| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 19, September 2022, pages 4-96
Novel marine bioactives: application in functional foods, nutraceuticals, and pharmaceuticals
Fereidoon Shahidi*, Sarusha Santhiravel
Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1C 5S7
*Corresponding author: Fereidoon Shahidi, Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1C 5S7. E-mail: fshahidi@mun.ca
DOI: 10.31665/JFB.2022.18316
Received: September 12, 2022
Revised received & accepted: September 28, 2022
| Abstract | ▴Top |
Functional food, nutraceutical, and pharmaceutical applications of natural products have gained growing attention as there is increasingly awareness of the association between bioactive compounds and improved health. Recently, food and biomedical scientists have focused more on marine resources to isolate bioactives since the marine ecosystem comprises unexploited resources with a wide range of organisms. Marine species produce a wide range of natural products, such as polysaccharides, peptides, polyunsaturated lipids, phenolic compounds, and pigments, with unique structures and diverse biological activities due to their extreme living environments. These active molecules reduce the risk of chronic diseases and improve health by exhibiting antioxidant, anticancer, anti-inflammatory, antimicrobial, antidiabetic, anti-obesity, antihypertensive, cardioprotective, and neuroprotective activities. This review summarizes the recent discoveries of bioactive compounds from marine invertebrates (sponges, cnidarians, echinoderms, molluscs, ascidians, and crustaceans), fishes, seaweeds, and marine microorganisms and their potential for functional foods, nutraceuticals, and pharmaceuticals applications.
Keywords: Marine sources; Bioactive compounds; Functional foods; Nutraceutical; Pharmaceutical; Biological activities
| 1. Introduction | ▴Top |
Recently, diet-related chronic diseases, like obesity, diabetes, cancer, hypertension, heart diseases, hyperlipidemia, and neurodegenerative disorders, have become a severe problem for the human population in developed and developing countries. The driving factors for the increasing rates of these disorders are changes in the environment, lifestyle, and dietary habits of humans as a result of globalization and industrialization (Paudel et al., 2019a). However, these diet-related issues could be addressed by developing functional foods and nutraceuticals by incorporating dietary bioactive compounds. The concept of the close association between diet and human health was originated several centuries ago. In the 1980s, the word “functional food” was first emerged in Japan to describe the novel food products developed through fortification with unique components with physiological benefits (Hamed et al., 2015). In 1989, Stephen DeFelice introduced the term “nutraceutical” by relating the words “nutrition” and “pharmaceutical” (Lobine et al., 2021).
According to Shahidi (2004), functional foods are defined as those that improve health condition beyond the basic nutritional value of conventional foods with a similar appearance to traditional foods and are consumed as a part of the usual diet, while nutraceuticals are those that provide protection against chronic diseases and other physiological benefits, which are produced from the compounds derived from foods but found in the medicinal forms of tablets, capsules, powder, solution, or portion, but if not necessarily derived from food, they are referred to as natural health products. Functional foods are developed either by raising the level of constituents (e.g., macronutrients, phytochemicals, dietary fibers, vitamins, minerals, etc.) that promote health or by decreasing the level of constituents (e.g., salt, saturated fat, sugar, etc.) related to adverse health conditions (Hosseini et al., 2022). Currently, functional foods and nutraceuticals have become the key focus in developing novel food products due to their effect on health improvement.
In the past years, food manufacturing, pharmaceutical, and cosmeceutical industries have demonstrated a growing interest in the natural sources of bioactive compounds with powerful health-promoting effects since those constituents could be used as an alternative for possibly harmful synthetic components (Šimat et al., 2020). Besides, modern consumers have become more health-conscious, resulting in their increased awareness of bioactive ingredients, dietary supplements, functional foods, and nutraceuticals. Terrestrial and marine-based animals, plants, and microorganisms are considered natural sources of bioactive or functional ingredients. These novel molecules are gaining more importance with the advancement in the field of biomedical, food science, and health related studies. Moreover, functional foods and nutraceuticals could delay the onset of certain disorders, improve the general physical and mental health condition of humans, and occasionally treat or cure some diseases (osteoporosis and cardiovascular disease) (Siró et al., 2008). In 2019, the global functional food market value was around US$ 177.77 billion, and it is expected to increase up to US$ 267.92 billion by 2027 (Statistica, 2022).
Over the past few years, the screening and extraction of bioactive molecules from marine resources has been a significant interest among food and biomedical scientists and nutritionists. Marine-derived constituents such as polysaccharides, proteins, lipids, and phytochemicals have received an incredibly increasing attention for functional food, nutraceutical, and therapeutic applications owing to their promising health promoting effects (Pangestuti and Arifin, 2018; Shahidi and Ambigaipalan, 2015). Around 71% of the surface area of our planet is occupied by oceans (Duan et al., 2018), and marine flora and fauna comprise nearly half of the global biodiversity. Generally, the terrestrial environment is the richest source of most bioactive molecules; however, there is a massive threat of the extinction of terrestrial species, and the discovery of new metabolites from them is gradually diminishing (Jensen and Fenical, 2000). In this regard, researchers started to target more unexplored and underutilized marine sources for natural products since marine ecosystem encompasses enormous biodiversity of creatures with the potential to produce biologically active metabolites.
The discovery of the therapeutic potential of marine sources goes back several centuries. The “father of modern medicine”, Hippocrates, demonstrated the medicinal properties of different marine invertebrates and their products on human health (Voultsiadou, 2010). Moreover, many countries have used marine-based natural compounds in traditional medicine since ancient times (Yuan et al., 2016). Compared to terrestrial species, marine organisms synthesize abundance of unique bioactive metabolites as they live in a diverse and extreme habitat, including varying salinity, pressure, temperature, oxygen concentration, nutrients, and illumination (Hosseini et al., 2022; Lordan et al., 2011). In this sense, the marine ecosystem is believed as a goldmine of bioactive natural products with promising applicability in functional foods, nutraceutical, and pharmaceutical industries. Besides, by-products and waste materials of the seafood industry can also be utilized to isolate active food ingredients (Durand et al., 2020).
Marine invertebrates, seaweeds, fishes, and microorganisms are identified as crucial marine organisms that produce their unique groups of biomolecules such as bioactive proteins and peptides, polysaccharides, polyunsaturated fatty acids, vitamins, minerals, pigments, and polyphenols (Lobine et al., 2021). These components are generally extracted from different parts of marine organisms: internal organs, skin, muscle, and bones. The biological activities exhibited by these compounds include antimicrobial, antioxidant, anti-diabetic, anticancer, lipid-lowering, neuroprotective, anti-obesity, wound healing, sleep-enhancing, and skin protection activities (Hosseini et al., 2022; Ghosh et al., 2022). Numerous studies have been conducted to discover new bioactive molecules from marine resources. For instance, more than 400 studies were conducted each year from 2015 to 2020 (Carroll et al., 2022, 2021, 2019; Blunt et al., 2018). Figure 1 shows the total number of papers published each year from 2010 to 2020 related to the exploration of marine-based novel natural components. However, there is a need to comprehensively review the recent studies on the bioactive compounds obtained from marine resources and their potential in functional food, nutraceutical, and therapeutic applications. Therefore, this review mainly considers aspects related to the biological activities of different natural compounds isolated from marine invertebrates, fish, seaweeds, and marine microorganisms and their importance in functional foods, nutraceutical, and pharmaceutical industries.
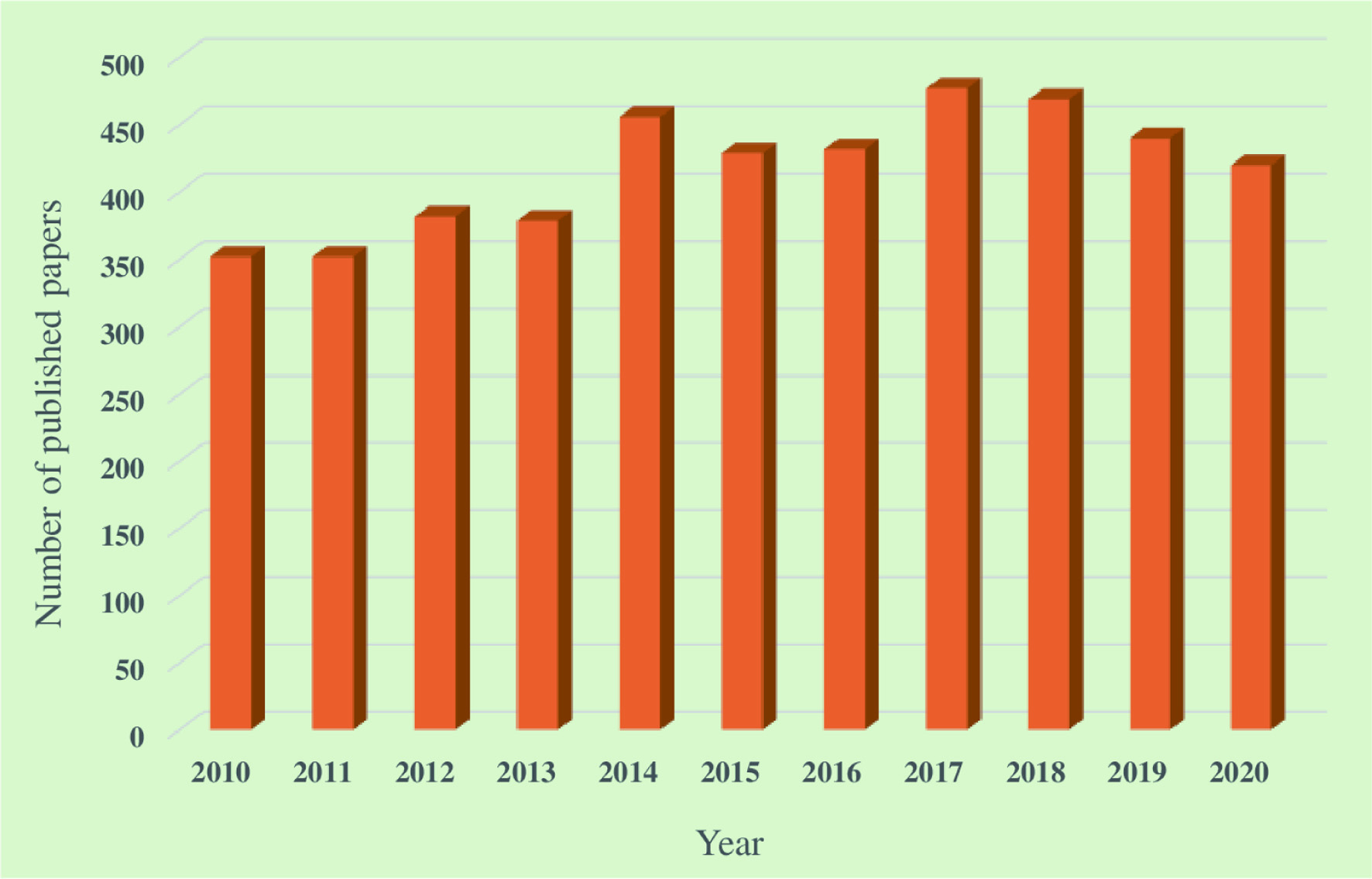 Click for large image | Figure 1. Total number of papers published in each year from 2010 to 2020 related to the exploration of marine-based novel natural components (Carroll et al., 2022, 2021, 2019; Blunt et al., 2018, 2017, 2016, 2015, 2014, 2013, 2012). |
| 2. Different classes of bioactive compounds derived from marine resources | ▴Top |
Bioactive compounds, obtained from either natural or synthetic sources, are coupled with a wide array of health benefits. Due to their potential for therapeutic applications, research interest has been increased in the search for novel bioactive compounds (Atanasov et al., 2021). Marine ecosystem is an outstanding source of biomolecules, including bioactive peptides, polyunsaturated fatty acids (PUFAs), polysaccharides, enzymes, polyphenols, pigments, collagen, gelatin, vitamins, and minerals with diverse biological activities (Barrow and Shahidi, 2007). The diversity and composition of bioactive metabolites produced by marine organisms vary with the species, season, temperature, and geographical location. The total number of new marine-based natural compounds identified each year has steadily increased from 332 in 1984 to 1,407 in 2020 (Carroll et al., 2022; Blunt et al., 2016). Figure 2 illustrates the trend of total number of novel marine-based natural compounds identified from 2001 to 2020. According to Figure 2, there was a 77% increase in the number of novel compounds in 2020 (1,407 compounds) compared to 2001 (793 compounds).
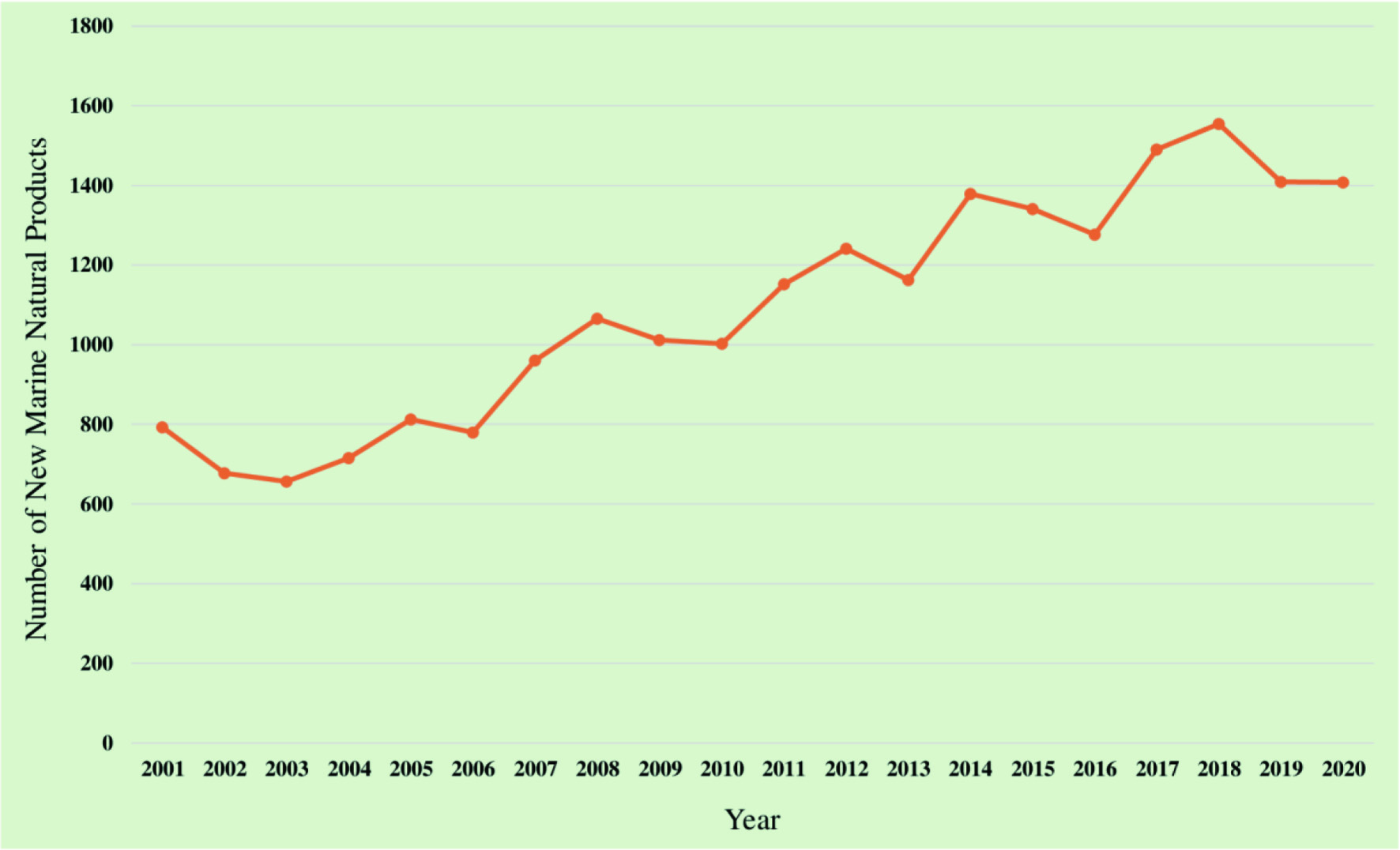 Click for large image | Figure 2. Trend of total number of novel marine-based natural compounds identified from 2001 to 2020 (Carroll et al., 2022, 2021, 2019; Blunt et al., 2018, 2017, 2016, 2015, 2014, 2013, 2012, 2011, 2009, 2008, 2007, 2005, 2004, 2003). |
The synthesis of thousands of bioactive metabolites by marine organisms is driven by several factors (Figure 3). The marine ecosystem is characterized by its harsh environmental conditions. Pressure and temperature of the ocean range from 1 to over 1,000 atm and from −1.5°C in the Antarctic zones to 350°C in the deep hydrothermal zones, respectively. The nutritional content of the marine environment varied from nutrient-spar to nutrient-rich, and the illumination of the ocean varied from extensive photic zones to complete darkness (Costa-Lotufo et al., 2009). Therefore, marine species have developed a broad spectrum of natural products as chemical weapons to withstand these extreme chemical and physical conditions. In addition, marine organisms possess specific biosynthetic mechanisms to produce structurally and chemically unique bioactive secondary metabolites to protect them from the pressure given by predators, competitors, and pathogens (Petersen et al., 2020; de Carvalho and Fernandes, 2010). The following section of this review discusses the physicochemical properties and biological activities of different bioactive metabolites.
