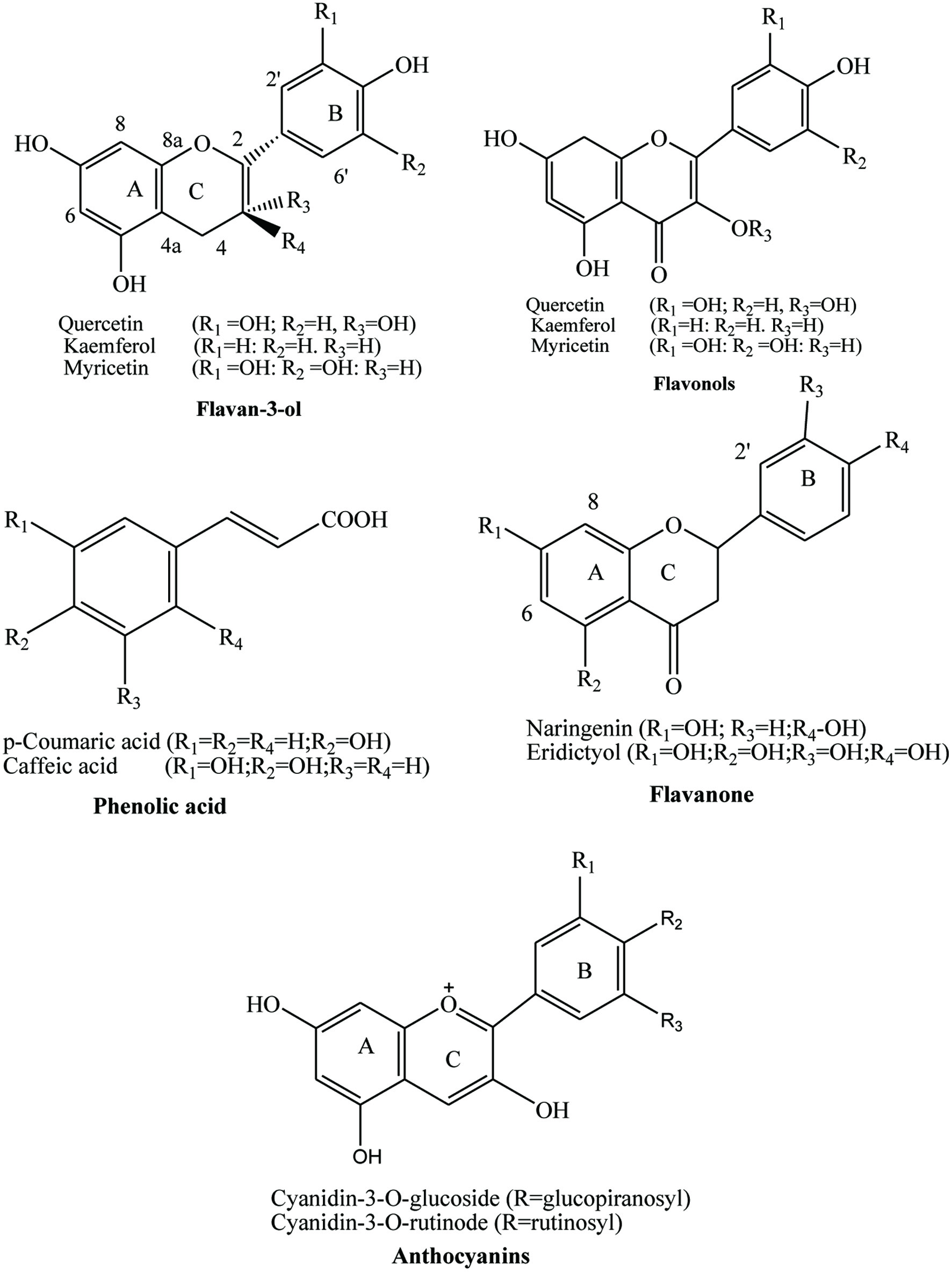 Click for large image | Figure 2. Chemical structure of bioactive compounds presents in fruits. |
1.2. Phytochemical profile of fruits
Plants food contains secondary metabolites, as anthocyanin, flavonoids, phenolic acids, terpenoids etc. These secondary metabolites have earned lots of popularity due to its antioxidant, antibacterial and anti-cancer properties as shown in Table 1. Generally, they are divided into 6 classes and the chemical structure of few phytochemicals is given in Figures 3 and 4 (Shahidi, and Ambigaipalan, 2015).