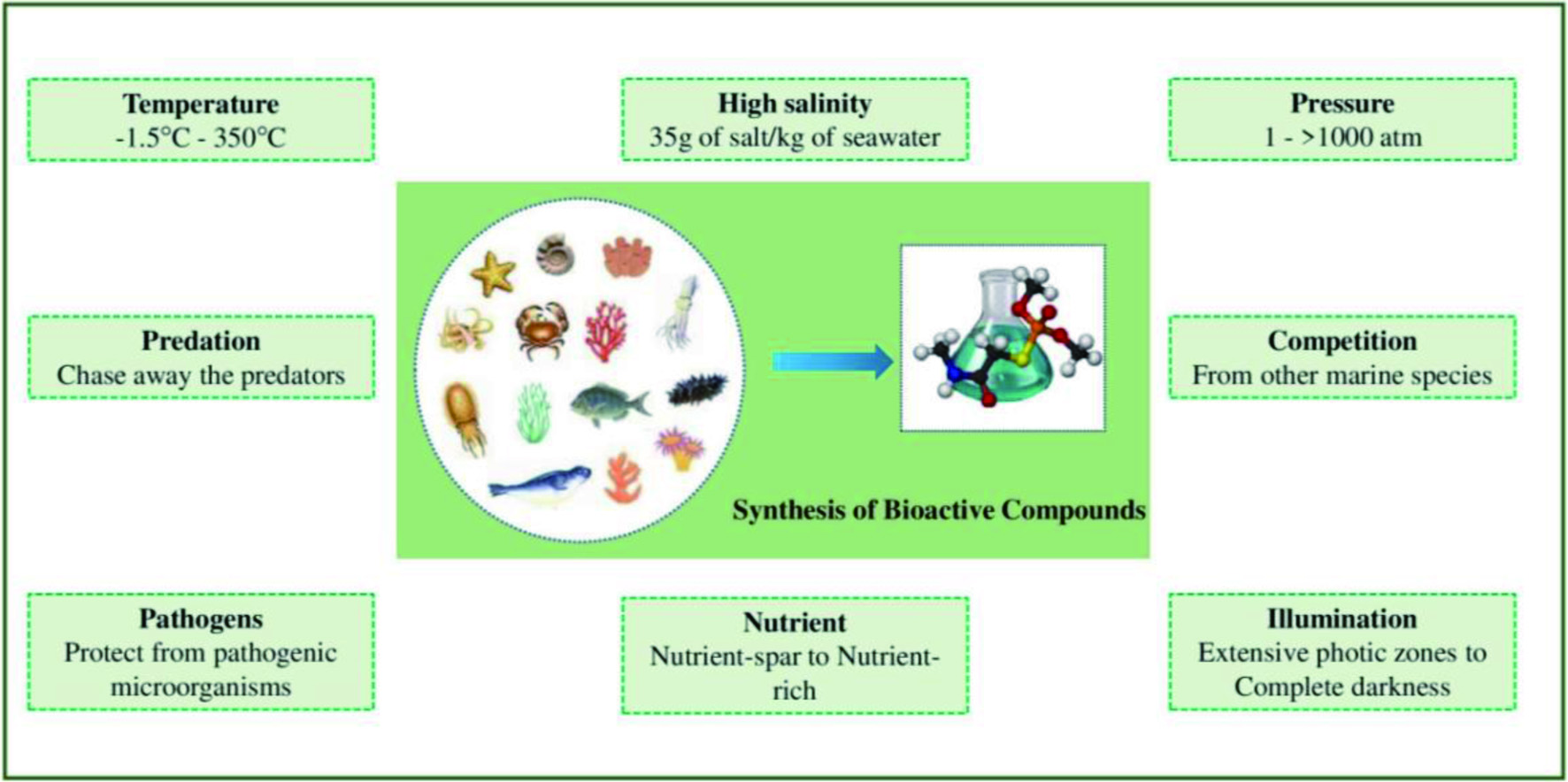 Click for large image | Figure 3. Factors contributing to the synthesis of diverse bioactive metabolites by marine species. |
2.1. Lipids
Marine sources are the key target for lipid extraction due to their unique lipid composition compared to terrestrial sources. The fatty acid composition of marine species is typically characterized by a relatively large proportion of PUFAs, substantial amount of monounsaturated fatty acids, and a lower content of saturated fatty acids (Larsen et al., 2011). There are two classes of PUFAs such as ω3 and ω6, which are distinguished by the position of their first double bond located from the methyl terminal of the fatty acids (Yu and Gu, 2015).
2.1.1. Omega 3 polyunsaturated fatty acids (ω3 PUFA)
Long chain ω3 PUFAs are mainly responsible for the health benefits exhibited by the lipids isolated from marine sources (Zheng et al., 2013). Indeed, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the two crucial active components of marine-derived ω3 PUFAs. The ω3 fatty acids have their first double in the third carbon from the methyl terminal of the fatty acid molecule. Both EPA and DHA are essential fatty acids for humans since they are unable to synthesize PUFA with more than 18 carbons. Thereby, humans need to obtain these PUFAs from seafood or supplements. Fish and other marine mammals are also incapable of synthesizing EPA and DHA. They acquire ω3 PUFAs that are initially synthesized by both unicellular and multicellular marine flora, namely algae and phytoplankton, through the food web and accumulate them in their body as the predominant lipid fractions (Shahidi and Ambigaipalan, 2015).
The significant sources of ω3 PUFAs are fish (salmon, sardines, tuna, herrings, anchovies, etc.), marine mammals, fungi, krill, microalgae, and macroalgae (Suleria et al., 2015). Along with EPA and DHA, seal blubber oil contains docosapentaenoic acid (DPA), which is present at minimal levels in fish oils. The chemical structure of EPA, DHA, and DPA are illustrated in Figure 4. Several epidemiological studies show that consumption of marine based PUFAs is linked to the reduced occurrence of certain chronic ailments such as diabetes, cardiovascular diseases, cancer, hypertension, obesity, and renal, neurodegenerative, and autoimmune diseases, primarily owing to their antithrombotic, anti-inflammatory, and anti-atherogenic properties (Jamshidi et al., 2020; Naqshbandi et al., 2012). For instance, a regular intake of 250 mg/day of EPA and DHA is recommended to prevent cardiovascular diseases (Kris-Etherton et al., 2009). According to Food and Drug Administration (FDA), taking 3 g/day of EPA and/or DHA is generally recognized as safe (GRAS). Besides, consumption of ω3 PUFAs is beneficial for mental health and functioning of the brain, visual, and nervous systems. According to in vitro and animal studies, cardiovascular health, blood lipid profiles, cell signaling cascades, eicosanoid (inflammatory mediators) biosynthesis, gene expression, and membrane lipid composition are influenced by the ingestion of ω3 PUFAs (Shahidi and Ambigaipalan, 2015).
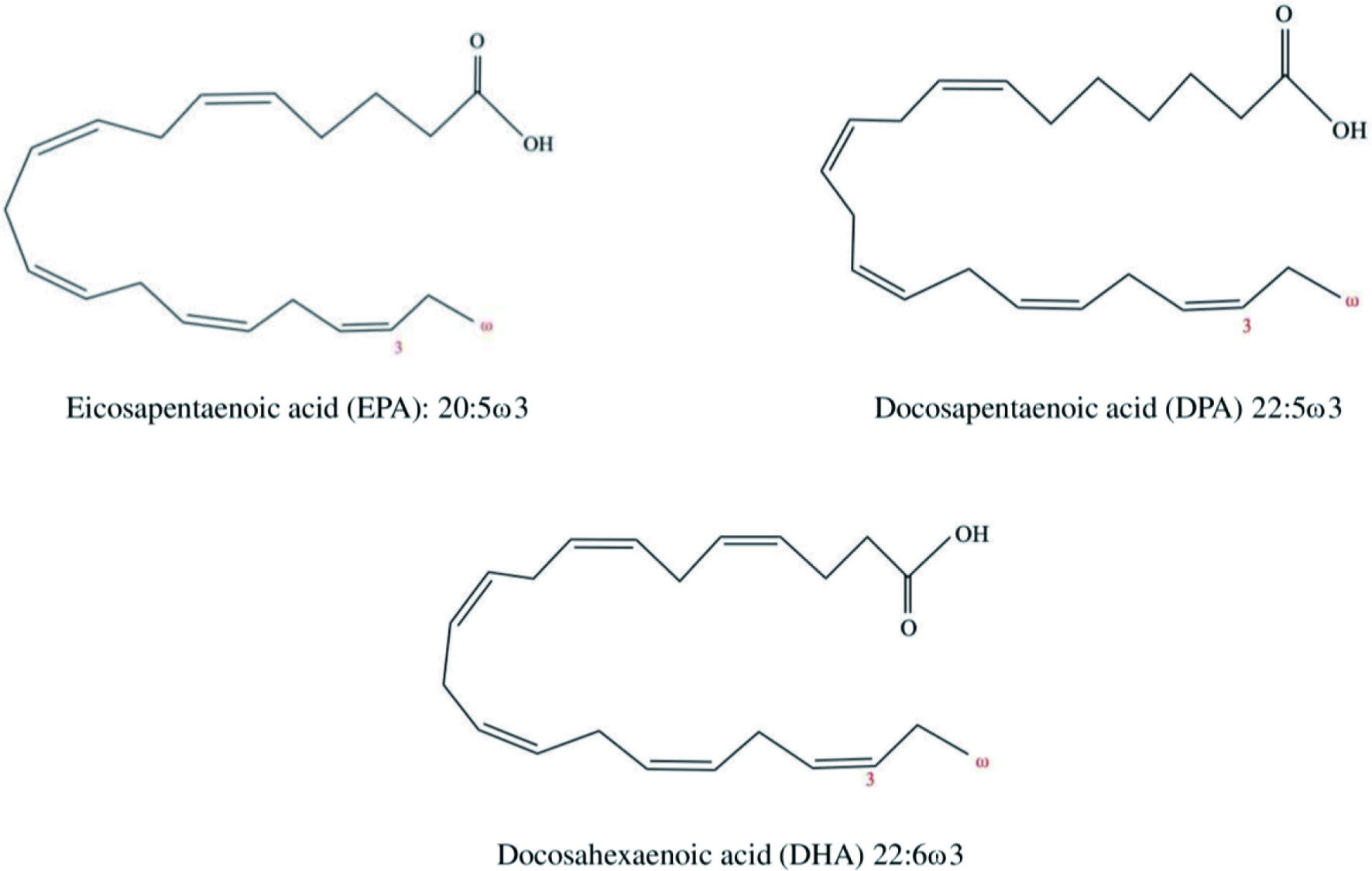 Click for large image | Figure 4. Chemical structures of EPA, DHA, and DPA. |
Humans consume marine-derived ω3 PUFAs either by directly eating seafood or by taking supplements in the form of tablets, powders, or capsules (Vestland et al., 2016; Das et al., 2009). It has been shown that consuming fish oil capsules containing with EPA and DHA reduces the risk of cardiovascular diseases by lowering the blood triacylglycerols and low-density cholesterol, while minimizing other contributing factors such as dyslipidemia, hypertension, and heart disorders (Jamshidi et al., 2020; Šimat et al., 2020). Naturally, breast milk contains DHA, which is necessary for an infant’s eye and brain development (Hoffman et al., 2009). It was reported that anti-aging effects and oxidative stress regulation of ω3 PUFAs from fish oil are due to the enhancement of superoxide dismutase (SOD) action in the heart and liver by the ω3 PUFAs (Zhang et al., 2016b). Moreover, the risk of inflammatory diseases (e.g., rheumatoid arthritis) was reduced by consuming ω3 PUFAs by lowering the levels of pro-inflammatory cytokines and C-reactive protein (Borges et al., 2017; Pipingas et al., 2015). However, PUFAs have somewhat limited utilization in the functional food industries as they are highly prone to oxidation. Thereby, they have generally been used along with other antioxidants to extend their shelf life.
2.2. Polysaccharides
Most marine natural carbohydrates are polysaccharides, while monosaccharides or oligosaccharides are present in lesser amounts. Polysaccharides are biological macromolecules or are sugar polymers found in different species of seaweeds, crustaceans, and other marine organisms with varying degrees of sulfation (Sharanagat et al., 2020). Traditionally, polysaccharides purified from marine resources, particularly algal polysaccharides, are used as stabilizers, emulsifiers, and texture modifiers in the food, beverage, pharmaceutical, and cosmetics productions. More recently, these polysaccharides were recognized as health-promoting functional ingredients due to their diverse biological effects, mainly antimicrobial, anticancer, antioxidant, anti-inflammatory, anticoagulant, antidiabetic, and immunomodulating properties (Fernando et al., 2019; Sanjeewa et al., 2018). Their antioxidant property is due to their ability to scavenge reactive oxygen species (ROS) (Suleria et al., 2016). However, these bioactivities are dependent on their molecular weight, hydrophilicity, bond and monomer type, charge density, and branching (Fernando et al., 2019). Chitin and chitosan, alginates, fucoidan, carrageenan, laminarin, glycosaminoglycans, fucans/fucanoids, and agar are the important polysaccharides obtained from marine sources (Sanjeewa et al., 2018; Šimat et al., 2020).
Generally, marine polysaccharides are not digested in the upper gastrointestinal tract but undergo fermentation in the lower region. Therefore, they could be used as prebiotics and dietary fiber (Shang et al., 2018). Sulfated polysaccharides, especially sulfated galactans (carrageenan) and sulfated fucans (fucoidan), are the major polysaccharides extracted from marine invertebrates and seaweeds (Sanjeewa et al., 2018). In addition to polysaccharides, there is also a disaccharide named trehalose present in shrimps and seaweeds. It controls the Nrf2 and insulin signaling pathway, thus exhibiting antiaging activity (Wang et al., 2021).
2.2.1. Chitin, chitosan, and their oligosaccharides
Chitin is one of the crucial polysaccharides widely present in different marine sources, including the exoskeleton of shrimps, crabs, and lobster, and the cell wall of seaweeds, fungi, and protozoa (Hu et al., 2016). It is a natural amino polysaccharide that is made up of (1→4)-linked N-acetyl-β-D-glucosamine monomers (Duan et al., 2018). Partial deacetylation of chitin results in several derivatives, such as chitosan, chitosan oligosaccharides (COS), and glucosamine (Shahidi and Ambigaipalan, 2015; Kaur and Dhillon, 2015). These derivates are characterized by their unique structures and diverse functional and biological properties. There has been a growing interest in chitin and chitosan derivatives for food, pharmaceutical, biomedical, and other industrial applications in recent years (Shamshina et al., 2019). Around 150 thousand tons of commercial chitosan were produced annually from chitosan obtained as a seafood processing by-product (Liaqat and Eltem, 2018).
Chitin and chitosan polymers are used as pharmaceutical ingredients for drug delivery and biomaterial for wound healing and tissue engineering. Moreover, their physicochemical and functional properties, including non-toxicity, biocompatibility, and biodegradability, make them a potential ingredient in several industries (Gurpilhares et al., 2019). For instance, they are utilized to manufacture different functional materials such as nanoparticles, nanofibers, membranes, scaffolds, films, gels, and sponges (Duan et al., 2018). These chitin-derived films and nanofibers are used in the packaging industry as eco-friendly packaging materials due to their biodegradability and better thermal, barrier, and mechanical properties (Hai et al., 2020). Compared to the fully acetylated insoluble form of chitin, derivatives of chitosan are efficiently utilized as nutraceutical agents due to their solubility in water (Shahidi and Ambigaipalan, 2015).
The bioactivities of chitosan and its derivatives include hypocholesterolemic, antibacterial, antioxidant, and antidiabetic properties. It has been proven that the levels of low-density lipoprotein (LDL), total cholesterol, and liver triacylglycerols are reduced by the chitosan with low molecular weight in high-fat diet-fed rats (Zhang et al., 2012b). Ardekani-Zadeh and Hosseini (2019) indicated that nanofiber mats, made up of chitosan, poly(ε-caprolactone), and oregano oil (5%), exhibited a strong antibacterial effect on Gram-positive (Listeria monocytogenes and Staphylococcus aureus) and Gram-negative (E. coli and Salmonella enteritidis) bacteria. Recently, it was observed that the viability of prebiotics Bifidobacterium animalis subsp. lactis Bb12 was enhanced by chitosan/ polyvinyl alcohol mats (Mojaveri et al., 2020). The polycationic nature of this biopolymer (due to the presence of NH3+ groups) is the reason for the antimicrobial property of chitin and chitosan derivatives. Therefore, its antimicrobial activity increases with the number of positively charged amino groups. Cell death occurs in microorganisms as a result of leakage of cellular substances caused by the interaction of negatively charged surface components with the NH3+ groups of the chitosan (Ma et al., 2017). These positively charge groups are able to interact with both teichoic acid in Gram-positive bacteria and lipopolysaccharides in Gram-negative bacteria (Raafat et al., 2008).
The average molecular weight of chitosan oligosaccharide (COS) is less than 3.9 kDa with 2 to 20 monomeric units. COS possesses bioactivities, such as easy absorption properties, and good water solubility due to its low molecular weight (Liaqat and Eltem, 2018). Several studies have shown that COS exhibits anticancer, antioxidant, antibacterial, and immunoenhancing activities (Kuroiwa et al., 2009). For instance, the human lung A549 cancer cell proliferation was decreased by the treatment with aminoethyl-COS due to stimulating cell apoptosis via up-regulation of caspase-3 and -9 and down-regulation of Bcl-2 (Ngo et al., 2019). Zhao et al. (2009) revealed the antitumor activity of COS against human cervical cancer cells was through autophagic and apoptotic pathways. Kumar et al. (2009) reported that lipid profiles, blood glucose, weight gain, and diet intake could effectively be controlled by COS in the insulin-resistant model of the genetically modified ob/ob model. Moreover, COS shows radical scavenging activity in human fibrosarcoma cells (HT1080) and inhibits oxidative damage to DNA by human lymphoma U937 (Shahidi and Ambigaipalan, 2015). COS can potentially act as a calcium-binding agent, thus increasing calcium solubility (Zhu et al., 2020). Thereby, low molecular weight COS could be utilized as nutraceuticals or food supplements.
2.3. Proteins and peptides
2.3.1. Proteins
Proteins are complex biopolymers consisting of more than 20 different amino acids linked via α-peptide bonds and coded by genetic code. Seafood has been regarded as a rich source of protein containing the correct proportion of all essential amino acids needed for humans. Marine species, including crustaceans, seaweeds, molluscs, marine mammals, and fish (e.g., herring, pollock, tuna, cod, hake, trout, and haddock) and their by-products produce significant amount of proteins (Suleria et al., 2015). The protein from seaweeds is identified as a high-quality protein with better nutritional properties in comparison to terrestrial proteins (Khalid et al., 2019). Besides their vital role as a nutrient, proteins possess several properties in the biological and food systems. In the biological systems, they act as antibodies (immunoglobins), transport proteins (hemoglobin, transferrin, etc.), enzymes, and hormones (growth factors, insulin, etc.) (Goodband, 2002). In the food systems, they play a key role in emulsification, anticoagulation, antioxidant, antimicrobial, and gel, foam, and film formation. For example, protamine, a marine-derived protein, is utilized as a natural antibacterial agent in the food industry (Suleria et al., 2015).
Moreover, proteins from marine sources play a significant role in functional foods and pharmaceutical applications to lower the risk of chronic diseases due to their diverse range of bioactivities. Examples of biological properties are anticancer, anti-inflammatory, immune-enhancing, antimicrobial, and antioxidant, among others (Šimat et al., 2020). Recently, a protein, chondrosin, extracted from marine sponge Chondrosia reniformis was reported as a novel cytotoxic protein (Scarfì et al., 2020).
2.3.2. Peptides
In recent years, there has been an immense interest in the extraction of bioactive peptides from marine sources and the analysis of their composition, structure, and bioactivities. Bioactive peptides are molecules with short-chain amino acid sequences, usually 3 to 20 amino acids linked via peptide bonds, produced from proteins during processing (chemical/enzymatic hydrolysis, fermentation, or cooking) and digestion (Ramezanzade et al., 2017; Ibañez et al., 2012). Extraction methods of bioactive peptides are shown in Figure 5. These bioactive peptides are isolated from several marine organisms, including fish species, seaweeds, crustaceans, sponges, cyanobacteria, bryozoans, tunicates, and marine mammals, among others. Bioactive peptides have been reported to exhibit numerous physiological functions in the human body, such as antioxidant, anticancer, antihypertension, antimicrobial, antithrombotic, antiparasitic, cardioprotective, and immunomodulation activities (Šimat et al., 2020; Hamed et al., 2015; Shahidi and Zhong, 2008). Therefore, these bioactive peptides can be potentially used as functional food ingredients, nutraceuticals, and pharmaceuticals.
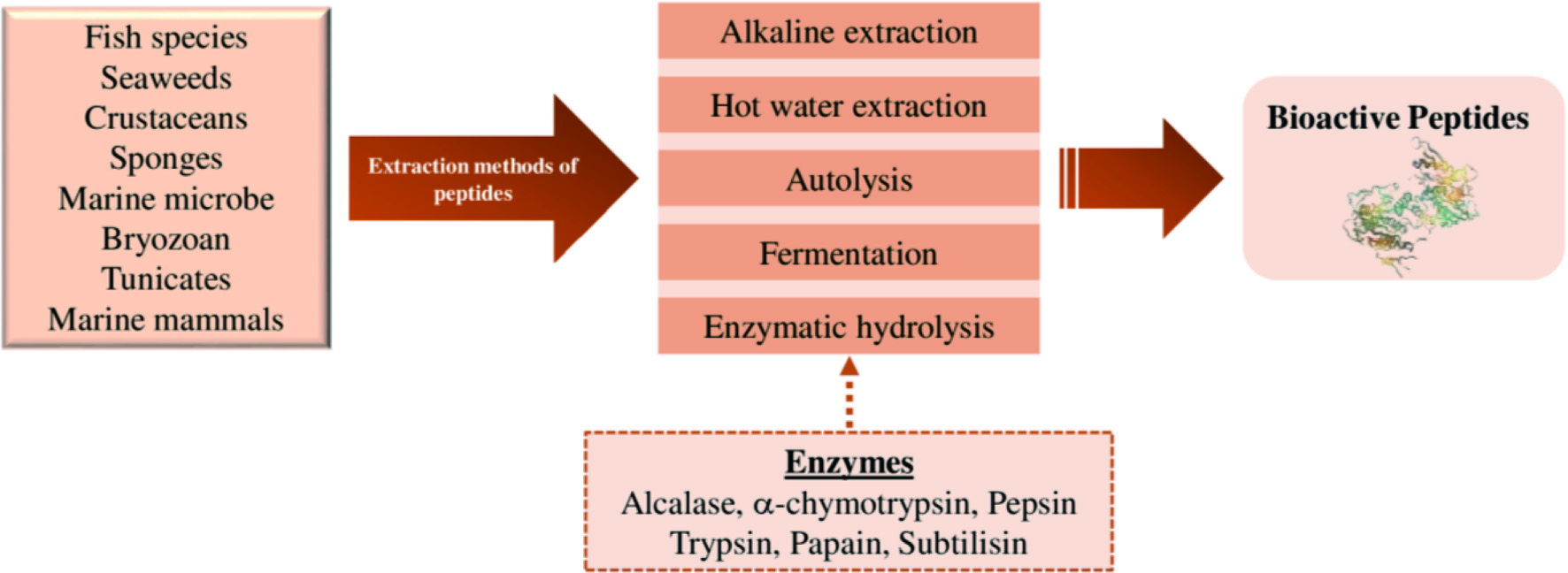 Click for large image | Figure 5. Different extraction methods of bioactive peptides. |
These biological activities are influenced by the amino acid composition (type, number, and sequence of amino acid) and molecular characteristics (charge, shape, size, and hydrophobicity) of peptide molecules (Najafian and Babji, 2012; Harnedy and FitzGerald, 2012). To illustrate, peptides with low molecular weights can easily permeate through the intestinal wall; as a result, the potential bioactivity is improved. Antihypertensive peptides inhibit the activity of the angiotensin-I converting enzyme (ACE) or renin, thereby regulating the blood pressure in humans. Antidiabetic peptides have specific amino acid sequences exhibiting dipeptidyl peptidase-4 (DPP-IV) inhibitory activity with the potential of treating type II diabetes (Shahidi and Zhong, 2008; Hu et al., 2019). Antimicrobial peptides (AMP) are fundamental components of the innate immune system and act as natural antibiotics in marine species protecting against pathogenic microorganisms. Generally, AMP has a relatively high proportion of hydrophobic amino acid residues with a net positive charge, facilitating their passage through the cell membranes of microorganisms and destroying them (Semreen et al., 2018). These bioactive marine peptides exhibit robust antimicrobial activity against Gram-positive bacteria (Streptococcus mutans and Bacillus subtilis) compared to Gram-negative bacteria and fungi (Shahidi and Zhong, 2008; Hosseini et al., 2022).
2.4. Pigments
Natural pigments include a collection of chemically diverse and biosynthetically irrelevant compounds with the common structural feature of the chromophore, which contribute to the unique color of each compound (Pereira et al., 2014). Initially, several researchers started to focus on marine-derived natural pigments due to the side effects (toxicity to the human body) of artificial synthetic colors used in the food, pharmaceutical, and cosmetic industries (Cho et al., 2002). Later, these natural pigments received a growing interest due to their potential health-promoting biological activities such as anticancer, anti-inflammatory, antioxidant, anti-obesity, antiangiogenic, and wound healing properties (Pangestuti and Kim, 2011). In recent years, marine-derived pigments have been primarily utilized as an active ingredient in developing functional foods, nutraceuticals, and pharmaceuticals, besides being used as a natural dying material. The most important marine natural pigment are carotenoids, chlorophylls, and phycobiliproteins, which are isolated from marine sources such as seaweeds, microorganisms, and marine mammals (Pereira et al., 2014).
2.4.1. Carotenoids
Carotenoids are C40 isoprenoid compounds imparting colors ranging from yellow to red and are the most abundant group of natural pigments (Gross, 1991). Based on their chemical structure, carotenoids are classified into two major groups: carotenes and xanthophylls. Carotenes constitute only hydrocarbons such as α, β, γ-carotene and lycopene. Xanthophylls are oxygenated derivatives of carotenes with one or more oxygenated groups, including zeaxanthin, lutein, astaxanthin, and cryptoxanthin (Chuyen and Eun, 2017). Marine organisms are also identified as a potential source of biologically active carotenoids that also contribute to their color. In the marine ecosystem, carotenoids are produced by certain photosynthetic organisms, like phytoplankton, algae, and bacteria. In these organisms, carotenoids are responsible for absorbing light and photoprotection in the photosynthetic system. However, marine animals cannot synthesize carotenoids and should acquire them through their diet (Saini and Keum, 2018).
The studies on the marine carotenoids and biological functions have been commenced very recently, although numerous research have been carried out on terrestrial carotenoid sources. Up to now, hundreds of carotenoid compounds have been discovered in marine ecosystems that significantly contribute to overall natural carotenoids (Chuyen and Eun, 2017). Health benefits of carotenoids include improving eye health and preventing cancer and cardiovascular diseases due to their diverse bioactivities, such as antioxidant, anticancer, anti-inflammatory, anti-diabetic, anti-obesity, provitamin A, photoprotection, and wound healing activities (Eggersdorfer and Wyss, 2018). The most common carotenoids present in marine organisms are astaxanthin, fucoxanthin, canthaxanthin, lutein, zeaxanthin, violaxanthin, and neoxanthin (Figure 6).
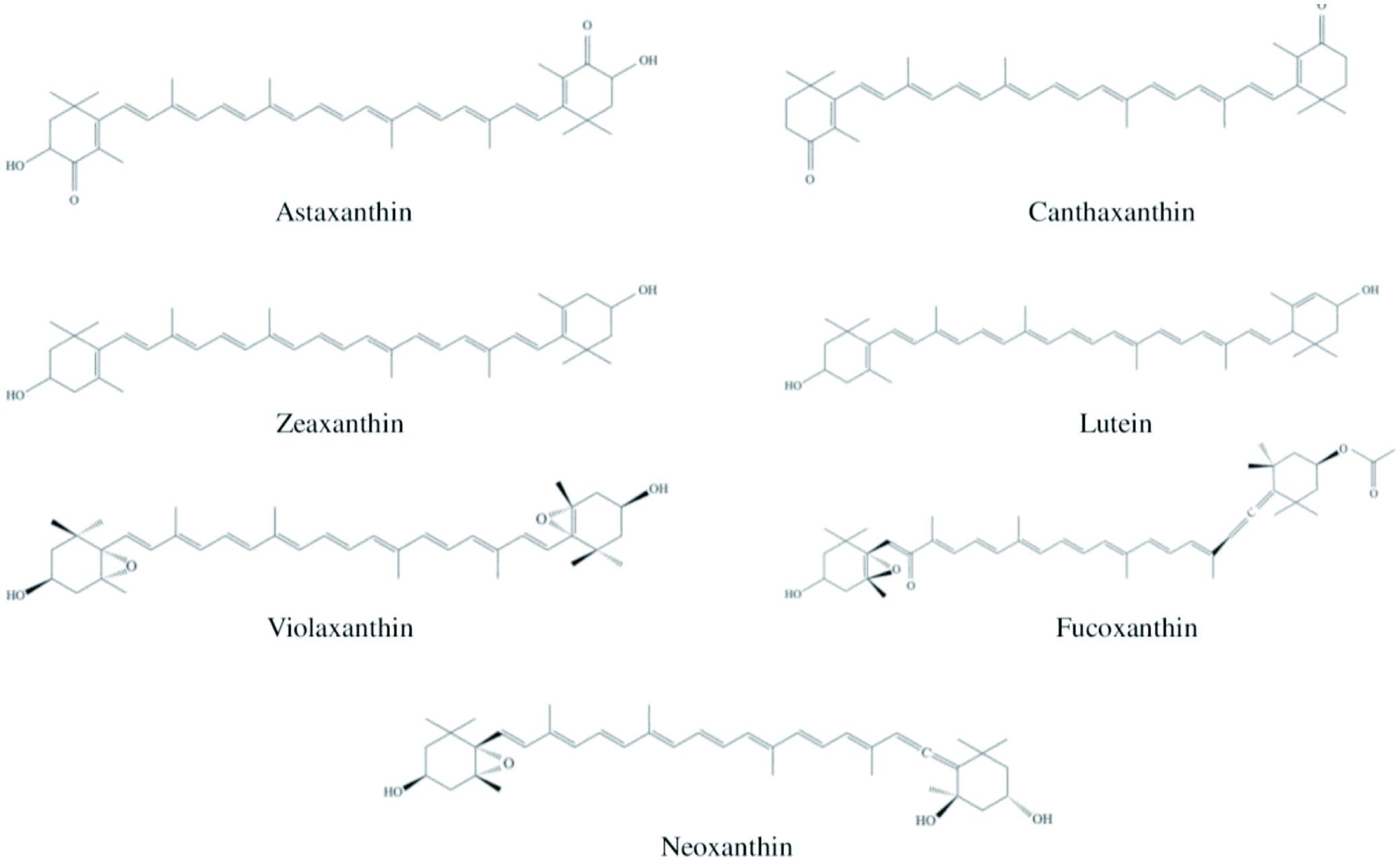 Click for large image | Figure 6. Chemical structures of the most common carotenoids. |
Zeaxanthin and lutein display the photoprotection activity by absorbing harmful blue and near-ultraviolet light, while β-carotene and β- cryptoxanthin exhibit provitamin A activity (Krinsky and Johnson, 2005). The free radicals trapping, and singlet oxygen quenching abilities contribute to the antioxidant property of carotenoids. They exhibit antitumor activities towards several cancer cell lines such as hepatic, breast, intestinal, leukemic, oral, and prostate due to their ability to induce apoptosis and suppress cell proliferation (Chuyen and Eun, 2017). Furthermore, the mechanism of their anti-inflammatory activity was investigated both in vivo and in vitro. One study revealed that inhibition of inflammatory mediators (prostaglandin E2 and nitric oxide) and suppression of pro-inflammatory markers (inducible nitric oxide synthase, cyclooxygenase-2, and TNF-α) in the body are responsible for the anti-inflammatory activity (Ohgami et al., 2003).
2.4.1.1. Astaxanthin
Astaxanthin (3,30-dihydroxy-β, β′-carotene-4,4′-dione) is a red-orange colored xanthophyll with the molecular formulae of C40H52O4. Its antioxidant capacity is higher than other carotenoids because it possesses oxygenated groups (hydroxyl and keto moieties) on each ionone ring structure (Ambati et al., 2014). Seaweeds, crustaceans, and microorganisms are rich sources of astaxanthin; remarkably the highest level is present in the green microalgae Haematococcus pluvialis (Hosseini et al., 2022). In nature, large percentages of astaxanthin exist as fatty acid esters (monoesters or diesters), and some as free form (Routray et al., 2019). Even though ester forms have greater bioavailability, thermal stability, and quenching (antioxidant) ability compared to free forms, the commercial value and functional food applicability are higher for free forms of astaxanthin (Zhou et al., 2019; Sun et al., 2011). In addition, they can also present in either a cis or trans form; still, trans forms are predominant in nature. For example, lipid extract of shrimp waste was reported to contain mainly trans isomers, two cis isomers, five monoesters, and ten diesters, and these compounds inhibited lipid oxidation during storage (Gómez-Estaca et al., 2017). In comparison to the trans form, the cis form (particularly 9-cis) shows a higher antioxidant activity (Fakhri et al., 2018). Galasso et al. (2018) have indicated that the only carotenoid that can penetrate through the blood-brain barrier is astaxanthin, thereby exhibiting essential neuroprotective functions. The antiproliferative activity of a common anticancer drug, carbendazim, towards MCF-7 breast cancer cells was higher when used along with astaxanthin (Atalay et al., 2019). Besides, they have the ability to decrease the expression of human matrix metalloproteinases, scavenge ROS and inhibit the peroxidation of lipids (Zhao et al., 2019).
2.4.1.2. Fucoxanthin
Fucoxanthin (C42H58O6) is an orange color xanthophyll carotenoid contributing to over 10% of the total carotenoids in nature. It is mainly extracted from brown seaweeds such as Sargassum fulvellum, Hijikia fusiformis, and Undaria pinnatifida, as well as from diatoms and haptophytes (Hosseini et al., 2022). The biological activities of fucoxanthin include antioxidant, antidiabetic, anticancer, antiangiogenic, anti-inflammatory, skin protective, and anti-obesity effects (Ravi and Baskaran, 2017; Peng et al., 2011; Miyashita, 2009). The antidiabetic activity of fucoxanthin isolated from brown seaweed is attributed to its ability to inhibit α-amylase and α-glucosidase and to stimulate insulin secretion (Hwang et al., 2015). The blood glucose level is lowered by the inhibition of α-amylase, resulting in the regulation of diabetes (Admassu et al., 2018). Moreover, fucoxanthin obtained from Undaria pinnatifida is useful in treating neurodegenerative diseases, namely Parkinson’s disease (Paudel et al., 2019a).
2.4.2. Chlorophyll
Chlorophyll is a widely distributed green color tetrapyrrole compound with four pyrrole moieties and a central magnesium atom (Ferruzzi and Blakeslee, 2007). Chlorophylls are the major pigments involved in photosynthesis. In the marine environment, it is synthesized by algae and cyanobacteria. The chlorophylls isolated from these species are generally converted into different forms, namely porphyrins, pheophorbides, chlorins, bacteriochlorins, porphycenes, phthalocyanines, porphycenes, and bacteriopheophorbides (Ormond and Freeman, 2013). In addition to the photosynthesis and colorant function of chlorophyll, these derivatives exhibit numerous biological activities with the potential for therapeutic applications such as anti-inflammatory, antioxidant, antibacterial, and antimutagenic activities (Manivasagan et al., 2018).
2.4.3. Phycobiliproteins
Phycobiliproteins are water-soluble and highly stable pigments found in the stroma or cytoplasm of the chloroplast. They can trap light energy in the visible light spectrum in the wavelength range of 450–650 nm (Viskari and Colyer, 2003). These pigments are commonly isolated from red algae and cyanobacteria. Examples of phycobiliproteins are phycoerythrin (red or pinkish-red color), phycocyanin (blue color), and allophycocyanin (blue color) (Manivasagan et al., 2018). The pharmacological properties of these pigments include antioxidant, ACE inhibitory, anti-inflammatory, antidiabetic, and immune-modulatory activities (Aryee et al., 2018). Traditionally, they have been used as a natural colorant in food products (Ice cream, soda pop, etc.).
2.5. Phenolic compounds
Phenolic compounds are an important class of secondary metabolites with one or more hydroxyl groups attached to (an) aromatic ring(s) along with their functional derivatives. Numerous phenolic compounds have been discovered to date, which mainly include phenolic acids and flavonoids. However, these compounds can be subdivided into different classes: simple phenols, flavonoids, benzoic acid derivatives, tannins, lignins, stilbenes, and lignans (Naczk and Shahidi, 2004). Although several studies have been carried out on the phenolics derived from terrestrial sources, the studies on marine-derived phenolics are still limited (Mateos et al., 2020). In the marine ecosystem, phenolics are mainly produced by seaweeds and macroalgae. They produce these compounds as a mechanism to protect themselves from oxidative stress and other external harmful factors, like predators, pathogens, biofouling, and the threat of consumption by herbivores (Shibata et al., 2008). Environmental conditions such as temperature, UV radiation, salinity, and nutrient availability play a significant role in synthesizing phenolic compounds by marine species (Generalić Mekinić et al., 2019). These metabolites are reported as powerful antioxidants along with other therapeutic properties, including anti-diabetic, anti-hypertensive, anti-inflammatory, antimicrobial, anti-tumor, and anti-allergic as well as reducing the risk of cardiovascular diseases. These biological properties are attributed to the presence of aromatic rings with hydroxyl groups. The phenolics present in marine sources range from simple compounds to highly complex molecules, with the majority of phlorotannins (brown algae), bromophenols, and flavonoids (green algae) (Mateos et al., 2020).
2.5.1. Bromophenols
Bromophenols are typically made up of monomeric and dimeric units of alkyl ethers and brominated 3,4-dihydroxybenzyl alcohols (Fernando et al., 2020). Brominated secondary metabolites are more common in seaweeds, particularly in red and green seaweeds, compared to other halogenated secondary metabolites (Cabrita et al., 2010). Bromophenols and their derivatives, such as polybrominated dibenzo-p-dioxins, hydroxylated and methoxylated bromodiphenyl ethers, and bromoanisoles are the widely present brominated compounds in seaweeds (Bidleman et al., 2019; Dahlgren et al., 2015). Besides, brominated sesquiterpenes are also found in macroalgae (Topcu et al., 2003). The pharmaceutical properties of bromophenols include antioxidant, antidiabetic, anti-Alzheimer’s, anti-obesity, anti-neurodegenerative disease, and antibacterial activities (Hosseini et al., 2022). Antidiabetic activity of bromophenols is mainly due to the inhibition of α-glucosidase and protein tyrosine phosphatase 1B (PTP1B) (Xu et al., 2016), while they prevent obesity via suppressing the adipogenesis of preadipocytes. Their anti- Alzheimer’s activity is attributed to their ability to inhibit cholinesterases (ChE), glycogen synthase kinase-3b (GSK3b), and b-site amyloid precursor protein cleaving enzyme 1 (BACE1) (Paudel et al., 2019c).
2.5.2. Phlorotannins
Phlorotannins belong to the tannin class of compounds produced via the acetate-malonate pathway by the polymerization of phloroglucinol (1,3,5-trihydroxybenzene) units (Lobine et al., 2021). They are widely distributed in brown algae seaweeds, notably found in their cell walls, forming a complex with other molecules (Catarino et al., 2019; Peng et al., 2015). Phlorotannins are highly soluble in water with molecular weights in the range of 126 to 6,50,000 Da. The major phlorotannins present in the brown algae are phloroglucinol, trifucol, triphlorethol A, phlorofucofuroeckol A, pentafuhalol B, diphlorethohydroxycarmalol, eckol, and dieckol (Figure 7) (Mateos et al., 2020). The tannins produced by terrestrial plants consist of 3 to 4 rings in their molecular structure, whereas phlorotannins have eight phenol rings, resulting in the strong antioxidant activity of phlorotannins. For instance, the antioxidant property of phlorotannins obtained from Eisenia bicyclis is ten times greater than that of α-tocopherol and ascorbic acid (Gupta and Abu-Ghannam, 2011a). Therefore, phlorotannins could be utilized as a natural antioxidant in the functional foods, pharmaceuticals, and cosmetics industries (Li et al., 2009). Besides, phlorotannins also exhibit cytotoxic (Shoeib et al., 2004), anti-inflammatory (Dutot et al., 2012), type-2 diabetic suppressing (Yan et al., 2019), UV-protective, chemopreventive activities and heavy metal detoxify activities (Kim et al., 2020; Hamed et al., 2015), as well as antimicrobial activities by attacking the proteins of microorganisms (bacterial inhibition) (Gupta and Abu-Ghannam, 2011b).