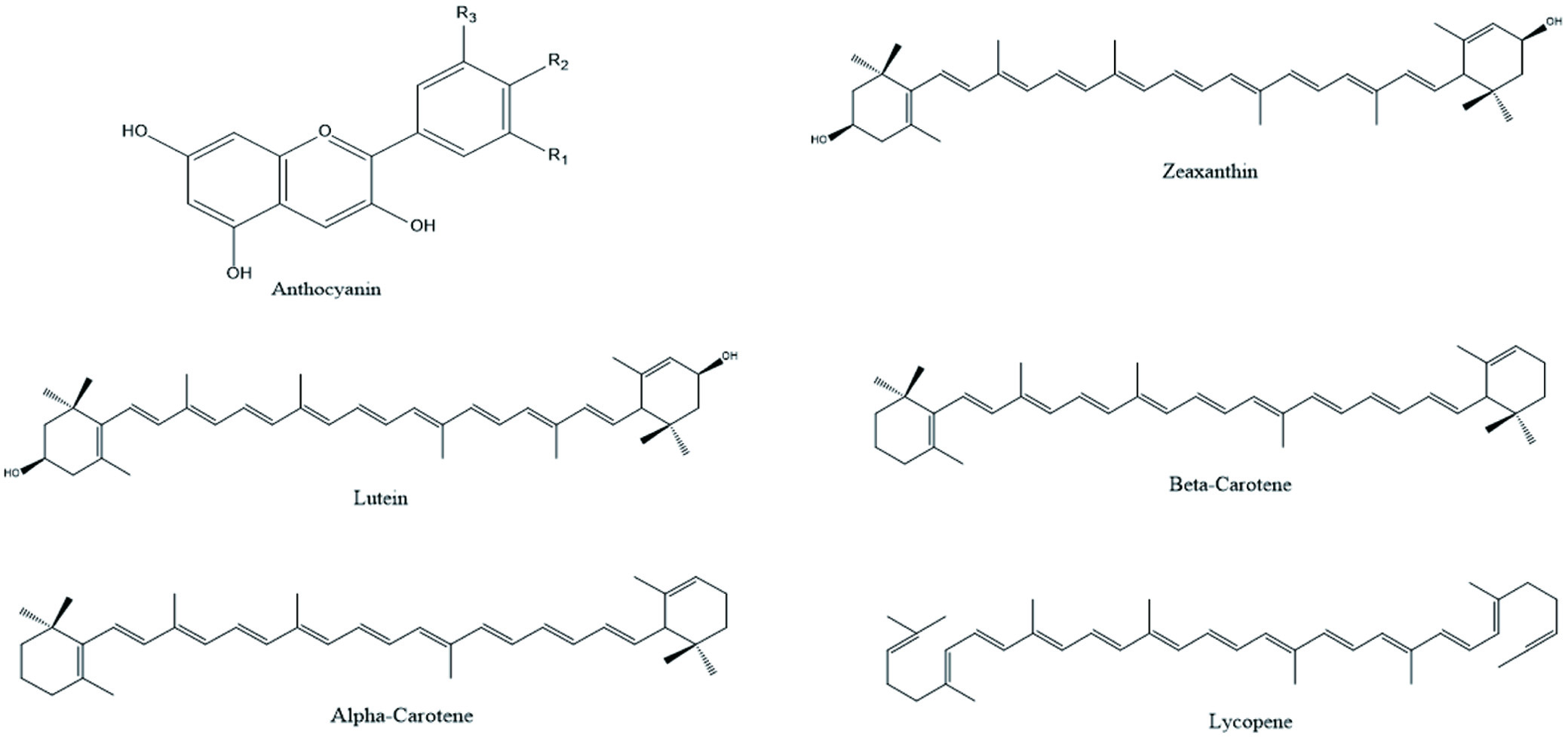 Click for large image | Figure 3. Illustration of different phytochemicals present in fruits. |
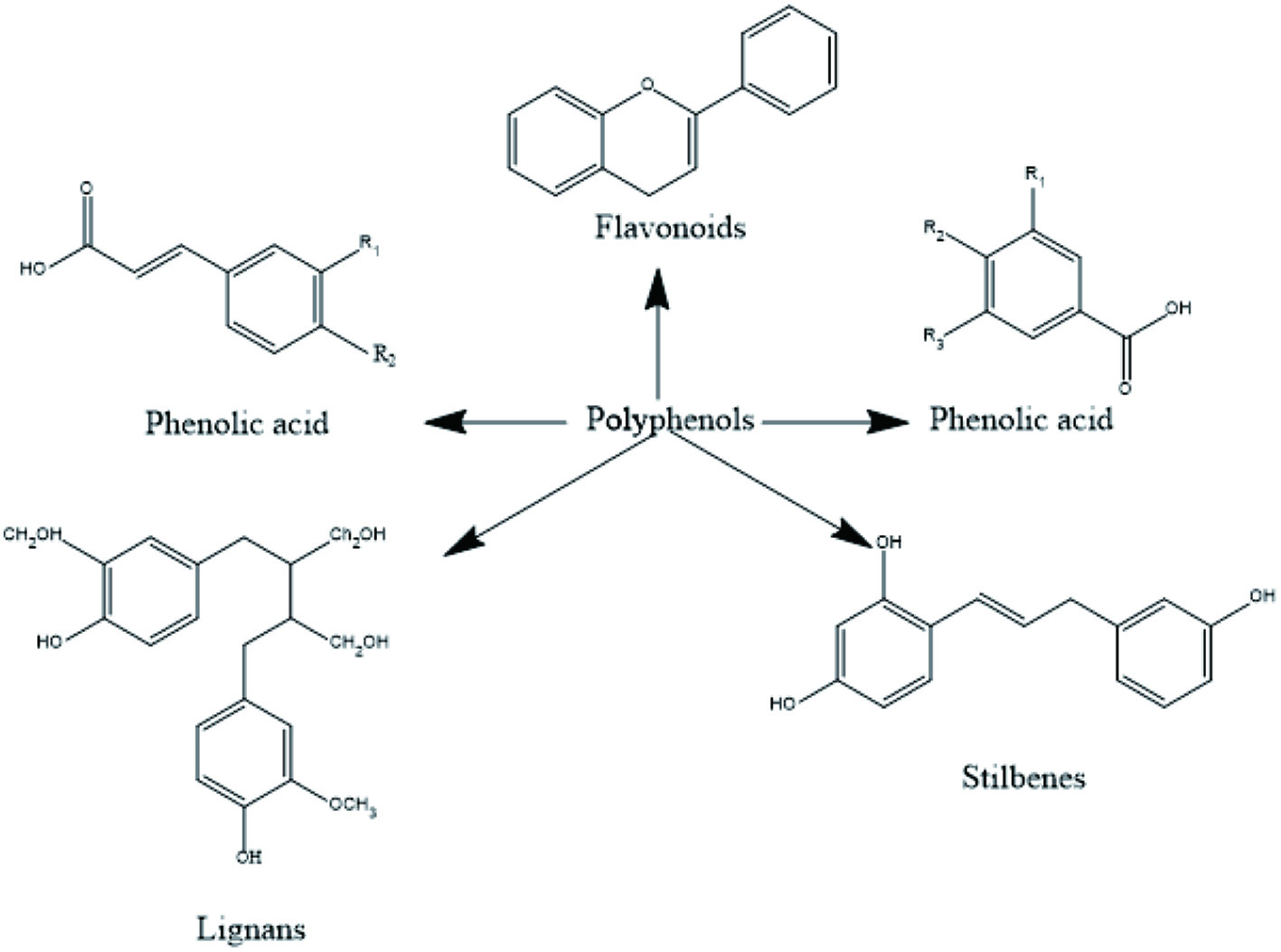 Click for large image | Figure 4. Different Polyphenols present in fruits. |
1.3. Active principles of different fruits
Bromelain is a protein-digesting enzyme obtained commercially from the pineapple fruit or stem. It is most frequently used as an anti-inflammatory, but scientists have found that it has anticancer and antibacterial properties as well as mentioned in Table 2. It has beneficial effects on the respiratory, digestive, and circulatory systems, and the immunological system. It’s an all-natural treatment for arthritic symptoms like joint pain and stiffness (Chakraborty et al., 2021).
 Click to view | Table 2. Health benefits and bioactive principles of the fruits |
Gallic acid is a phenolic chemical that can be found in a variety of fruits and medicinal plants. It also seems to contain several health-promoting properties. Gallic acid is a polyphenol component that can be found in fruits, vegetables, and herbal medications. There are numerous biological properties associated with gallic acid, including antioxidant, anticancer, anti-inflammatory, and antibacterial properties. Its derivatives not only improve gut microbiome (GM) activities but also modify immune responses. As a result, gallic acid has a great potential to help the body’s natural defence system against infections and alter the immune response (Yang et al., 2020).
Ellagic Acid-rich foods and formulations have been linked to cancer, diabetes, and diabetic complications, chronic tissue inflammation, metabolic syndrome, obesity-mediated metabolic complications, cardiovascular, gastrointestinal, kidney, liver, and eye diseases, depression, Alzheimer’s, and other neurodegenerative diseases (García-Niño et al., 2015; Anantharaju et al., 2016).
Resveratrol (RV), a naturally occurring polyphenol (trans-3,4,5-trihydroxystilbene) found in grapes, has anti-inflammatory, anti-tumorigenic, and antioxidant properties that could be used to prevent chronic disease (Ramírez-Garza et al., 2018). Obese animals have been the subject of numerous researches that shown a reduction in age-related symptoms and the prevention of early mortality (Miller et al., 2011), specific age-related characteristics such as impaired glucose metabolism were delayed (Poulsen et al., 2013).
Chlorogenic acid is widely distributed in plants, and it is one of the most common polyphenols in the human diet that has several health-promoting properties (Santana-Gálvez et al., 2017). It has been found that it contains antioxidant, anti-inflammatory, anticancer, antilipidemic, antidiabetic, hypertension, antihypertensive, and anti-neurodegenerative properties (Dhingra and Gahalain, 2016).
Papain is found in papayas that are green in hue. Papain is obtained by removing the peel of immature papaya and extracting the fluid (latex), which is then assembled and dried. Papain helps the immune system fight tumors, Joint and prostate swelling and redness are reduced. Papain reduced indigestion allergies such as leaky gut syndrome (stomach related), hydrochloric acid production, and the ability to tolerate gelatin (intestinal related) etc. (Shouket et al., 2020).
Quercetin (Que) is an antioxidant flavonol that belongs to the flavonoid family. It is most normally found as Que glycoside (David et al., 2016). Quercetin (Que) and its derivatives are phytochemicals that occur naturally and have promising biological properties. Que has been studied extensively for its anti-diabetic, anti-inflammatory, antioxidant, antibacterial, anti- Alzheimer’s, anti-arthritic, cardiovascular, and wound-healing properties, as well as its anticancer effects against various cancer cell lines (Salehi et al., 2020).