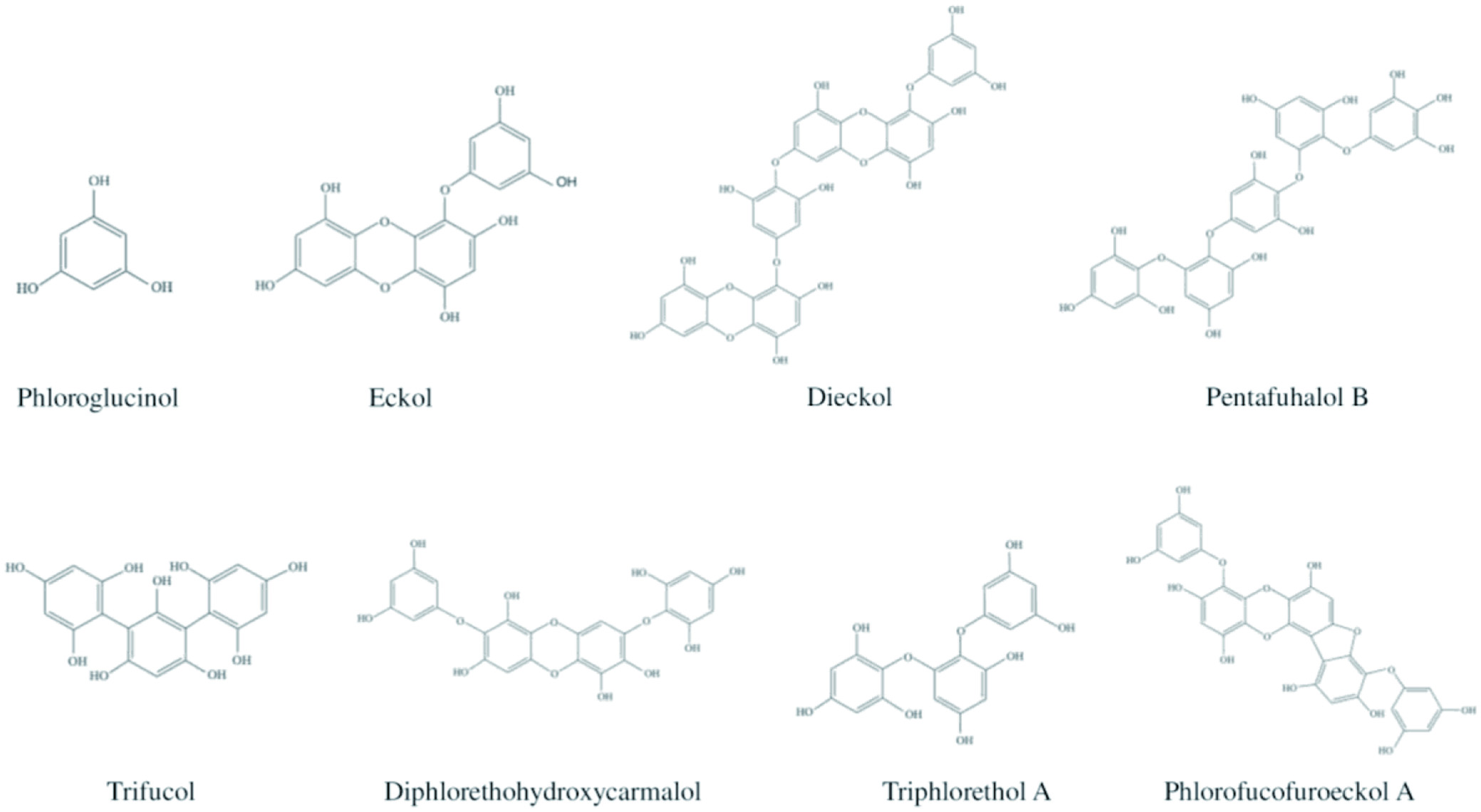 Click for large image | Figure 7. Chemical structures of phlorotannins present in brown algae. |
| 3. Biological activities exhibited by marine natural products | ▴Top |
Seafood is considered a highly nutritious food for humans due to the presence of high-quality protein with all essential amino acids and easily absorbable lipids, vitamins, and minerals. In recent years, marine functional ingredients have been widely used in nutraceutical and pharmaceutical applications due to their numerous health-promoting effects. These bioactive compounds can influence the health of humans by changing the gene expression of a host at the cellular level (MacArtain et al., 2007). The diverse range of biological activities of marine products includes antioxidant, anti-diabetic, anticancer, antihypertensive, anti-inflammatory, neuroprotective, lipid-lowering, and antimicrobial activities.
3.1. Antioxidant activity
Natural and synthetic antioxidants protect cells from oxidative damage by harmful free radicals. An excessive amount of reactive oxygen species (ROS) in biological systems promotes oxidative stress, which leads to the onset of diabetes, cancer, inflammatory and neurodegenerative, because ROS can cause cell and tissue damage due to their ability to react with macromolecules, including proteins, DNA, and membrane lipids (Cornish and Garbary, 2010; Zubia et al., 2007). For several decades, synthetic antioxidants, namely butylated hydroxytoluene, butylated hydroxyanisole, tertiary-butylhydroquinone, and propyl gallate have been used to inhibit lipid oxidation. These synthetic molecules possess harmful effects on human health, thus their utilization is discouraged or strictly regulated in different countries (Park et al., 2001).
Most marine species synthesize antioxidants to deactivate ROS in their bodies, which is produced when exposed to a harsh oceanic environment. Examples of antioxidants produced by marine species are sulfated polysaccharides, proteins and peptides, organic acids, pigments (carotenoids), and phenolic compounds (Domínguez, 2013; Balakrishnan et al., 2014). They exhibit antioxidant effects by trapping free radicals, quenching singlet oxygen, or chelating metal ions. These compounds are extracted from marine sources as natural antioxidants and used in nutraceutical and pharmaceutical industries to prevent diseases promoted by ROS and improve the body’s health condition (immune response). Besides, these components could also be utilized in the cosmetic industry to produce anti-aging products (Cornish and Garbary, 2010).
3.2. Antihypertensive activity
Hypertension, also known as high blood pressure, is one of the important risk factors for the onset of cardiovascular diseases. In the human blood, angiotensin II causes blood pressure to increase by constricting the small blood vessels. The inactive angiotensin I is converted into its active form angiotensin II by the action of the angiotensin-I converting enzyme (ACE). Therefore, hypertension could be prevented by inhibiting the ACE, and this inhibition is the primary target for antihypertensive activity (Šimat et al., 2020; Kim et al., 2012). Among marine bioactive compounds, peptides, PUFAs, COS and phlorotannins exhibited strong antihypertensive activities (Šimat et al., 2020; Wijesekara and Kim, 2010). ACE-I inhibitors from natural sources are safer due to some side effects of chemically synthesized ACE-I inhibitors (captopril, alcacepril, lisinopril, and enalopril) (Hamed et al., 2015).
3.3. Cardiovascular beneficial effects
Cardiovascular diseases (CVD) are a group of illnesses associated with the heart and blood vessels, including coronary heart diseases (myocardial infarction), heart failure, stroke, and peripheral vascular disorders. The risk factors contributing to CVD are hyperlipidemia and hypertension. Hyperlipidemia is a condition with elevated levels of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL), reduced levels of high-density lipoprotein (HDL), and high plasma cholesterol and triacylglycerol levels (Suleria et al., 2016). ω3 PUFAs present in marine sources reduce these risk factors and hypertension (by inhibiting the ACE-I activity) (Chang and Cho, 2009). Some marine-derived polysaccharides and other compounds have also proven to modify fat absorption, raise the generation of LDL receptor mRNA, and activate lipid metabolic enzymes, resulting in the reduction of total fat levels in the blood, thereby preventing CVD development (Qin, 2018).
3.4. Anticancer activity
Cancer is a condition triggered by the uncontrolled division and growth of cells in the body due to intrinsic (inherited mutations) and extrinsic factors (pathogens, smoking, radiation, malnutrition, and some chemicals). These cells are known as abnormal cells, which cease responding normally to chemical signals from other cells and are capable of invading and manipulating normal tissues (American Cancer Society, 2006). Although radiation and/or several chemotherapy drugs are used to treat cancer, the search for natural substances that could prevent cancer development has been gaining significant attention in the past decades (Subramaniam et al., 2019). Induction of apoptosis in cancer cells is the primary target for bioactive compounds with anticancer properties. Apoptosis is the death of cells occurring as the normal process in the body’s growth and development, and it is morphologically characterized by DNA fragmentation and cell shrinkage (Nkwe et al., 2021). Numerous potent bioactive molecules exhibiting anticancer activities have been identified from marine species. There has been a rising number of preclinical anticancer marine-based compounds subjected to human clinical trials since the early 1900s (Newman and Cragg, 2004). For instance, in Europe, trabectidin (Yondelis®), extracted from Caribbean marine tunicate Ecteinascidia turbinate, has been approved as an anticancer agent (Rinehart, 2000).
3.5. Anti-inflammatory activity
Inflammation is an integral part of the host’s response to tissue damage or infection (microbial invasion). It is associated with several biological pathways guided by internal and external stimuli. Long-term inflammation or misdirection and exaggeration of host response could result in adverse health effects, including arthritis, inflammatory bowel disease, and asthma (Lunn and Theobald, 2006). However, anti-inflammatory agents can modulate the biological pathways; remarkably, they alter macrophages, which is the crucial factor for the development of inflammation (Fujiwara and Kobayashi, 2005). Diet modification could alleviate various inflammatory conditions by inhibiting the inflammatory mediators (Lunn and Theobald, 2006). The anti-inflammatory effects of marine sources are mainly due to the ω3 PUFAs, which are capable of inhibiting inflammatory mediators (Calder, 2009). It was observed that ω6 PUFAs-derived eicosanoids exhibit immunoactivity and pro-inflammatory properties, whereas ω3 PUFAs derived eicosanoids show anti-inflammatory effects. Therefore, by raising the ω3 to ω6 fatty acids ratio, inflammation could be reduced (Ghosh et al., 2022).
| 4. Marine sources of Bioactive molecules | ▴Top |
The biodiversity of the marine environment has not yet been fully explored. Nearly 230,000 marine organisms have been discovered and described until now (Kiuru et al., 2014; Blunt et al., 2007). However, it has been reported that over 2 million undiscovered species exist in the marine ecosystem (Mora et al., 2011). The ocean is an excellent source of plants, animals, and microorganisms with the capability to produce a wide range of biologically active primary and secondary metabolites as an adaptation mechanism to their specific habitats. In the following section, the marine species are categorized into marine invertebrates, fishes, seaweeds, and marine microorganisms, and the bioactive compounds produced by these species are discussed in detail.
4.1. Marine invertebrates
More than 92% of the marine species are invertebrates with immense biological diversity and are primarily responsible for the chemical and biotic composition of the oceans (Diniz et al., 2014). They are abundantly distributed in all ocean parts, inhabiting from hydrothermal vents to the unexplored Arctic (Eisenhauer et al., 2019; Snelgrove, 2016). Marine invertebrates are characterized by their complex, multi-stage life cycle, hard outer covering for their structure and protection, and the absence of an internal bony skeleton. The larval stages of marine invertebrates are free-swimming, but they become a sessile benthic adult after metamorphosis (Fuchs et al., 2020; Pandori and Sorte, 2019). Most marine invertebrates belong to eight phyla such as Porifera, Cnidaria, Arthropoda, Mollusca, Echinodermata, Annelida, Bryozoa, and Chordata (Ascidians), as shown in Figure 8 (Leal et al., 2012a). Each phylum is further categorized into different classes, which encompass numerous species.
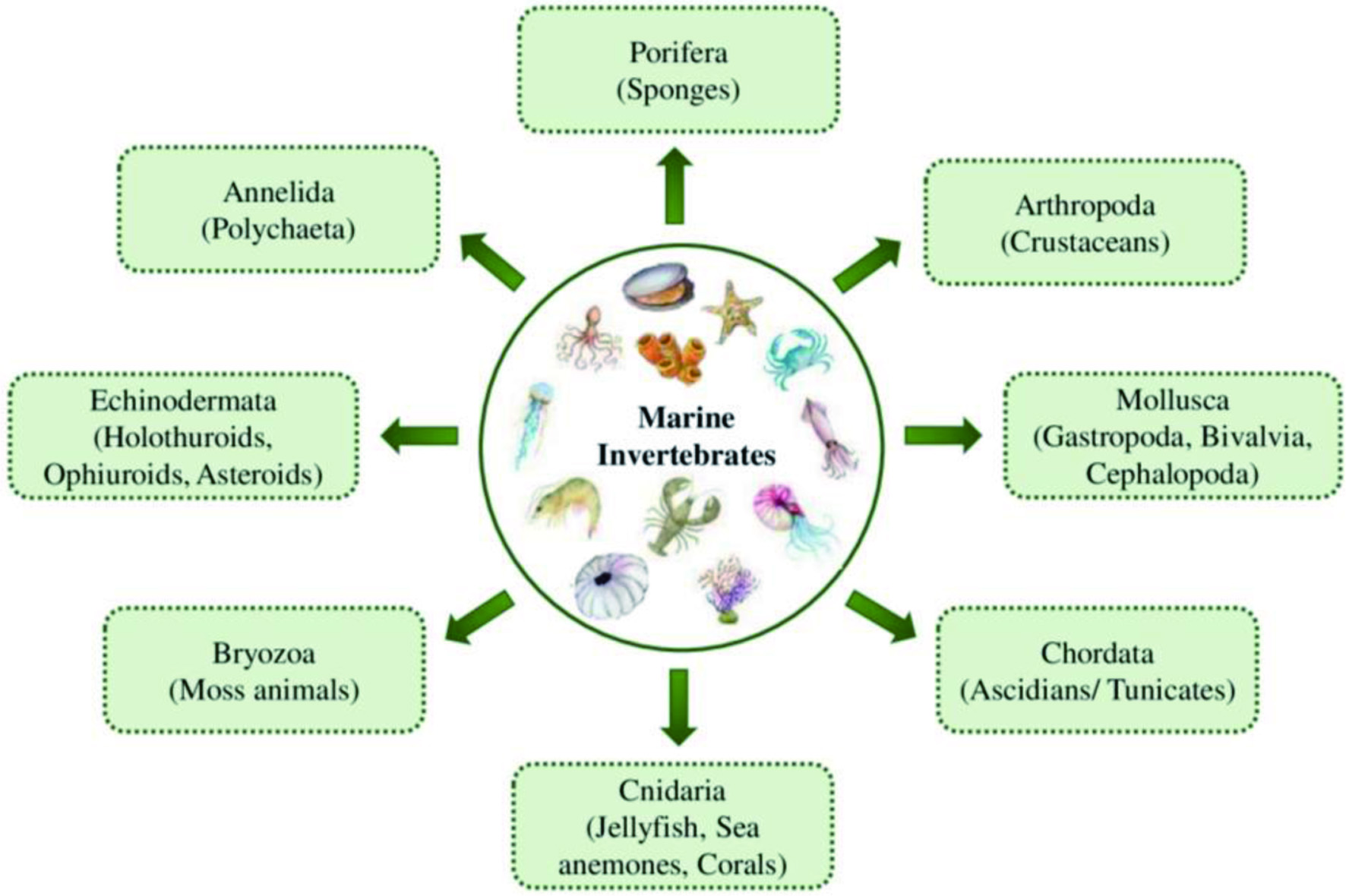 Click for large image | Figure 8. Eight major phyla of marine invertebrates. |
The majority of the species of marine invertebrates are soft-bodied and sessile, which makes them dependent on the secondary chemical metabolites as a chemical defense mechanism to protect them from predators and competitors (Haefner, 2003; Faulkner, 2000b). These species produce an abundance of chemically diverse and unique natural products with potent biological activities. Approximately 9,812 novel natural products were discovered from marine invertebrates from 1990 to 2009. There was an increase of 17.7% in the isolation of new bioactive compounds in the 2000s compared to the 1990s (Leal et al., 2012b). Among all marine invertebrates, natural products with biological activities have been discovered from 11 phyla, six subphyla, 20 classes, 20 subclasses, 74 orders, 253 families, 569 genera, and 1,354 species (Leal et al., 2012b). Moreover, phylum Porifera is predominant in producing secondary metabolites contributing to about 48.8% of marine invertebrates-derived natural products, followed by Cnidaria (28.6%), Echinodermata (8.2%), Chordata (6.9%), and Mollusca (5.8%). Other phyla such as Arthropoda, Annelida, and Bryozoa contribute to the remaining 1.7% of natural products extracted from marine invertebrates (Figure 9). Different classes of bioactive compounds derived from the major phyla of marine invertebrates, such as Porifera, Cnidaria, Echinodermata, Mollusca, Arthropoda and Chordata and their biological activities are discussed in the following section.
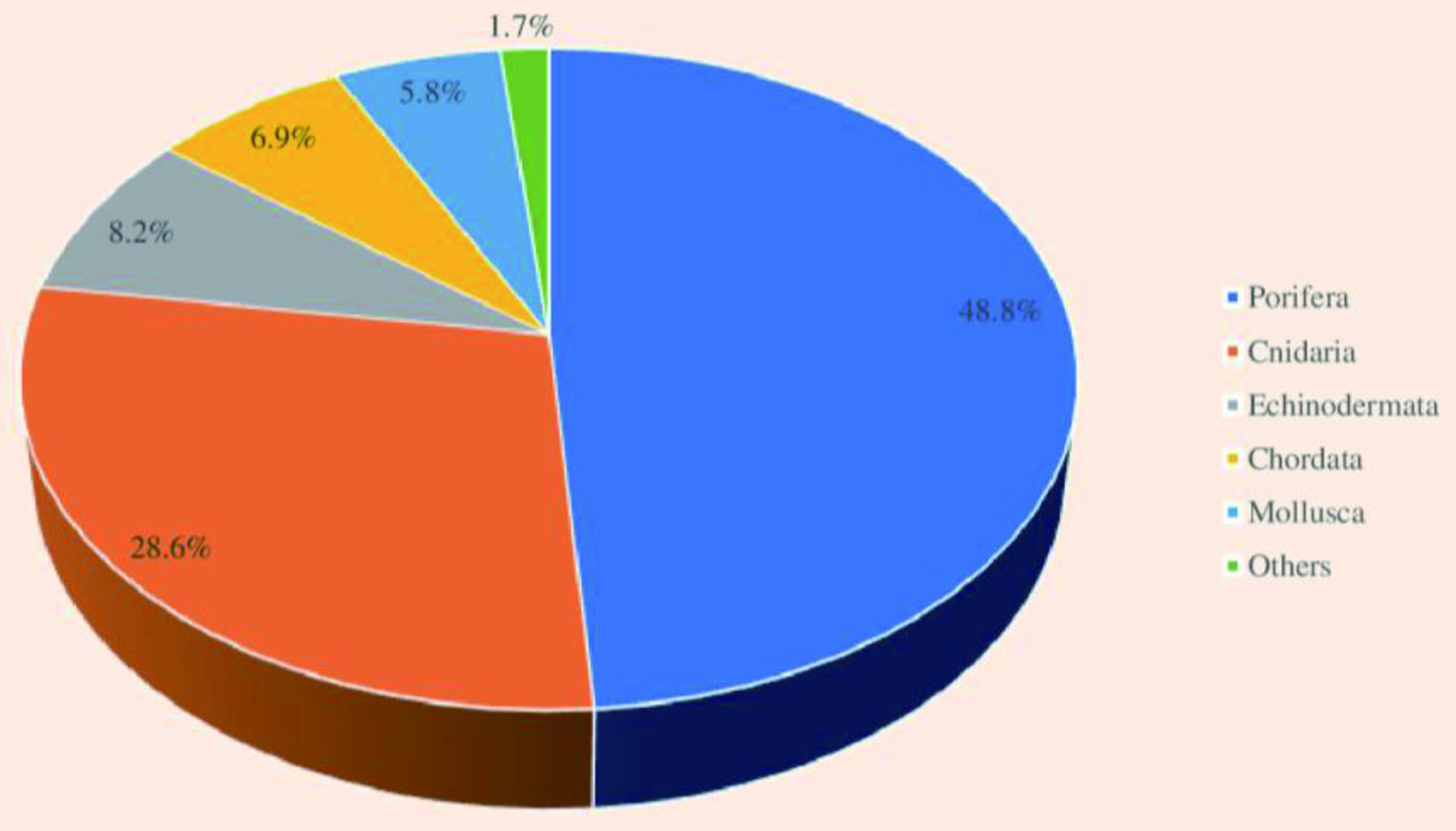 Click for large image | Figure 9. Contribution by different phyla for the overall production of natural products by marine invertebrates. |
4.1.1. Porifera (Sponges)
Porifera is one of the most diverse taxonomic groups of marine sessile invertebrates, with more than 9,000 existing species belonging to an ancient metazoan lineage (Simion et al., 2017). The term Porifera means “pore bearing,” which means the body is full of pores for water circulation through the body. Phylum Porifera includes four classes: Calcarea (five orders and 24 families), Demospongiae (15 orders and 92 families), Hexactinellida (six orders and 20 families), and Homoscleromorpha (one order and two families). Sponges comprise two clades of Hexactinellida + Demospongiae and Homoscleromorpha + Calcarea to form a monophyletic group (Ereskovsky and Lavrov, 2021; Gazave et al., 2012).
Sponges are sessile, multicellular, filter-feeding primitive marine invertebrates that attach to the solid surface. They are mainly found in freshwater and marine habitats, as well as in tropical, temperate, and polar environments (Petersen et al., 2019; Pronzato et al., 2017). Over 8,000 species of sponges have been discovered yet (Varijakzhan et al., 2021). Sponges are miscellaneous and exist in different sizes, colors, and shapes, namely caliculate (cup-shaped), tubular (tube-like), flabellate (fan-shaped), globular (ball-shaped), arborescent (plant-shaped), and amorphous (shapeless) (Martins et al., 2019). Sponges have a basic level of body organization, which lacks definite tissues and organs, with relatively independent specialized cells performing a range of biological functions. The body of the sponges is composed of connective tissues known as mesohyl of external and internal layers of cells. The exterior surface, covered with pinacoderm, consists of flattened or T-shaped cells called pinacocytes. The internal system of canals and microscopic chambers is surrounded by choanocytes comprised of flagellated collar cells (Soest et al., 2012).
The inner choanocytes are separated by a jelly-like mesohyl layer, which harbors the skeleton of mineral spicules comprised of silica or calcium carbonate and protein fibers of collagen, known as spongin (FAO, 2017). The species of the sponges could be identified depending on the shape of the spicules and sizes. The most significant structure of the sponges is the internal water spaces, through which water circulates, influencing the reproduction, gas exchange, feeding (providing nutrients), and expulsion of sponges. The water-current system of canals and chambers is connected to the external environment via excurrent Oscula and incurrent Ostia (Dahihande and Thakur, 2021).
The studies of screening and isolation of marine-based bioactive compounds commenced with the discovery of C-Nucleosides, spongothymidine, and spongouridine from Caribbean sponge Cryptothecaa crypt by Bergmann and Feeney (1951). From this preliminary stage of research on the marine-derived natural components, numerous bioactive compounds have been identified from marine invertebrates. Notably, sponges are the richest source of secondary metabolites with varying biological activities and chemical diversity (Singh and Majik, 2019). Hundreds of new compounds have been discovered from marine sponges annually (Ebada and Proksch, 2012; Laport et al., 2009). Recently, studies on sponges are gaining growing attention due to (i) their wide distribution in different geographical regions (nearly in seas of 31 countries), (ii) their symbiotic relationship with a range of microorganisms, (iii) their richness of structurally diverse secondary metabolites with different biological activities, and (iv) therapeutic potential of these compounds to treat human diseases (Abdelaleem et al., 2020).
Around six orders of sponges such as Dictyoceratida, Haplosclerida, Poecilosclerida, Halichondrida, Astrophorida, and Lithistida contribute to nearly over 20, 14.2, 14, 10.7, 9.2 and 5.5% of the discovery of new bioactive compounds from sponges, respectively (Mehbub et al., 2016). Moreover, biologically active secondary metabolites have been isolated from around 11 genera of sponges, including Discodemia, Haliclona, and Petrosia. Microbial symbionts also contribute to the synthesis of the wide range of bioactive compounds produced by sponges (Ebada and Proksch, 2012), and these compounds have proven to exhibit potential immunosuppressive, anticancer, antibiotic, anti-inflammatory, and antiviral activities (Frota et al., 2012; Jha and Zi-rong, 2004). Perdicaris et al. (2013) have reported the isolation of over 15,000 natural products from marine sponges from 1992 to 2012. These belong to diverse groups of chemical compounds such as sterols, nucleosides, alkaloids, peptides, glucosides, terpenes, polyphenols, polyketides, amino acid derivatives, macrolides, and peroxides, and fatty acids. Among these compounds, terpenes, terpenoids, alkaloids, peptides, sterols, and steroids are produced in large numbers (Mehbub et al., 2014).
Marine sponges are primitive filter feeders with symbionts attached to them and have a natural chemical defense mechanism against potentially hazardous factors, including predation, space competition, microbial growth, and overgrowth of fouling organisms. These factors lead to the synthesis of an abundance of bioactive compounds. Sponges can adapt and respond to varying environmental conditions because they have been living in the ocean for more than 600 million years and encounter similar adverse conditions as those faced by the oceans (Li et al., 1998). Figure 10 illustrates the factors contributing to the production of a diverse range of novel bioactive compounds by sponges.
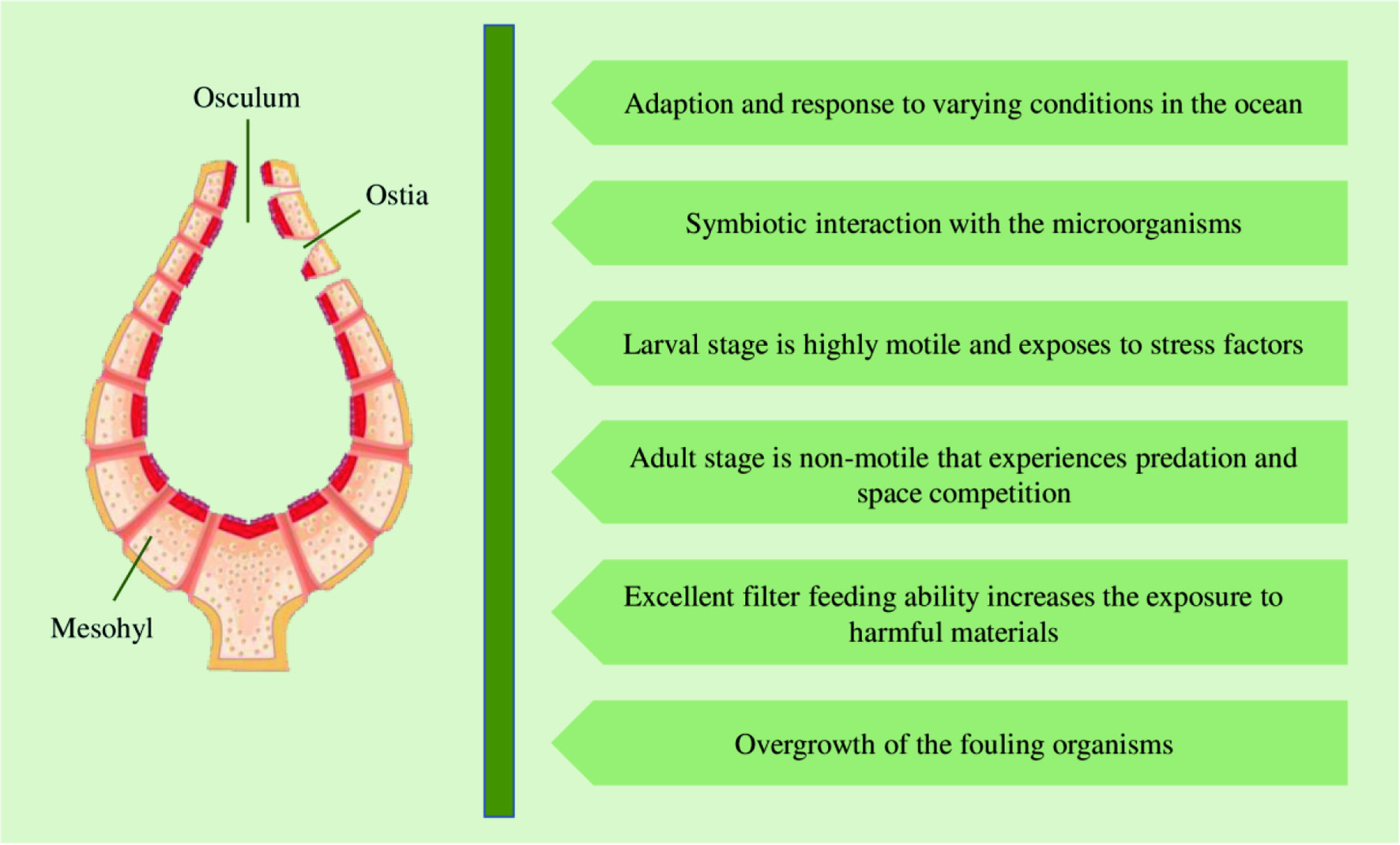 Click for large image | Figure 10. Factors contributing to the production of bioactive compounds by sponges. |
The specific metabolic pathway of synthesis of bioactive compounds by sponges is associated with the specific bacterial symbionts of the bacteria (Wilson et al., 2014). The outer layer of the sponges harbors photosynthetic microbes. At the same time, the mesohyl matrix of the sponges accommodates extracellular autotrophic and heterotrophic microorganisms entering through the Ostia, resulting in the symbiotic interaction between sponges and the microbes (McClintock and Baker, 2010; Taylor et al., 2007). The symbiotic species associated with sponges include cyanobacteria, heterotrophic bacteria, archaea, facultative anaerobes, fungi, yeast, dinoflagellates, virus, and some larger organisms, namely crustaceans, molluscs, nematodes, etc. (Kiran et al., 2018; Schippers et al., 2012). Nearly 50% of the biomass of the sponges is comprised of a dense microbial population, which includes a large proportion of bacteria, around 38% of the sponge biomass in the extracellular mesohyl matrix (Steinert et al., 2018; Wang, 2006; Usher et al., 2004). These symbiotic microbes are not only responsible for the biosynthesis of secondary metabolites, but they can also contribute to several physiological functions, including photosynthesis, dehalogenation, nitrogen, and carbon cycles, protecting the sponges against UV radiation, and stabilizing their skeleton (Thoms et al., 2003). Moreover, symbiotic cyanobacteria are the reason for the bright colors of the sponges (Taylor et al., 2007).
Sponges are able to multiply either by asexual reproduction by budding or sexual reproduction. During the sexual reproduction, sperms released by male sponge, move towards and enter the female sponges resulting in the fertilization and release of a larva into the water. The larvae are highly mobile and exposed to diverse groups of abiotic and pathogenic stress factors. Then the larvae find a suitable substrate for attachment and start to grow into an adult sponge, which is non-motile, and encounter predation stress from natural predators and intense competition for space among other non-motile species (Steinert et al., 2018). When the predators threaten sponges, they secrete high levels of mucus containing secondary metabolites (toxins) with the antibiotic, cytotoxic, and feeding deterrent abilities or nasty odors and tastes, creating a clear zone surrounding them against other species. It has been proven that bacterial symbionts of sponges contribute to the production of chemical compounds to protect against predators (Pawlik et al., 2002).
Further, sponges possess a unique chemical defense mechanism against pathogenic bacteria, parasites, viruses, fungus, and other predators, which is helpful in the development of novel components against parasitic, fungal, and viral diseases (Mehbub et al., 2014). Sponges produce some bioactive compounds such as terpenoids, steroids, alkaloids (brominated alkaloids), and polyacetylene derivatives to prevent the settling of fouling organisms on their surfaces (Qi and Ma, 2017). The water circulation property of the sponges is protected by restricting the settlement of bryozoans and barnacles on the surface and the formation of biofilms, which block the osculum and other systems and could result in the death of sponges (Varijakzhan et al., 2021; Stowe et al., 2011).
The excellent filter-feeding ability of the sponges is also a contributing factor to their high production of secondary metabolites. The aquiferous system of the marine sponges can filter an enormous quantity of water, which is around 0.002–0.84 mLs−1cm−3 of sponge tissue (24 m3kg−1day−1) (Weisz et al., 2008; Hentschel et al., 2002). The flagellated cells (choanocytes) are responsible for water movement in one direction by coordinating the flagella. Further, amoebocyte cells engulf the particles entering the water, and sponges can retain a diverse group of organic matter in size range of 0.1–50 µm, such as bacteria, heterotrophic eukaryotes, viruses, and phytoplankton. Eventually, the sponges produce an abundance of bioactive molecules due to the increased risk of exposure to potentially harmful materials caused by their filter-feeding ability (Wehrl et al., 2007).
The crude extract derived from the marine sponges contains chemically diverse groups of natural compounds which are either produced by the sponges or by other species associated with the sponges (He et al., 2020; Cheng et al., 2020). A few examples of groups of compounds, including peptides, alkaloids, and terpenes synthesized by the marine sponges are discussed below. Apart from these three groups of compounds, the examples and biological activities of other groups are listed in Table 1. Considering the different biological activities of compounds isolated from marine sponges, most of the metabolites exhibit cytotoxic activities against cancer cell lines. For instance, Figure 11 depicts the diverse biological activities of natural products isolated from Dictyoceratida sponges, where the majority of the metabolites exhibited cytotoxic effects (53%) followed by antimicrobial properties (17%) (Abdelaleem et al., 2020).
 Click to view | Table 1. Different bioactive compounds (except peptides, alkaloids, and terpenes) produced by sponges and their biological activities |
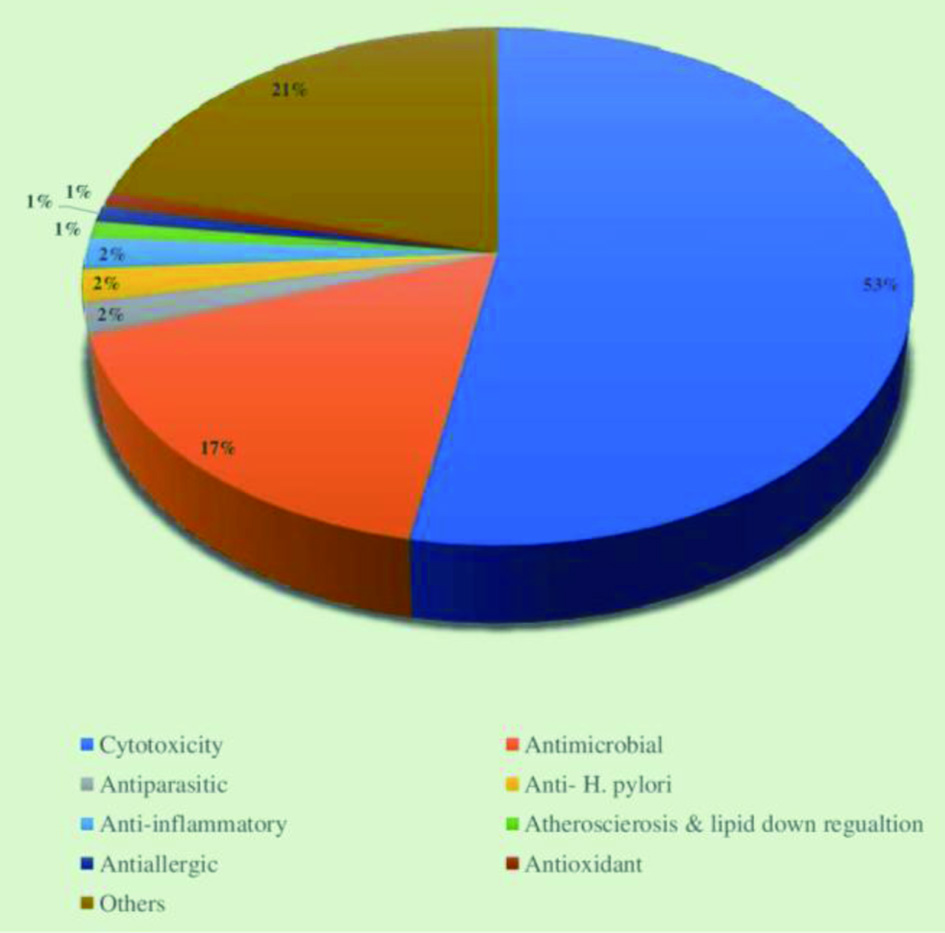 Click for large image | Figure 11. Distribution of different biological activities exhibited by natural products isolated from Dictyoceratida sponge. |
4.1.1.1. Peptides
Marine species are the most abundant source of biologically active peptides with various functionalities, including antimicrobial, antidiabetic, antioxidant, antihypertensive, anticancer, etc. (Mehbub et al., 2014). In 1969, cytarabine was approved by the US FDA for commercial use, becoming the first marketed marine-based anticancer compound. It is a synthetic analog of a C-nucleoside extracted from the sponge Tethya crypta and applied to treat acute myelocytic leukemia, acute lymphocytic leukemia, and non-Hodgkin’s lymphoma. Bioactive peptides from sponges with anticancer activities are particularly associated with the above discovery (Macedo et al., 2021; Sagar et al., 2010).
Hoshino (1977) discovered the first bioactive peptide Discodermin A, a tetradecapeptide, with antimicrobial property from the marine sponge Discodermia kiiensis in the Shikine Island, Japan (Nakao et al., 2003). Later, subsequent studies identified various analogs such as discodermin B, C, and D. All analogs of discodermins (A–D) discovered from the sponge possess potential inhibitory activity against phospholipase A2 (Negi et al., 2017). The tetradecapeptide contains anomalous amino acid residues such as 3-methyl-L-proline, 3-methyl-D-valine, and formyl-D alanine, which are the characteristic features of the tetradecapeptide AMPs (Vitali, 2018).
It was observed that marine sponges derived bioactive peptides consist of unique structures among those derived from other natural sources. Most amino acids in the peptides extracted from sponges are almost absent or rarely present in the peptides obtained from microbial and terrestrial species. They are in either linear or cyclic shapes (Wang et al., 2017b; Yeung et al., 1996). Proline-rich cyclic peptides and depsipeptides are the two major groups of peptides produced by the sponges (Vitali, 2018). Depsipeptides exist in cyclic and linear forms, while proline-rich cyclic peptides are only present in the cyclic form with several proline residues (Wang et al., 2014a; Plaza et al., 2010). These proline rings provide rigidity and decrease the conformational flexibility, thus contributing to the structural stability of the peptide (Zou et al., 2013). Proline-rich cyclopeptides show anti-inflammatory, immunosuppressing, and anticancer activities. Callyaerins A–F and H derived from the ethyl acetate extract of Callyspongia aerizusa are examples of proline rich cyclopeptides, where Callyaerin A exhibited robust antifungal activity for Candida albicans (Vitali, 2018). Specifically, sponges are the rich source of novel AMPs having antibacterial, antifungal, and anti-HIV activities.