Catechins are a type of polyphenol present in many plants and a key component of tea leaves, fruits, and powerful antioxidants (Bae et al., 2020). Among other things, it has anti-cancer, anti-obesity, anti-diabetic, anti-cardiovascular, anti-infectious, hepatoprotective, and neuroprotective properties (Isemura, 2019).
| 2. Different modes of extraction of phytochemicals | ▴Top |
2.1. Percolation method
Coarse particles of plants should be fed into a percolation device and submerged in a suitable solvent for 24–48 hours, after that percolate should be collected at the bottom of the percolation apparatus. At the time of the percolation process, a solvent should be constantly supplied to the tip of the percolation apparatus. Due to persistent concentration differences during the process, it is more efficient than the immersion approach. However, this technique is time-consuming and requires a lot of solvents (De-Silva et al., 2017). To extract Undaria pinnatifida, Zhang et al. (2014) evaluated the percolation and refluxing extraction procedures. They discovered that the percolation extraction approach yielded larger amounts of the principal component, fucoxanthin, than the refluxing method, while there was no significant difference in extract yield between the two methods.
2.2. Maceration method
In a sealed container, the entire powdered material is allowed to come into contact with the solvent for a specified period while being agitated regularly. The solvent is emptied at the end of the procedure, and the leftover miscella is separated from the plant material by pressing or centrifuging. Because the active compounds have not been completely removed, maceration is not a sophisticated procedure (Akuru and Amadi, 2018). Using 50 percent ethanol, a solid–solvent ratio of 1:20, and a particle size of 0.75 mm. Ćujić et al. (2016) obtained high yields of total phenols and total anthocyanins from chokeberry fruit, indicating that maceration was a simple and effective method for extracting phenolic compounds from chokeberry fruit. The most effective method for extracting catechin from Arbutus unedo L. fruits was microwave-assisted extraction (MAE), but a lower temperature was used in maceration with virtually comparable extraction yields, which can be converted into economic benefits (Albuquerque et al., 2017).
2.3. Supercritical fluid extraction method
The supercritical fluid comes into touch with the plant tissues while it is in its supercritical form. It is also known as green technology. The technique uses different solvents as fluid under the pressure of about 200 to 400 bars and temperatures of 40 to 60°C as a form of solvent extraction (Gullón et al., 2020). When a fluid is subjected to biomass, it is under the supercritical condition, which allows it to behave both as a liquid and as a gas. A gas such as carbon dioxide is typically used as the fluid; it is converted to the supercritical stage beyond its critical point under pressure of 7.38 MPa and a temperature of 31 °C (Ding et al., 2020). Under these conditions, the solvent’s polarity declines, and its solubility increases, enabling the fluid to penetrate the cell wall more rapidly. As these bioactive compounds dissolved in the supercritical fluid and were extracted from it as mentioned in Figure 5 (Torres-Ossandón et al., 2018). The major advantage of SFE is that processing can be carried out at near-room temperature while keeping almost all of the active compounds in the product. There is no organic solvent left over after the operation. The product was extremely pure and had a great yield. Furthermore, the procedure was easy and energy-saving (Tyśkiewicz et al., 2018).
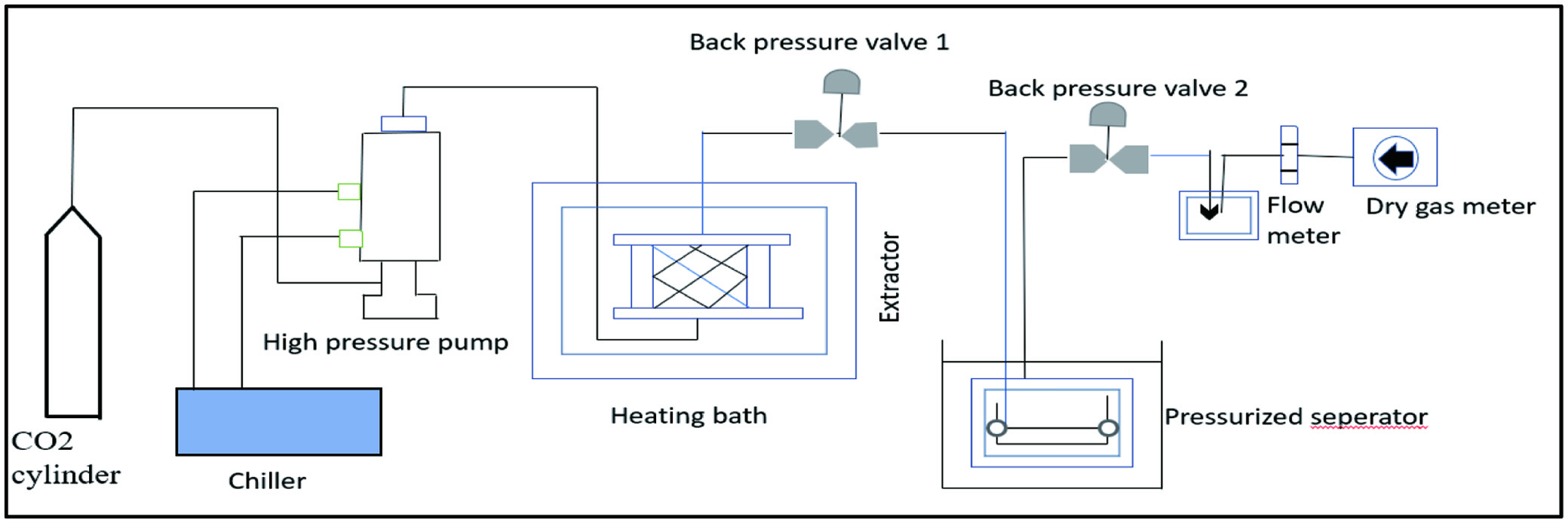 Click for large image | Figure 5. Digramatic representation of supercritical fluid extraction method. |
Using soxhlet extraction, ultrasound-assisted extraction, and SFE with CO2 as solvent and ethanol as cosolvent, Cruz et al., (2017) analyzed and compared the extraction of phenolic compounds from jelly palm seeds. Carbon dioxide, ethanol, and water added to the SFE resulted in a higher extraction yield. At optimum conditions of temperature 52°C at 35.7 MPa (density of CO2: 895 kg/m3) at a constant flow of 30 g CO2/min for 150 min, it was found that SFE was superior to Soxhlet extraction for extracting oil containing fatty acids and phenolic compounds from guava seed. Guava seed oil is found to contain the major fatty acids linoleic acid and oleic acid during extraction. This oil contained cinnamaldehyde, vanillic acid, cinnamic acid, phytosterols, tocopherols, and *-sitosterol as well as phenolic compounds (Narváez-Cuenca et al., 2020).
2.4. Ultrasound-assisted extraction
This new technology is often known as a green technology because of its benefits. The most common cause of extraction is cavitation, which is produced by ultrasonication at frequencies ranging from 20 to 40 kHz. Cavitation is the process of microbubbles forming, enlarging, and collapsing. This cavitation facilitates mass transfer between the liquid extracting fluid and the solid plant materials, which improves the extraction process as shown in Figure 6 (Alirezalu et al., 2020; Ninčević Grassino et al., 2020) The key disadvantage of this technology is that the number of repetitions required to accomplish the extraction process results in significant time and energy waste.