4.1.1.1.1. Antiviral peptides
Mirabamides, neamphamide A, callipeltins, stellettapeptins, papuamides, and celebesides are cyclic depsipeptides that show antiviral properties. The above peptides are characterized by the presence of a tyrosine-free hydroxyl group, aliphatic tails, N-terminal moieties of polyketide, unusual amino acid residues, and a 3,4-dimethylglutamine residue (Giordano et al., 2018). These properties of the cyclic depsipeptides are responsible for their influence on the surface components of viruses, which could affect of phosphatidylserine phospholipids on the viral surface (Andjelic et al., 2008). The aliphatic tail and atyrosine hydroxyl group of these peptides are responsible for the penetration of peptides into the viral membrane and interaction with the cholesterol membrane, respectively (Plaza et al., 2007).
Mirabamides A–D and new mirabamides E–H, extracted from Siliquariaspongia mirabilis and a sponge genus Stelletta, exhibited potential anti-HIV activity (Plaza et al., 2007; Lu et al., 2011b). Mirabamide A contains the rhamnosylated β-methyltyrosine residue resulting in the powerful antiviral effect of Mirabamide A in comparison to mirabamide B, C, and D. The only mirabamide peptide which lacks a 2,3-diaminobutanoic acid residue is mirabamide B, thus it has the least antiviral activity (Plaza et al., 2007). S. mirabilis is also a rich source of celebesides A–C, consisting of unusual amino acids 3-carbamoyl threonine and phosphoserine and shows anti-HIV action due to the presence of phosphoserine (Giordano et al., 2018).
Shin et al. (2015) evaluated the anti-HIV activity of the stellettapeptins A and B, which were discovered from Stelletta sp., using an XTT-based cell viability assay. They found that stellettapeptins A and B effectively inhibited the HIV-1 infection at the half-maximal concentrations of 23 and 27 nM, respectively. Moreover, cyclic depsipeptides koshikamides F–H and linear peptides koshikamides C–E were isolated from deep sea sponges Theonella swinhoei and T. cupola. The entry of HIV-1 was prevented by the low concentrations of koshikamides F and H due to the presence of a lactone ring with ten amino acid residues. In contrast, koshikamides C and E did not show any inhibitory activity (Plaza et al., 2010).
4.1.1.1.2. Antifungal peptides
Several active peptides, such as motuporins (de Silva et al., 1992), polytheonamides (Hamada et al., 1994, 2005), theonegramides (Bewley and Faulkner, 1994), theonellapeptolides (Kobayashi et al., 1994) and cyclolithistide A (Clark et al., 1998) have been isolated from the marine sponge Theonella swinhoei. Among these peptides, theonellamide F, an unusual bicyclic peptide, exhibited potent antifungal action against several pathogenic strains of fungus such as Trichophyton sp., Aspergillus sp., and Candida sp. at 3–12 mg/mL levels (Matsunaga et al., 1989a; Wang et al., 2014a). This peptide acts as an antifungal agent by damaging the plasma membrane due to the binding of 3-β-hydroxysterols with the histidine-alanine bridge of the peptide (Nishimura et al., 2010; Giordano et al., 2018). Furthermore, theonellamide G also showed potent inhibition against the amphotericin-resistant strain (ATCC 90873) and wild strain (ATCC 32354) of fungus (Youssef et al., 2014).
4.1.1.1.3. Anticancer peptides
It has been reported that sponge derived peptides show cytotoxic effects against several cancer cell lines. Carteritins, hymenamides, euryjanicin A, and phakellistatin, a group of prolein rich cyclic peptides with D-, L- and unnatural amino acids in their composition, were shown to suppress the viability of human hepatoma (BEL-7402), human colon cancer cell line (HCT116), HeLA (human cervical carcinoma) cancer cells and human lung carcinoma cells (A-549) (Anand et al., 2019; Li et al., 2018b). Further, a sponge glycopeptide Theonellamide G showed a cytotoxic effect on human colon adenocarcinoma cell lines (HCT-16) (Youssef et al., 2014).
Callyaerin, a peptide with linear and cyclic parts, was isolated from the sponge Callyspongia aerizusa. Ibrahim et al. (2010) tested the cytotoxicity of callyaerins A–F and H on the PC12 (rat brain tumor), HeLa, and tumor cell line L5178Y (mouse lymphoma). They found that callyaerin B was the most potent and callyaerin F was the least powerful anticancer agent (Giordano et al., 2018). It has also been observed that callyaerin B possesses higher toxicity when evaluated using the MRC-5 (human fetal pulmonary fibroblast) and THP-1 cell line (acute human monocytic leukemia) (Daletos et al., 2015).
Phakellistatins, cycloheptapeptides discovered from the sponge genus Phakellia, have gained the attention of researchers as they have significant cytotoxicity against murine leukemia cells (Meli et al., 2017). The cycloheptapeptides fuscasins A–D were isolated from Phakellia fusca and evaluated for cytotoxic activity against six human cancer cell lines. It was found that only fuscasin A has the cytotoxic effect towards the human cancer cell line HepG2, and it could be utilized in the development of antitumor drugs (Wu et al., 2019).
The marine sponge Pipestela candelabra was found to be the source of peptides hemiasterlin D and milnamides E–G which exhibited cytotoxic and antiproliferative activity (Tran et al., 2014). Besides, several peptides, including geodiamolide D–F (Coleman et al., 1999), hemiasterlin A (Gamble et al., 1999), and milnamide A–D (Sonnenschein et al., 2004; Chevallier et al., 2003) were isolated from the same sponge. These peptides were also identified as potent anticancer agents, which prevented the proliferation of human prostate cancer cells (PC3). Among the peptides extracted from P. candelabra, milnamide A and milnamides E–G showed potent anticancer activity.
4.1.1.1.4. Anti-inflammatory peptides
The marine sponge based natural products were also proven to show anti-inflammatory activity. Characellides A and B, extracted from Characella pachastrelloides, showed strong anti-inflammatory activity. These peptides contain a rare sugar unit and a central tripeptide connected to an alkyl chain with a 2,3-dimethyltetrahydropyran terminal unit. The anti-inflammatory action of characellides A and B was assessed in microglia BV-2 cells induced with lipopolysaccharide. It was observed that characellides suppressed ROS production by 50% in the tested cells (Afoullouss et al., 2019).
Stylissatin A is a proline rich peptide extracted from the sponge Stylisha massa consisted of cis- and trans- proline units as its structural components (Kita et al., 2013). This peptide was shown to reduce the production of nitrogen oxide in murine RAW264.7 macrophages stimulated with lipopolysaccharide contributing to its anti-inflammatory activity (Zhang et al., 2019a).
4.1.1.2. Alkaloids
Alkaloids represent the largest group of bioactive compounds derived from marine sponges. Numerous classes of alkaloids with unique chemical structures have so far been isolated from marine sponges. This group of compounds exhibits strong biological activities such as antiviral, antifungal, antioxidant, antibiotic, antimalarial, anti-inflammatory, neuro-suppressive, and immune-modulating (Elissawy et al., 2021; Casertano et al., 2020; Johnson et al., 2012; Xu et al., 2011b). In 1986, the sponge derived alkaloid manzamine was first discovered from Haliclona sp. It is a polycyclic alkaloid with a bridged and fused tetra- or pentacyclic ring unit attached to the β-carboline (Baldwin and Whitehead, 1992). Manzamine alkaloid has exhibited cytotoxic, antibacterial, and antimalarial activities (Ang et al., 2000; Sakai et al., 1986). Pyrrole and its derivatives, a unique and diverse sponge-derived alkaloid group, show a broad spectrum of biological activities such as antiangiogenic, anti-inflammatory, antitubercular, and antimicrobial effects (Singh and Majik, 2019).
Moreover, marine sponges are the only sources of a few compounds that belong to bromopyrrole alkaloid group. Oroidin, isolated from Agelas oroides, was the first compound discovered in this class. Pyrroleimidazole unit, a derivative of oroidin, is present in all bromopyrrole alkaloid compounds, making oroidin the precursor for these compounds. Examples for marine sponge derived bromopyrrole alkaloids are clathrodin, hymenidin, and sventrin. The bromination pattern of the pyrrole moiety is responsible for the bioactivity of these components (Ebada and Proksch, 2012).
Most of the natural compounds originating from marine sponges have been recognized as potential cytotoxic agents with cytotoxic chemical structures. These structures have shown in vitro cytotoxic effects against several tumor/cancer cell lines and gained attention for future in vivo assays. Elissawy et al. (2021) listed 20 different chemical classes of alkaloids with potent cytotoxic activities, namely acridine, β-carboline, brominated, bromotyrosine, manzamine, imidazole, dimeric aaptamine, indole, guanidine, pyridine, piperidine, peptide, pyrimidine, pyrroloiminoquinone, pyrrole, steroidal, quinoline and quinolizidine, terpenoids, sesquiterpene quinones/hydroquinones, and tetrahydroisoqouinoline alkaloid. Table 2 summarizes the different cytotoxic activities of diverse classes of alkaloids extracted from marine sponges. Among the different classes of alkaloids with cytotoxicity, guanidine alkaloid (Crambescidin 814, Crambescidin 816, and Unguiculin A–C), pyrroloiminoquinone alkaloid (Dihydrodiscorhabdin A and L, and Discorhabdin A), quinolone alkaloid (Renierol, Renieramycin, Renieramycin J and Jurunnamycin A) and acridin alkaloid (Dercitine) have been shown to display strong cytotoxic activities in micromolar to nanomolar concentrations (Elissawy et al., 2021). It was reported that pyrroloiminoquinone alkaloids have more potential for developing novel anticancer drugs due to their pharmacological properties and safety (Lin et al., 2017).
 Click to view | Table 2. Cytotoxic activities exhibited by diverse classes of alkaloids extracted from marine sponges |
Sponge derived alkaloids also serve as a source of enzyme inhibitors. For instance, apatamines, extracted from genus Aaptos, showed inhibitory activity against monoamine oxidase (Ioffina et al., 1990), sortase A (Jang et al., 2007), 1,3-glucanase of marine mollusks (Sova and Fedoreev, 1990), and enzymes that damage proteasomes (Tsukamoto et al., 2010). Apatamines have either an N-methylated or a non-N-methylated 1,6-naphthyridine central moiety joined to a benzenoid unit. Utkina et al. (2021) evaluated the direct and indirect inhibitory effects of aaptamine, aaptanone, 9-demethylaaptamine, isoaaptamine, damirones A and B, zyzzyanone A, and makaluvamines H and G on the α-N-acetylgalactosaminidase (α-NaGalase) from human cancer cells and α-D-galactosidase (α-PsGal) from the marine bacterium Pseudoalteromonas sp. using in vitro studies. It was found that 9-demethylaaptamine, isoaaptamine, zyzzyanone A and makaluvamines G had irreversible slow-binding inhibitory activity on α-PsGal. At the same time, none of the compounds showed direct inhibition on the activity of cancer α-NaGalase. The chemical structures of these molecules dictate their inhibitory activity against α-PsGal. Aaptamine alkaloids have an N1-methyl group on a benzo-1,6-naphthyridine skeleton and a hydroxyl group at the C-9 position, which relates to the inhibitory activity against α-PsGal. The presence of hydroxyphenyl ring in zyzzyanone A and makaluvamine G contributes to the inhibition of α-PsGal (Utkina et al., 2021).
The sponge derived alkaloids also act as antioxidants in response to oxidative stress conditions, which are associated with several disease conditions, including neurodegenerative diseases, aging, and cancer. It was found that aromatic alkaloids aaptamine and isoaaptamine, extracted from the marine sponge Aaptos aaptos, and bromo-2′-deN-methylaplysinopsin, isolated from the sponge Hyrtios sp., exhibited antioxidant activities due to their ability to release both H atom and electrons. Alonso et al. (2016) evaluated the antioxidant activity of seven makaluvamines (A, F, G, H, J, K, and P), pyrroloiminoquinones extracted from Zyzzya genus sponges in primary cortical neurons and neuroblastoma cells using an in vitro oxidative stress model. It was found that makaluvamines J was the most active one that reduced the damage to mitochondria caused by the stressor H2O2, and its antioxidant activity enhanced the endogenous defenses of glutathione and catalase. Moreover, the release of ROS was reduced, and the mitochondrial function was regulated by a low level (10nM) of makaluvamines J. The strong antioxidant property of makaluvamines J, among other makaluvamines, is characterized by a p-hydroxyphenethyl moiety connected to an aromatic ring and a pyrrole with non-substituted nitrogen in its structure. Remarkably, the presence of non-substituted nitrogen is responsible for the antioxidant activity of makaluvamines J since other compounds that have methyl substitutions have little effect or are inactive (Alonso et al., 2016).
4.1.1.3. Terpenes
Terpenes are another group of secondary metabolites that have been significantly identified from marine sponges. Steroidal terpenoid was the first terpene identified from a marine sponge. The isoprene unit (C5H8) is the building block of terpenes, connected repetitively in a head to tail order. The linear and cyclized non-polar terpenes and polar terpenoids are two components included in the terpenes (Cox-Georgian et al., 2019). A large number of derivatives with diverse chemical structures and biological properties are obtained via modifying the structures of terpene-based compounds. The terpenes with varying chemical structures are isolated depending on the polarity of the solvents (Jiang et al., 2016). Sesterterpenoid (C25) and triterpenoids (C30), important groups of terpenoids usually extracted from marine sponges, display several biological activities (Proksch, 1994; Selvin and Lipton, 2004).
Manoalide, a compound of the sesterterpene class, is the primary metabolite produced by marine sponges. It was discovered from sponge L. variabilis and displayed a wide range of biological activities (de Silva and Scheuer, 1980). For example, it showed anti-inflammatory activity and potent inhibitory activity against the enzyme phospholipase A2 (PLA2), which provides a substrate for pro-inflammatory mediators (Ortiz et al., 1993), and it acts as an antibacterial agent for S. aureus and Streptomyces pyogenes (Melander et al., 2016). Unique functional groups present in the structure of manoalide are responsible for its varying bioactivities (Ebada and Proksch, 2012).
Sesquiterpenoid compounds, extracted from sponge Hyrtios erectus, showed an antimalarial effect on chloroquinone-resistant Dd2 strain P. falciparum (Ju et al., 2018). During the investigation of terpene-based compounds of Mediterranean Spongia officinalis, a series of sesterterpenes and four new molecules such as 7,8-epoxyfurospongin-1 (C21 furanoterpene), officinoic acid A and B (linear carboxylic acids), and isofurospongin-4 (linear furanosesterterpene) were isolated. These compounds exhibited antifungal and antibacterial activities (Manzo et al., 2011). Furthermore, cytotoxic effect on the murine leukemia P388 cell lines (cancer cells) was observed for stelletins, an isomalabaricane triterpenes with a γ-pyrone group that was extracted from sponges Stelleta tenuis and J. stellifera (Su et al., 1994). The sponge genus Stelletta is one of the rich sources of terpenoids. McCormick et al. (1996) discovered four cytotoxic isomalabarican triterpenes, such as stellettins C–F, which have been proven, in vitro, to exhibit cytotoxic effects against human tumor cell lines. Later in 2007, two more cytotoxic triterpenes, stellettins L and M, were extracted from S. tenuis in Hainan Province, China. These two molecules showed cytotoxic effects on HL-60 human leukemia cell line and stomach (AGS) cancer cell lines (Lin et al., 2007). Stellettin D, extracted from Hainan sponge Stelletta sp., exhibited significant protein-tyrosine phosphatase 1B inhibitory activity, which is beneficial for treating obesity and type 2 diabetes (Xue et al., 2013).
4.1.2. Cnidaria
Cnidaria is a diverse, extensive, ecologically, and biologically important animal phylum containing over 11,000 species worldwide (Rocha et al., 2015). Most Cnidarian species live in marine habitats, while around 40 species (commonly hydrozoans) live in freshwater environments (Jouiaei et al., 2015). Molecular phylogeny recognized Cnidaria as a sister group of Bilateria, which explains the animal relationships and evolution of basic bilaterian features, including the bilaterality, the central nervous system, and the third germ layer (the mesoderm) (Bosch et al., 2017; Han et al., 2016).
The body of Cnidaria is composed of two epithelial monolayers, ectoderm, and endoderm, throughout their life. Unlike bilaterians, they lack the third germ layer, the mesoderm in their body (Cheng, 2021; Technau et al., 2015). The general characteristic features of the Cnidarians are the presence of an extracellular matrix (fluid mass of protein and cells) called mesoglea, multiple sensory, and neuromuscular systems between the ectoderm and endoderm layers (Technau and Steele, 2011). Bilateral symmetry is absent in Cnidaria; however, most possess rotational symmetry. The digestive system of Cnidaria is incomplete and contains only one opening, known as the oral disk which acts as both the mouth and the anus. Cnidarians are called polyps if the mouth is oriented upward, whereas they are known as medusa in the downward orientation of the mouth (Figure 12) (Cheng, 2021). Another characteristic feature of cnidarians is the presence of stinging cells around the mouth and in the tips of tentacles that are used to protect them from predators and hunt down their prey. The coiled-shaped stinging cells inject and eject toxins through a dark-like tip (Technau et al., 2015).
 Click for large image | Figure 12. General body plan of Polyp and Medusa forms of Cnidarians. |
The phylum Cnidaria consists of two main groups, the Anthozoa and the Medusozoa (Figure 13). Sea anemones and corals are examples of Anthozoa, which are commonly sessile and exist solitary or in colonial polyps due to the metamorphosis of a planula larva. Anthozoa can be categorized into two classes, the Octocorallia and the Hexacorallia. The group medusozoa (hydroids, jellyfish) form gamete-bearing (free-swimming) medusa along with solitary or colonial polyps. Within the Medusozoa, four classes are distinguished, Scyphozoa, Hydrozoa, Cubozoa, and Staurozoa (Leclère and Röttinger, 2017; Technau et al., 2015). Hydrozoans usually contain a small-sized hydra that can filter water to obtain food materials (fish eggs) or attack small animals using their stinging tentacles (Santhanam, 2020). Scyphozoans are recognized as true jellyfish, whereas cubozoans consist of box jellies. Both of these classes are characterized by their bell-shaped soft bodies. Recent molecular phylogenies and specific gene similarities identified the parasitic Myxozoa as cnidarians, remarkably as a sister group of Medusozoa (Cheng, 2021).