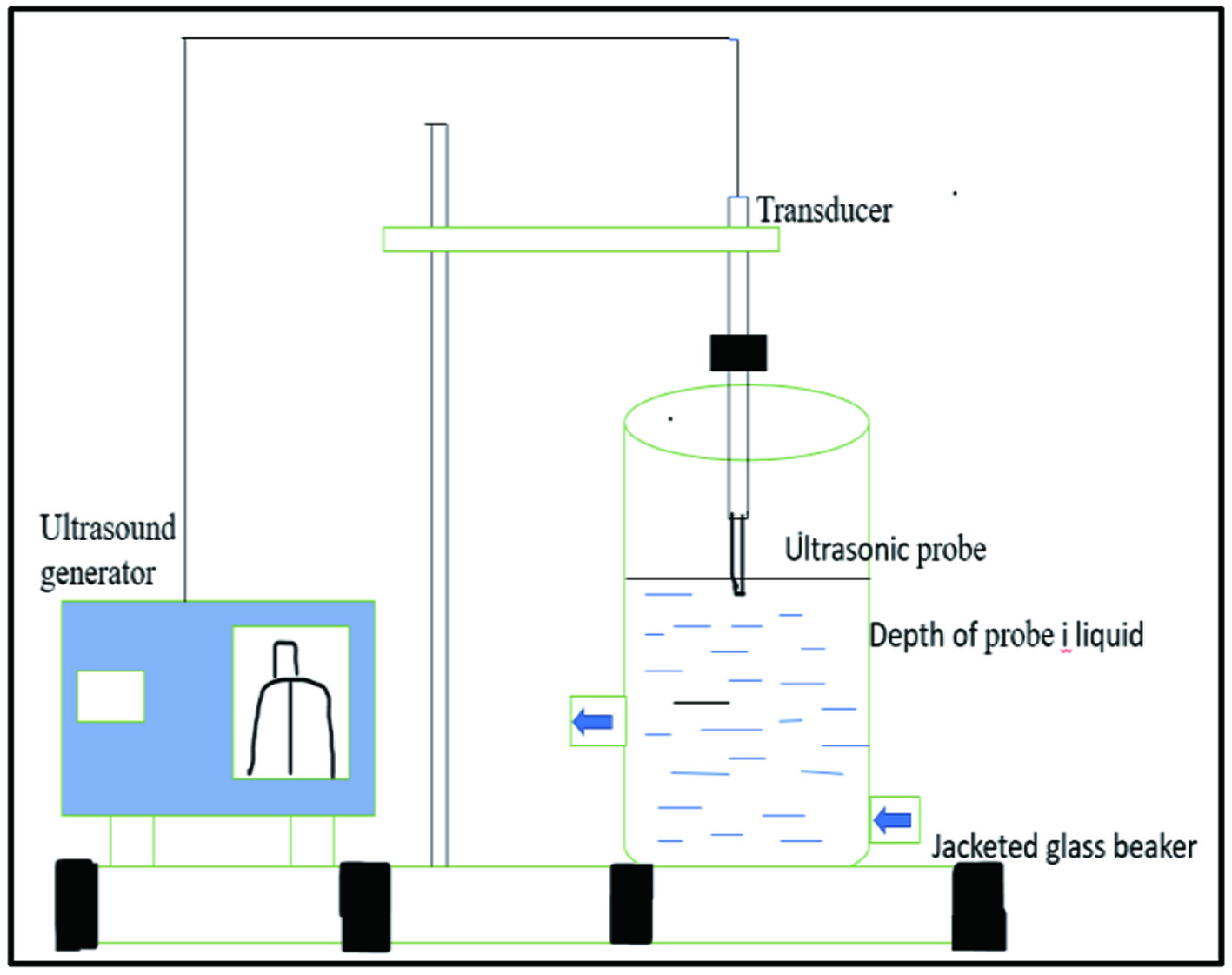 Click for large image | Figure 6. Ultrasound assisted extraction equipment. |
The negative pressure created during UAE generates cavitation, which results in the creation of bubbles that collapse near solid materials. Brittle materials are split due to localised heating caused by the high speed of the liquid jet. When the plant cell is ruptured by turgor pressure caused by the cell wall due to the solvent medium’s entry, the required chemicals are eroded from the plant cell (Chen et al., 2019; Machado et al., 2019)
2.5. Microwave-assisted extraction method
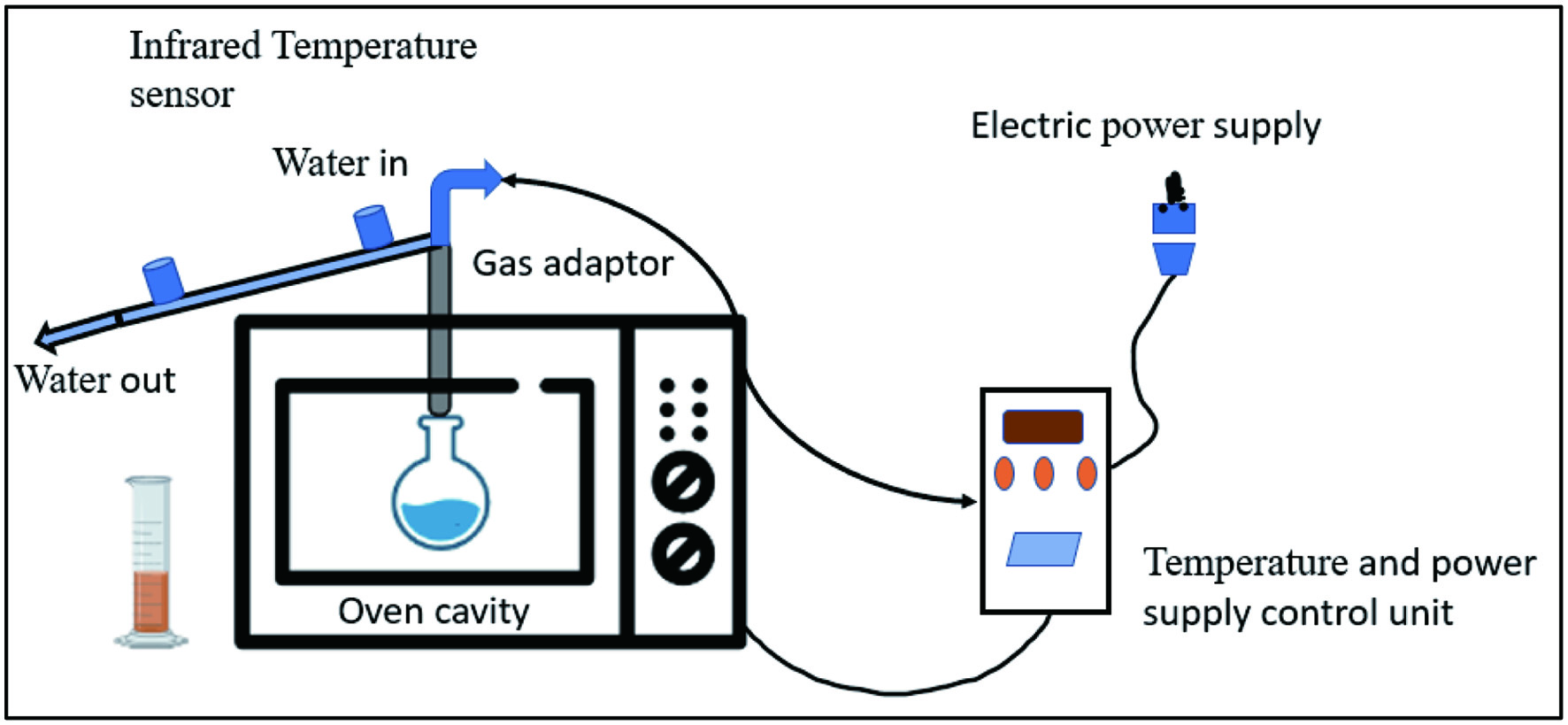
Microwave is a type of electromagnetic wave that has a wavelength of 0.1–100 centimeters and a frequency of 300–300,000 Megahertz, falling between infrared and radio waves. In the presence of microwave radiation, rotation and displacement of ions and molecules cause localized heating from inside to out of the sample as mentioned in Figure 7 (Mena-García et al., 2019). Mass and heat are transferred between cells primarily through dipole rotation and ionic conduction. Water evaporation breaks the bonds between tissues and molecules, leading to the extraction of volatile and non-volatile compounds. The microwave treatment causes the cell wall to rupture, which then allows the bioactive compounds inside the wall to penetrate the cell (Adetunji et al., 2017). As a result of the moisture present in biomass and microwave power, compounds are rapidly extracted from the product (Mena-García et al., 2019) Avocado seeds are agro-industrial waste products that contain antioxidants, phenolic acids, procyanidins dimer B, catechin, and epicatechin. Araújo et al. (2020) found that by utilizing microwave-assisted solvent extraction with two distinct solvents, acetone (72.18 °C and 19.01 min) and ethanol (71.64 °C and 14.69 min) a maximum number of bioactive chemicals could be recovered from avocado seeds. Similarly, watermelon peel has a significant amount of pectin, which may be extracted with higher yields using Microwave assisted extraction (MAE) at 477 W microwave power, 128 Sec irradiation time, pH 1.52, and a solid/liquid ratio of 1:20 g/mL, with distilled water as the solvent (Maran and Priya, 2014). Further, the pineapple peel contains pectin and polyphenolic components, which can be extracted with MAE at 420 W power and 30-minute irradiation duration, as opposed to CE at 60 minutes (Rodsamran and Sothornvit, 2019).
 Click for large image | Figure 7. Schematic representation of extraction by microwave equipment. |
2.6. Enzyme assisted extraction (EAE)
Enzyme-assisted extraction is typically used for bioactive substances that are difficult to extract using traditional methods and are strongly bound in the cell wall. The cell wall is dissolved by enzymes in this method, and the bioactive substances found in the cell plasma flow out of the cell (Nadar et al., 2018) In the extraction process, the enzyme plays an important role. It binds to the active site of the cell wall, which is made up of a polysaccharide–lignin network, and hydrolyzes the polysaccharide and lipid by breaking the bonds of glycoside in the cell wall as well as proteolytic bonds in the middle lamella (Ninčević Grassino et al., 2020). The bioactive compounds found within the cell and middle lamella are thereby extracted via the cell wall. The choice of extracting enzymes is based on the isolation of target molecules that degrade pectin, and polysaccharides or break the cell wall to isolate those (Lombardelli et al., 2020). According to Vasco-Correa and Zapata (2017) pectin extracted from passion fruit peel utilizing the proto-pectinase enzyme yields more than standard chemical extraction. By using a 30 U/ml enzyme at pH 3 at 37 °C, the pectin extraction was increased by nearly 40%. Similarly, in the juice industry pomegranate seed contains a significant proportion of fatty acids (22.9%), protein (13.2%), and dietary fibres (97.2%). When utilizing protease enzyme at a concentration of 50 U/g for 14 hours at 45 °C and pH 7.2, the enzyme-assisted extraction yields a better yield of oil recovery (4%) than the Soxhlet method (Talekar et al., 2018).
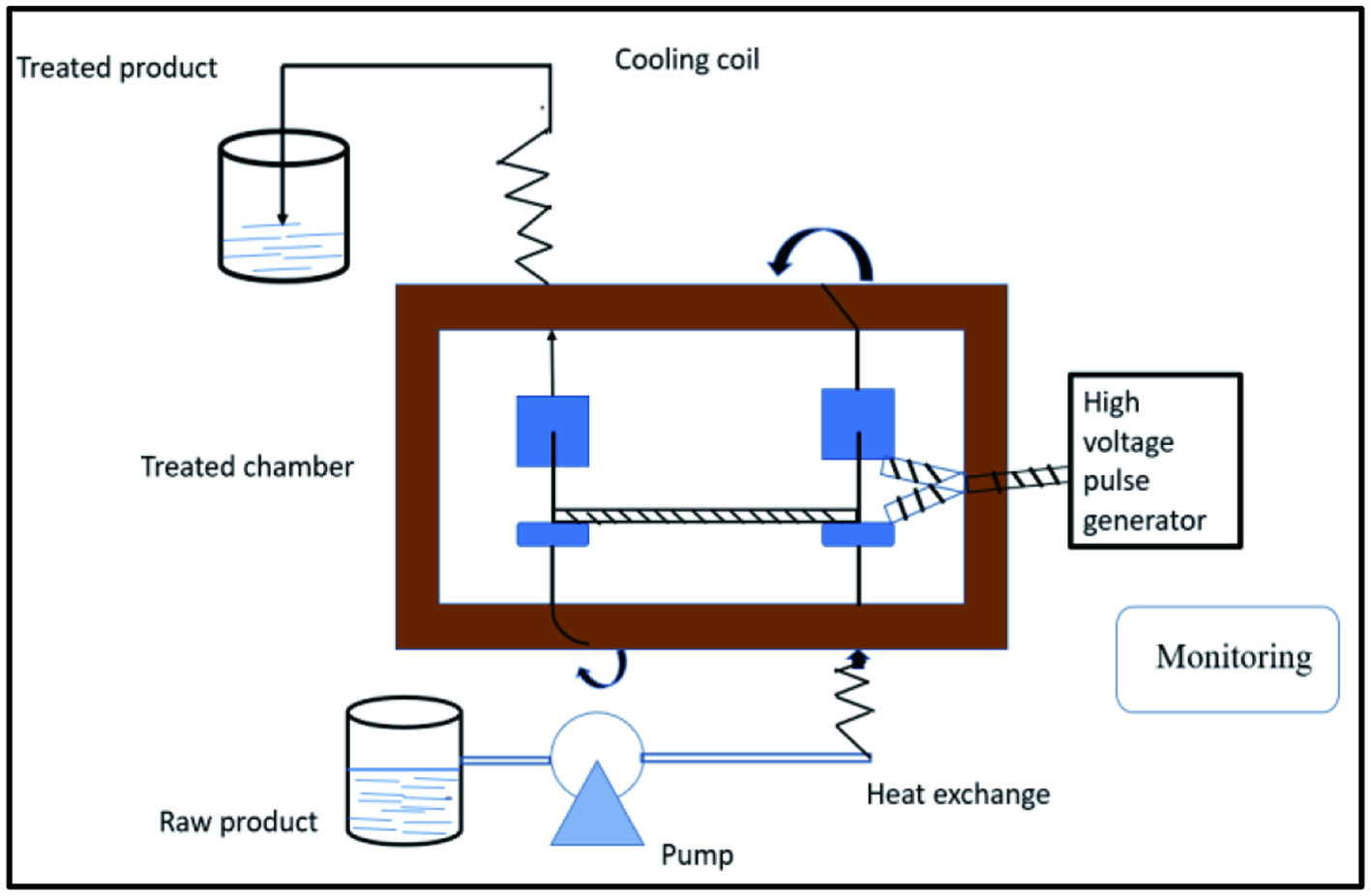
2.7. Pulsed-electric field-assisted extraction (PEFAE)
PEFAE, is pure, has low energy requirements, and has environmentally friendly solvent usage. PEFAE can be regarded as a new extraction technology for bioactive component extraction. Because the natural components are recovered at a low temperature without losing quality or nutritional value, it’s also known as a non-thermal extraction procedure (Figure 8) (Andreou et al., 2020). Pulsed electric field extraction is mostly accomplished using electroporation. PEFAE uses electrical energy to create nano/micro-poration in the cell membrane, allowing bioactive chemicals present in the cell plasma to be extracted (Shorstkii et al., 2020). The electric pulses induce ions and molecules to be transferred from inside the cell to the cell membrane (phospholipids attached molecule), which acts as an insulator (Plazzotta et al., 2021). According to Parniakov et al. (2016), the yield of antioxidants, protein, and carbs from mango peels was