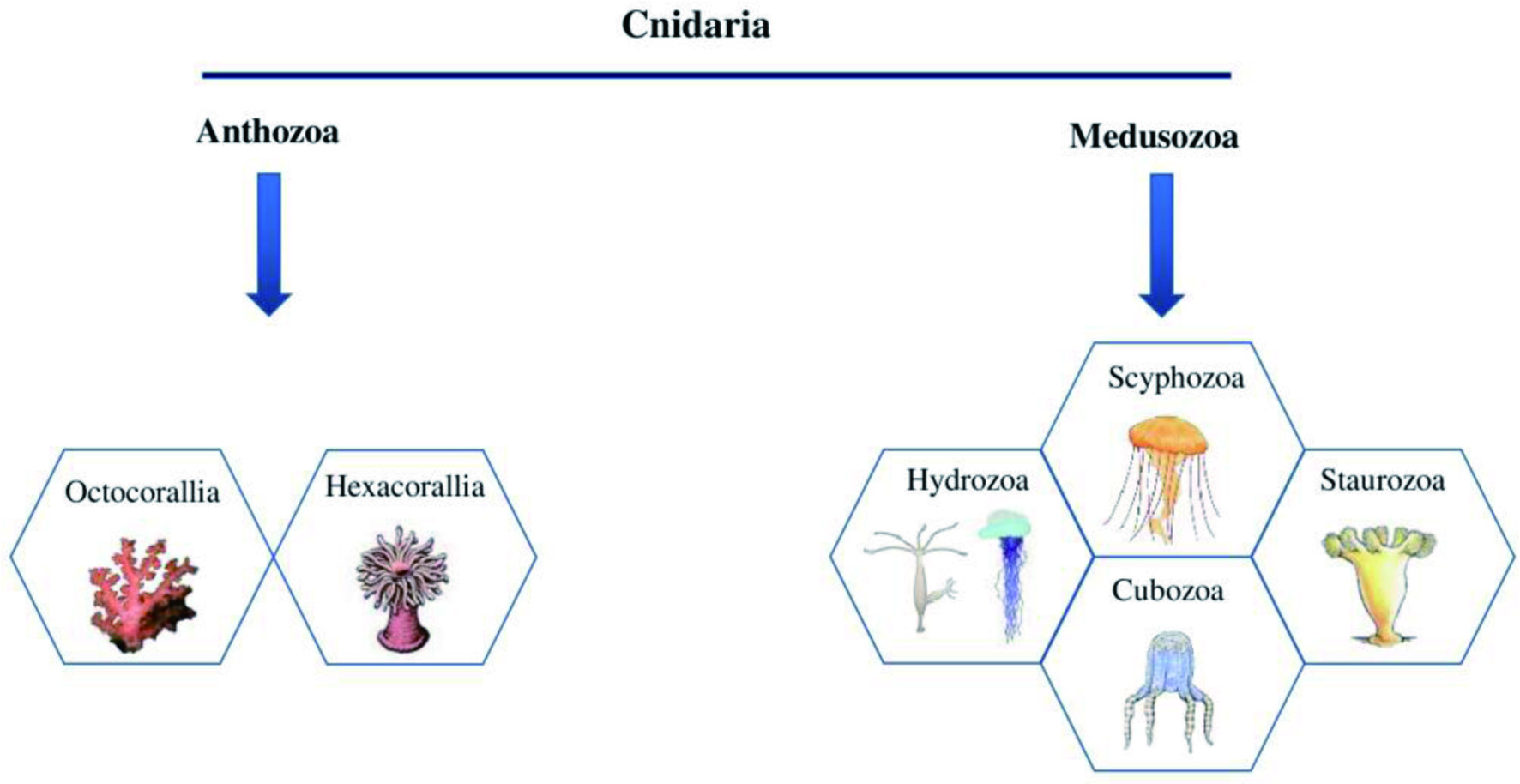 Click for large image | Figure 13. Different classes of Cnidarians. |
Phylum Cnidaria has been extensively studied as they are classified as the top group among all marine invertebrates that produce toxic substances and the oldest hierarchy of existing venomous animals (Turk and Kem, 2009). Most of the species of Cnidarians are recognized to cause envenomation threats to human beings. Toxin producing cells or glands are present in Cnidarians, which produce and encapsulate toxic polypeptides (Jayathilake and Gunathilake, 2020). Although Cnidarian toxins cause hazards to humans, these toxins are also proven to be utilized as a source of beneficial bioactive compounds for the development of novel nutraceuticals and pharmaceuticals, making Cnidarians as the predominant area of research interest (Mariottini and Grice, 2016; García-Arredondo et al., 2016). New natural products have only been discovered from 337 (3.1%) Cnidarian species, although around 11,000 Cnidarian species are known thus far (Rocha et al., 2015).
In the early 1970s, Cnidarians were recognized as a potential source of natural bioactive compounds (Mariscal, 1974). Numerous metabolites and compounds with varying structures and biological properties were isolated in later years. Notably, a total of 3,244 compounds were discovered from 1990 to 2011 (Rocha et al., 2015). It was observed that the number of new natural products isolated from Cnidarians has been greater than those from sponges since early 1990, and the trend of new bioactive compound discovery is still continuously increasing (Leal et al., 2012b). While over 20,000 compounds, belongs to the classes of terpenoids, steroids, alkaloids, polyketides, among other, have been identified since the mid of 1960, only a few compounds were utilized to develop materials of biomedical importance (Mayer et al., 2010). The majority of the new products have been discovered in the tropical regions (Asian territories) including Japan, China, and Taiwan (Rocha et al., 2015).
Cnidarians occur in many geographical habitats such as tropical reefs, polar seabed, and deep waters near hydrothermal vents. They have evolved to use their stinging cells with potent toxic substances to catch their prey and deter predators since they lack a mechanical way to prey (Paul et al., 2011). Besides, Cnidarians living in a vastly biodiverse tropical ecosystem, specifically coral reefs, produce abundant chemical compounds helpful in driving off predators and other competitors (Leal et al., 2012a). The extreme physical and chemical environmental factors of the habitats of the Cnidarians results in the secretion of different compounds with diverse biological function and structural properties. For instance, they produce compounds with bodily functions such as defensive mechanisms against fouling organisms, pathogens, microorganisms, and herbivores (Paul and Puglisi, 2004).
The bioactivities of Cnidarians based compounds and their potential for developing drugs are evidenced by their crucial role in the traditional medicine of ancient civilizations in Asian countries, particularly in India and China, to treat various disease conditions such as genito-urinary and respiratory diseases, anemia, vertigo, and headache (Gopal et al., 2008; de Zoysa, 2012). The primary group of compounds produced by Cnidarians is terpenoids (66%), followed by alkaloids, steroids, carbohydrates, and aliphatic compounds, among others, and the biological activities exhibited by these compounds include antitumor, anti-inflammatory, antimicrobial, antifoulant, anticancer, and antiulcer effects (Leal et al., 2012b). Figures 14 and 15 illustrate the percentage of different compounds isolated from phylum Cnidaria in the twenty-first century and the distribution of biological properties of these compounds, respectively.
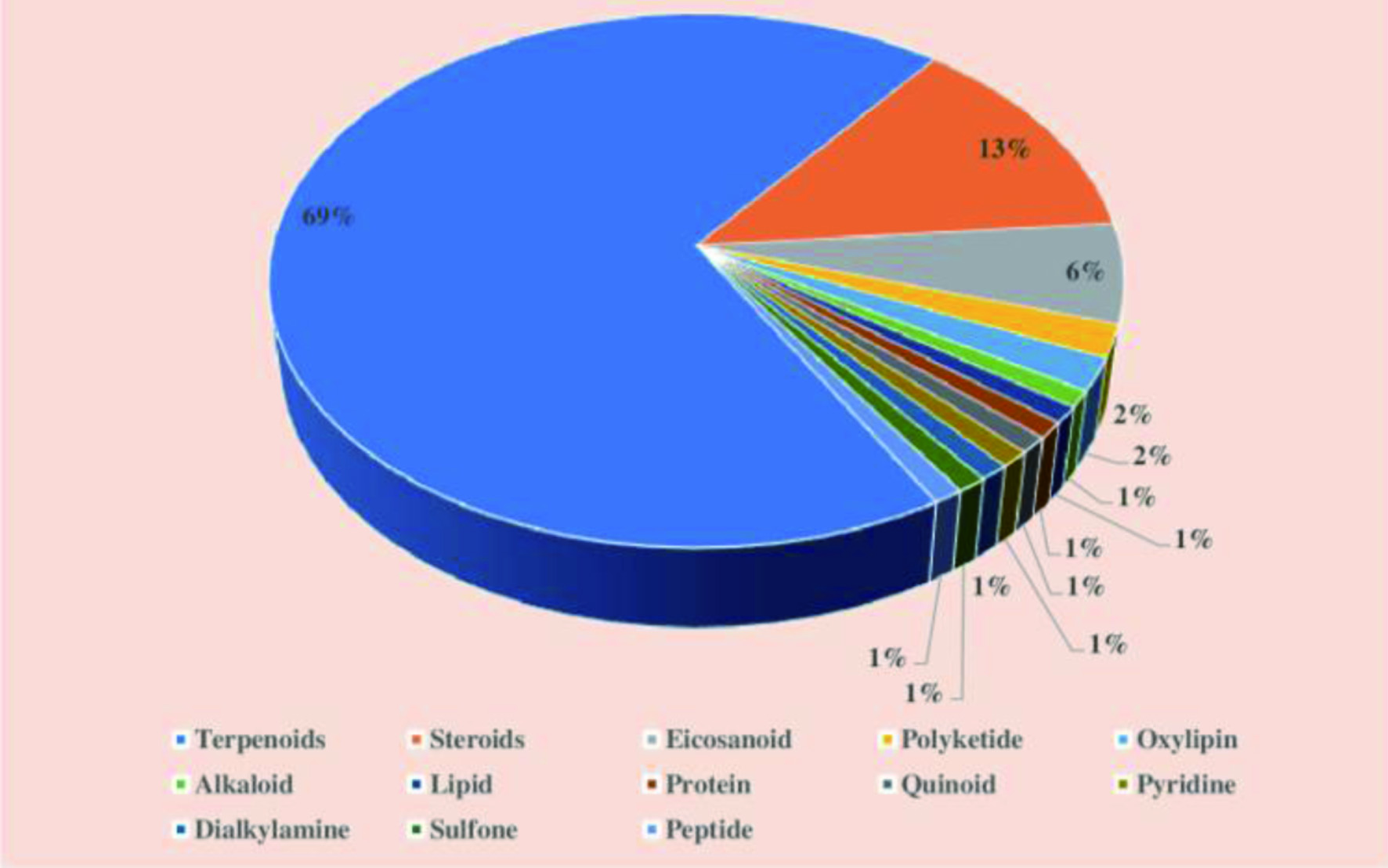 Click for large image | Figure 14. Percentages of different compounds isolated from phylum Cnidaria in the twenty-first century. |
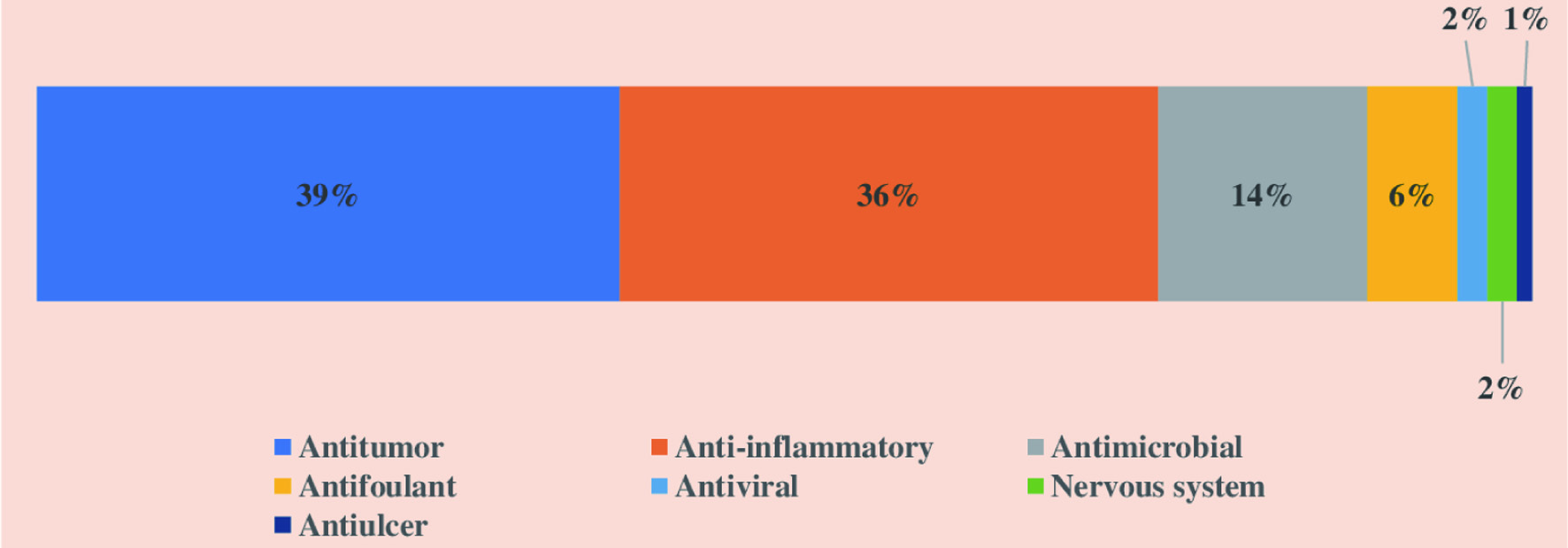 Click for large image | Figure 15. Distribution of biological properties exhibited by the compounds isolated from phylum Cnidaria in the twenty-first century. |
4.1.2.1. Class anthozoa
Out of the 3,244 new compounds isolated from Cnidarian species from 1990 to 2011, the species of class Anthozoa produced around 99%. It was found that 1,071 and 1,758 new compounds were discovered in the 1900s and 2000s, respectively (Rocha et al., 2015). Anthozoans are the class of greater biodiversity consisting of 10 orders and more than 7,500 species. The greater chemical diversity of compounds produced by Cnidarians might be due to the vast biodiversity of species of this group. Two orders of this class, Alcyonacea (soft corals) and Gorgonacea (sea fans), produce the maximum number of bioactive natural compounds. Besides, other orders, namely Scleractinia (hard corals) and Actiniaria (sea anemones), also contribute to the bioactive molecule extraction (Meyer et al., 2009; Miyaoka et al., 2006).
4.1.2.1.1. Order Alcyonacea
Soft corals, which resemble trees or plants, are typically colorful with bright colors and consisting of nutrient rich components. They produce numerous toxic substances to deter predators (Hooper and Davies-Coleman, 1995). Around 94% of the compounds produced by Cnidarians belong to the order Alcyonacea. Most of the compounds produced by soft corals have proven to possess therapeutic potential (Bhakuni and Rawat, 2005). They produce several secondary metabolites, including terpenoids, diterpenes, furanoditerpenes, sesquiterpenes, steroids and capnellane, which exhibit cytotoxic, anticancer, anti-inflammatory, HIV-inhibitory and antimicrobial activities (Rocha et al., 2015). Table 3 summarizes the biological activities of different compounds isolated from the order Alcyonacea.
 Click to view | Table 3. Biological activities of different compounds isolated from the order Alcyonacea |
Recently, a group of cembrane type diterpenoids, including four new cembranoids such as sinulacrassins A–C and ent-xishaflavalin G, as well as five known analogs were isolated from South China Sea soft coral Sinularia crassa. The cembrane type diterpenoids contain a 14-membered carbocyclic skeleton with one isopropyl and three methyl groups having varying degree of oxidation, which results in a number of analogs with unique structures. Out of the compounds discovered, S-(+)-cembrane A and sinulacrassins B exhibited α-glucosidase inhibitory activity with IC50 values of 30.31 ± 1.22 and 10.65 ± 0.16 μM, respectively (Wu et al., 2020).
Chemical investigation on cultured soft corals Sinularia flexibilis and Sinularia sandensis have resulted in five new cembranoids namely 7-acetylsinumaximol B, 4-carbomethoxyl-10-epigyrosanoldie E, dihydromanaarenolide I, isosinulaflexiolide K, and diepoxycembrene B, along with 11 known related compounds. Within the identified compounds, isosinulaflexiolide K, sinulaflexiolide K, (−)-sandensolide, 11-dehydrosinulariolide, sinulariolide and 3,4:8,11-bisepoxy-7-acetoxycembra-15(17)-en-1,12-olide (Figure 16) suppressed the accumulation of pro-inflammatory proteins, iNOS and COX-2, in an in vitro anti-inflammatory study using lipopolysaccharide induced macrophage-like cell line RAW 264.7. Moreover, it was revealed that potential anti-inflammatory activities were displayed by the cembrane type compounds with one seven-membered lactone moiety at C-1 (Tsai et al., 2015).
 Click for large image | Figure 16. Structures of important Cembranoids compounds identified from soft corals Sinularia flexibilis and Sinularia sandensis. |
Different soft corals of the eastern Red Sea were screened for antibacterial activities and among the analyzed soft corals, the crude extracts of Sarcophyton spp. and Sinularia polydactyla exhibited antibacterial activities against two-gram positive bacterial pathogens, Staphylococcus aureus and Bacillus spp. (Afifi et al., 2016). Several compounds with bioactivities were extracted from soft coral, Sinularia maxima (Metwally et al., 2020). Among the isolated compounds, ergosta-5,24-dien-3-ol, phthalic acid, di-(2-propylpentyl) ester and bis-(2-ethylhexyl) phthalate showed antioxidant activity, and E-10-methyl-11-tetradecen-1-ol propionate was proven to act as oligosaccharide provider, methyl donor, catechol-O-methyl transferase-inhibitor, methylguanidine inhibitor, and increase zinc bioavailability (Sivakumaran et al., 2020).
Abdel-Lateff et al. (2019) reported different terpenoids isolated from sixteen species of the genus Alcyonium of the order Alcyonacea, including 25 diterpenes, 42 sesquiterpenes, and 25 steroids. The renowned metabolites discovered from Alcyonium are sesquiterpenoids, which could be classified into eleven carbo-skeleton types such as cadinene, aphanmalane, bulgarane, aromadendrane, furanosesquiterene, bicyclogermacrane, guaiazulene illudalane, eudesmane, triprenylhydroquinone, and paesslerane. Further, their diterpenoids are categorized under six groups, namely cladiellin, cembrane, prenylbicyclogermacrane, eunicellin, xenicane and xenicin. It was stated that considerable number of alcyonacean metabolites are not yet biologically screened (Abdel-Lateff et al., 2019).
4.1.2.1.2. Order Actiniaria
Actinarians are commonly known as sea anemones, which is a single polyp with its base attached to a hard surface, some floats near the water surface and few live in soft sediments. Sea anemones produce more natural biologically active compounds compared to the toxins. They contain a wide range of bioactive polypeptides and proteins. Besides, they have neuropeptides, protease inhibitors, and cytolytic toxins (Štrukelj et al., 2000). During chemical investigations of sea anemone Actinia equina, Equinatoxin II, Equinatoxin III, Acrorhagin I and II, and cytolysin Equinatoxin II, Na+ channel neurotoxin and potent cytolytic proteins were isolated (Thangaraj et al., 2019; Lenarcic et al., 1997). Papain-like cysteine proteases play a major role in the onset of several diseases of the central nervous system, including Alzheimer’s disease, brain tumors, neurological autoimmune diseases, certain forms of epilepsy and cerebral lesions (Brömme and Petanceska, 2002). Equistatin was identified as a powerful inhibitor of papain-like cysteine proteinases and specific inhibitor of the aspatic proteinase cathepsin D, which is involved in the pathogenesis of breast cancer (Lenarcic and Turk, 1999). The extracts of Actinia equina were also reported to control the CVD and exhibit haemolytic activities (Suput et al., 2001).
The compounds isolated from Anemonia viridis displayed antioxidant effect and cancer inhibitory activity against A375, PLC/PRF/5 and PC3 tumor cell lines (Merle et al., 2007). The extracts of Urticina crassicornis showed antioxidant and hemolytic activity (Lee et al. 2015). Moreover, a novel biopeptide τ-AnmTx Ueq 12-1 was discovered from Urticina eques. This peptide exhibited antibacterial activity against Gram-positive bacteria, and it is useful for developing antibacterial analgesic drugs (Logashina et al., 2017).
4.1.2.1.3. Order scleractinia
The chemical investigation of Tubastraea sp. resulted in the identification of a bis(indole) alkaloid, cycloaplysinopsin C. It showed inhibitory activity against the growth of chloroquine-sensitive and chloroquine-resistant strains of P. falciparum (Meyer et al., 2009). Another species of this order yielded Cladocorans A and B, which are marine sesterterpenoids having a hydroxybutenolide moiety. This moiety plays a key role in the biological activity of these compounds (Fontana et al., 1998).
4.1.2.2. Class hydrozoa
The species of class hydrozoan occur as solitary or colonial and their representative species are hydroids and the Portuguese man-o-war. This class consists of 7 orders and nearly 3,500 species, however, biologically active compounds are isolated only from few species (Rocha et al., 2015). The marine hydroid Garveia annulate (order Anthoathecata) yielded polyketides annulins A, B, and C (Pereira et al., 2006). This annulins were proven to inhibit indoleamine 2,3-dioxygenase, which has been proposed to be responsible for the evasions of T-cell mediated immune rejection and they are more powerful than 1-methyltryptophan, one of the currently used potent inhibitor of indoleamine 2,3-dioxygenase (Muller et al., 2005). Further, the cleavage of the 2,3-bond of tryptophan is catalyzed by indoleamine 2,3-dioxygenase and it is the rate limiting and first step in the kynurenine pathway of tryptophan catabolism in mammalian cells (Grohmann et al., 2003). Marine hydroid Solanderia secunda belongs to the order Anthoathecata produced cyclopropyl oxylipins Solandelactones C, D, and G, which act as inhibitors against farnesyl protein transferase. The farnesyl protein transferase is related to differentiation and proliferation of cells and its inhibitory activity is useful to develop new anticancer drugs (Seo et al., 1996).
4.1.2.3. Class scyphozoa
The class Scyphozoa includes three orders and approximately 200 species. Leone et al., (2015) investigated the biochemical composition and nutraceutical properties of three Mediterranean Sea jelly fish species Aurelia sp.1, Cotylorhiza tuberculata and Rhizostoma pulmo. Out of the analyzed species, R. pulmo and C. tuberculate protein extracts consisted of essential amino acids such as leucine, isoleucine, histidine, methionine, lysine, threonine, phenylalanine, and valine. The phosphate buffered saline extracts of R. pulmo and C. tuberculate were shown to have high phenolic contents, which is related to the presence of phenolic amino acid residues of protein. The radical scavenging activity analysis of the aqueous and hydroalcoholic protein extracts of these three species of jelly fish and the hydrolyzed peptides resulting from pepsin and collagenase digestions exhibited significant antioxidant activities. The analysis lipid content of above three species showed ω3 EPA was more prevalent in Aurelia sp.1, whereas the ω3 EPA and ω6 arachidonic acid were abundant in R. pulmo. Besides, C. tuberculate contained ω6 linoleic acid as the major component along with EPA, arachidonic acid, ω3 eicosatetraenoic acid, and docosahexaenoic acid (DHA). The microalgal symbionts (Symbiodinium spp.) of these species are responsible for the presence of PUFAs (Leone et al., 2015).
The collagen hydrolysate extracted from ribbon jellyfish Chrysaora sp., and Rhopilema esculentum was found to exhibit anti-hypertensive and antioxidant activities (Barzideh et al., 2014; Zhuang et al., 2012). The oral ingestion of collagen and collagen hydrolysates from proteins of edible jellyfish Rhopilema was reported to reduce the photoaging of skin in mice via anti-melanogenic, antioxidant, and immunity-enhancing biochemical activities (Leone et al., 2015). Very recently, moon jellyfish Aurelia coerulea of Mediterranean Sea was investigated for lysozyme antibacterial and antioxidant activities shown by the extracts from different parts of medusa form. The soluble fractions isolated from the oral arms contained more proteinaceous and phenolic compounds exhibiting significant lysozyme-like and antioxidant activities (Stabili et al., 2021).
4.1.3. Echinodermata
Echinoderms are a phylum of marine invertebrates consisting of around 7,000 existing species and 13,000 extinct species (Gomes et al., 2014). The phylum Echinodermata is categorized into five classes: Holothuroidea (sea cucumbers), Crinoidea (sea urchins and sand dollars), Ophiuroidea (brittle stars and basket stars), Asteroidea (starfishes), and Crinoidea (sea lilies and feather stars) as shown in Figure 17 (Arnone et al., 2015). Echinoderms are widely abundant in almost all latitudes and depths of marine habitats, also present in shallow shores and reef environments (Gomes et al., 2014). One of the characteristic features of the echinoderm is the skeletal system (endoskeleton), which is made up of calcite and skeletal elements such as plates, spicules, or ossicles. The echinoderm endoskeleton is known as stereo, with a unique porous, lattice-like organization that varies between different classes. The echinoderms have a rigid body, where the plates are tightly interconnected by connective tissue ligaments (Arnone et al., 2015).
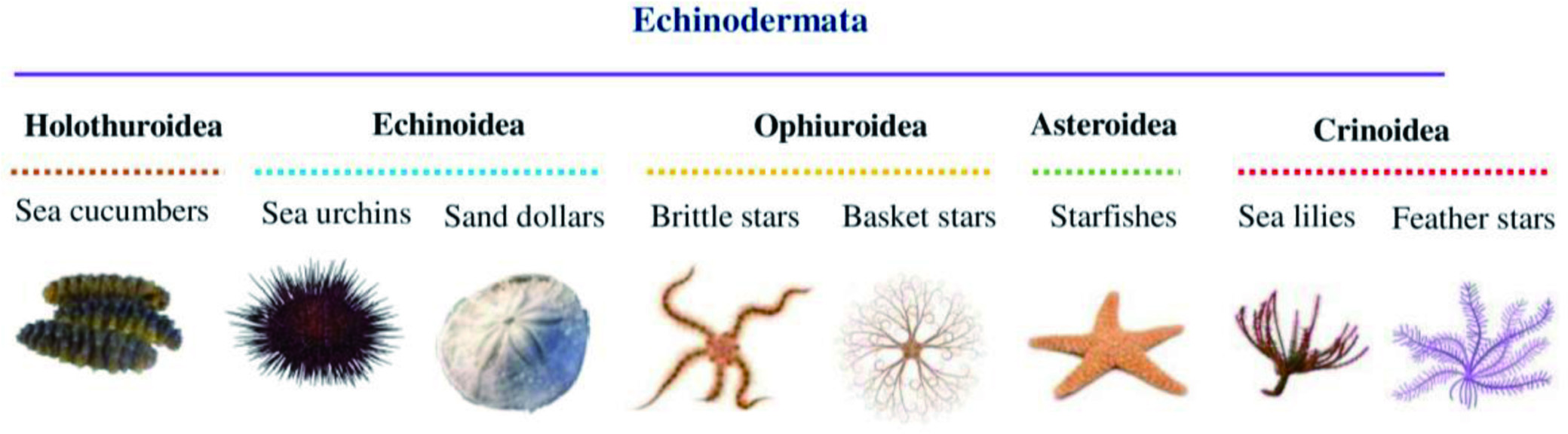 Click for large image | Figure 17. Examples of different classes of Echinodermata. |
A fivefold (pentameric) symmetry characterizes the adult body of echinoderms. This radical symmetry body plan appears after the mesoderm formation; the larval stages of echinoderm possess a distinct bilateral symmetry lost during metamorphosis. The adult echinoderms contain a water vascular system with fluid-filled reservoirs and canals useful for internal transport and locomotion. The water vascular system in echinoderms communicates with the external environment via the madreporite, a distinct skeletal plate on the surface of the body (Arnone et al., 2015). Commonly, this system bears an oral water ring and five canals, each having tiny side branches connected to the locomotory tube feet and their ampullae. The regeneration ability is also a unique feature of echinoderms. Generally, starfishes, sea cucumbers, and sea lilies lose and regenerate parts of their arms intentionally during asexual reproduction and when they are threatened by the predators. Echinoderms have the ability to reproduce asexually by transverse fission and sexually (Gomes et al., 2014).
Numerous natural compounds have been identified from echinoderms with a wide array of promising biological activities and potential for nutraceuticals and pharmaceutical applications (Mayer et al., 2013). The biological activities exhibited by these compounds include antitumor, anti-inflammatory, antibacterial, antifungal, antiviral, antiprotozoal, antituberculosis, anticoagulant, antimalarial and anti-HIV activities (Mayer et al., 2013; Laurienzo, 2010). Schoenmakers (1979) reported that vertebrate-type steroids are synthesized by echinoderms to regulate their reproduction, growth, and development. This discovery led to a hypothesis that echinoderms are capable of producing bioactive secondary metabolites that can substitute the synthetic compounds and improve human health. Echinoderms possess varying defense mechanisms such as the presence of cuvierian tubules, spine, evisceration, unpalatability, and toxic secretion. However, some species, particularly holothuroids, do not have a specific mechanism for defense. Therefore, they are primarily dependent on their chemical substances, triterpene glycosides, to escape from predators (Iyengar and Harvell 2001; Bahrami and Franco, 2016).
Out of the 28,609 marine natural products discovered until 2016, over 35% of the total products were identified from the species of phylum Echinodermata. Among the classes of echinoderms, Asteroidea and Holothuroidea were found to be the major sources of natural products. The chemical diversity of the bioactive compounds isolated from echinoderms is low in comparison to other phyla (Blunt et al., 2018). The echinoderms-derived natural products are generally sulfated compounds, categorized into the main two groups of aromatics and saponins. Saponins are the most abundant secondary metabolite extracted from holothuroids, echinoids, and asteroids. At the same time, crinoids and ophiuroids have been identified as sources of aromatic sulfated compounds, which are pigments purified from naphthoquinones or anthraquinones (Kamyab et al., 2020). Table 4 lists some of the bioactive compounds isolated from different classes of Echinodermata and their biological activities.
 Click to view | Table 4. Bioactive compounds isolated from different classes of Echinodermata and their biological activities |
4.1.3.1. Holothuroidea
Sea cucumbers are holothuroids with a soft and elongated body lacking pentaradial symmetry and a definite skeleton. Around 100 of 1,500 known species of sea cucumber are consumed by humans (Eckert, 2007; Purcell et al., 2010). Holothuroids are considered a valuable food source due to their enriched nutrition profile (vitamins, minerals, and essential amino acids), higher protein content, and lower sugar and fat contents with ω3, ω6 fatty acids (Wen et al., 2010; Bordbar et al., 2011). Sea cucumbers have been utilized in the traditional medicine in Asian countries due to their richness of marine natural components. Particularly in East Asian countries, sea cucumber is used as a natural therapy to cure eczema, arthritis, hypertension, wound injuries, rheumatism, impotence, back pain, and kidney problem, among others (Althunibat et al., 2013; Wen et al., 2010). Therefore, sea cucumber has gained immense attention as a functional food ingredient because of its bioactive compounds with therapeutic potential.
The bioactive compounds discovered from sea cucumber include polysaccharides (glycosaminoglycans, sulfated polysaccharides, fucoidan) (Marques et al., 2016; Liu et al., 2012b), peptides (Song et al., 2016; Zhao et al., 2009), lectins (Mojica and Merca, 2004), saponins, polyunsaturated fatty acids (ω3 and ω6 fatty acids) (Hu et al., 2014a; Yahyavi et al., 2012), phenolics (Mamelona et al., 2007), sterols, cerebrosides (Sugawara et al., 2006), and ceramides as well as gangliosides (Ikeda et al., 2009). Even though various secondary metabolites have been identified from sea cucumber, only a few compounds have been studied clinically (Mayer et al., 2010).
Sea cucumber contains phenols as one of the abundant class of secondary metabolites exhibiting potent antioxidant activities. Different tissues of sea cucumber have varying contents of phenols. The primary phenolic compound in sea cucumber is chlorogenic acid (about 93% on weight basis). They contain other phenolics such as rutin, coumaric acid, catechin, pyrogallol, and a minimal amount of ascorbic acid (Hossain et al., 2020; Xu et al., 2018; Fahmy, 2015). Moreover, sea cucumbers are also a good source of triterpene glycosides known as saponins. The carbohydrate chain of saponins comprises six monosaccharides, namely, D-glucose, D-xylose, 3-O-methyl-D-glucose, 3-O-methyl-Dxylose, and D-quinovose. Up to 300 triterpene glycosides have been isolated from different species of sea cucumber, and around 60% of the compounds have attached sulfate groups to the monosaccharide groups. The major triterpene glycosides identified from sea cucumber are holostane type triterpene glycosides, which are lanostane derivatives with an 18(20)-lactone (Hossain et al., 2020; Honey-Escandón et al., 2015; Kalinin et al., 2015).
The biological activities exhibited by these compounds are antioxidative (Zou et al., 2016; Ghanbari et al., 2015), anticancer (Salimi et al., 2017; Silchenko et al., 2017), anti-inflammatory (Wang et al., 2016; Li et al., 2015), anti-thrombotic (Li et al., 2017a; Luo et al., 2013), anti-diabetic (Hu et al., 2014b; Nguyen and Kim, 2015), anti-obesity (Tian et al., 2016; Liu et al., 2015b), antimicrobial (Mashjoor and Yousefzadi, 2017; Schillaci et al., 2013), anti-fatigue (Ye et al., 2017), ACE inhibitory (Ghanbari et al., 2015), anti-hyperlipidemic (Li et al., 2017a; Zou et al., 2016), hepatoprotective (Esmat et al., 2013) and memory or learning improvement (Che et al., 2017; Zhou et al., 2016) activities.
4.1.3.2. Asteroidea
Starfish and sea stars are included in the class Asteroidea, and their body is composed of a central disc, usually with five or sometimes more arms radiate. Over 1,500 species of Asteroids have been identified to date, which are widely distributed in tropical coral reefs, tidal pools, rocky shores, and deep-sea floor (Kamyab et al., 2020). The bilateral symmetry shown by the larva stage is lost during the metamorphosis and develops into radial symmetry. They are opportunistic feeders, and a majority of them are carnivorous, feeding on ascidians, sponges, mollusks, bryozoans, snails, and bivalves (Gomes et al., 2014). Asteroids show physical (spines, camouflage, autotomy, shedding, and quick locomotion) and chemical defense mechanisms to protect them from predators.
Starfishes produce several secondary metabolites such as peptides, phospholipids, alkaloids, anthraquinones, steroids, steroidal glycosides (polyhydroxylated glycosides, asterosaponin, and macrocyclic glycosides), and fatty acids. Most of these compounds are lipid-like or lipid-soluble molecules (Xia et al., 2020). Asteroids also contain numerous saponins in different organs, and saponins play a significant role in defense against predators, digestion, and reproduction (Demeyer et al., 2014). For instance, sea star Pteraster tesselatus produce a mucous-like substance containing saponin or saponin-like nature as a chemical defense. The biological properties displayed by these compounds include antiviral, antifungal, cytotoxic, hemolytic, and antimicrobial activities (Dong et al., 2011).
4.1.3.3. Ophiuroidea
The class Ophiuroidea is widely distributed, including brittle stars and basket stars. Brittle stars are the largest group of the phylum Echinodermata, containing more than 2,000 species (Kamyab et al., 2020). The body plan of the brittle star is almost similar to the starfish, but the central circular or pentagonal disc is sharply marked off from the highly flexible arms. They have varying feeding behavior such as predation, deposit-feeding, or suspension-feeding (Stöhr et al., 2012). Even though most species of brittle stars escape from predators using a physical defense mechanism, some species depend on a chemical defense system.
Brittle stars are reported to produce different classes of biologically active secondary metabolites, including brominated indoles, carotenoids, gangliosides, steroids, phenylpropanoids, and varying groups of terpenes (Nuzzo et al., 2017). For example, ophioxanthin (carotenoid) exhibiting antioxidant properties and polyhydroxysterols (steroidal compound) with antiviral properties were isolated from Ophioderma longicauda (D’Auria et al., 1985) and Astrotoma agassizii (Comin et al., 1999), respectively. Moreover, Ophiomastix mixta produces 2,3-dimethyl butanolide, a terpene compound, displaying antitumor properties (Lee et al., 2007).
4.1.3.4. Echinoidea
Sea urchins and sand dollars are included in the class Echinoidea, typically a disc, globular, or hemispherical shaped and free moving (Clemente et al., 2013). They possess tube feet that are moved by a water vascular system by pumping water in and out of the tube feet. Nearly 800 species of sea urchins have so far been discovered, and most species are utilized for human consumption, largely in Asian and Mediterranean countries (Sibiya et al., 2021). Sea urchins primarily feed on algae as they are slow-moving. Since ancient times the dry cancerous shells of sea urchins have been incorporated into the Chinese indigenous medicine to treat phlegm, inflammation, and sputum accumulation due to the therapeutic potential of their active compounds (Lu et al., 1994).
Sea urchins are found to be a rich source of fatty acids (long chain PUFAs), polar and nonpolar lipids, essential polysaccharides (sulfated fucose and galactose), and proteins (dyneins, actin, myosin, and filamentous protein). The bioactive natural products of sea urchins include polysaccharides, proteins, pigments, and minerals found in their spines, shells, gonads, testes, or pedicellaria (Jiao et al., 2015; Shang et al., 2014). Also, shells of sea urchins possess a comparatively large quantity of naphthoquinone pigments (163 mg/ 100 g in green and 121 mg/ 100 g in red species) (Amarowicz et al., 2012). The biological properties exhibited by these compounds include antioxidant, antimicrobial, anti-fatigue, anticancer, anti-inflammatory, and anti-ulcer activities (Vasconcelos et al., 2018; Soleimani et al., 2016). The scientific studies on sea urchins are currently increasing due to their remarkable medical properties and potential use in pharmaceutical industries.
The shells, gonads, and intestine of sea urchins contain polysaccharides, for example, the intestine of sea urchins was found to have water-soluble polysaccharides (Zhang et al., 2003), and bioactive polysaccharides were isolated from the gonads of Strongylocentrotus nudus, and sulfated polysaccharides were extracted from the shells of Hemicentrotus pulcherrimus (Shang et al., 2014). Moreover, the teeth and spiny shells of sea urchins are the rich sources of several bioactive proteins (Kuwahara et al., 2009; Veis et al., 2002). Importance bioactive proteins such as Strongylostain 1 and Strongylostain 2 were extracted from the gonads of the sea urchin Strongylocentrotus droebachiensis (Lu et al., 1994).
The gonads of sea urchin also serve as a rich source of several fatty acids, around 40 different fatty acids containing alcohol, glycol, cholesterol, sterol, sitosterol, phosphatidylinositol, phosphatidylcholine, phosphatidylserine, and cardiolipin were extracted from sea urchin gonads (Shang et al., 2014; Wang et al., 2012b). Besides, sea urchins were identified as a rich source of pro-vitamin A, sulfolipids, β-carotene, and certain xanthophylls exhibiting several therapeutic properties (Pozharitskaya et al., 2015). Numerous antioxidative compounds like bryostatin, didemnin, and dolasstatin were extracted from spines, shells, and pigments of red sea urchin Pseudocentrotus depressus, purple sea urchin