highest when PEFAE at intensity 13.3 kV/cm was mixed with aqueous extraction at50 °C for 5 h at pH 6, which was found to be greater than aqueous extraction at 60 °C and pH 6. However, when PEFAE or aqueous extraction were used independently, and the yield was significantly lower. Similarly, Luengo et al. (2013) reported that extracting polyphenols and flavonoid components from orange peel using an electric field of 7 kV/cm strength and a pulse rate of 20 in 60 microseconds was successful. In a study, the phenolic components in apple peels were extracted using a pulsed electric field with various electric intensities and periods. The extraction is examined using confocal laser scanning microscopy and electrical conductivity (disintegration index) (microscopic cell disintegration index). Results showed that the extraction was reliant on the cell integration index and the electric field strength. The researchers also discovered that the higher the intensity (1,200 V/cm) at a constant cell integration constant, the better the soluble matter recovery (Wang et al., 2020).
 Click for large image | Figure 8. Representation of pulse electric equipment. |
The chemical based testing and advanced techniques for determination of phytochemicals have been discussed in brief in Tables 3 and 4 respectively.
 Click to view | Table 3. Testing for the determination of phytochemicals |
 Click to view | Table 4. : Advanced techniques for determination of phytochemicals |
| 3. Conclusion | ▴Top |
Fruits are high in bioactive components because they provide additional health benefits beyond basic nutrition and also help to prevent disease. Nutraceuticals and phytochemicals play a significant part in health enhancement and prompted a rise in global interest. Many publications mentioned the benefits of phytochemicals with regards to microbial outbreak. Moreover, it is also beneficial for human health. There are various methods of extraction of phytochemicals present in the fruits. For the extraction process, several kinds of solvents and temperatures are used like solvent extraction method, maceration method, etc. By extraction and using the different methods of determination like a test of phenols, alkaloids, and tannins it is easy to estimate the phytochemicals present in the fruits and their health benefits. However, improved approaches can be utilized to discover them qualitatively and quantitatively at the same time without the need for multiple separate experiments.
Acknowledgments
The authors would like to thank scientists and researchers whose work has been cited in the manuscript.
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |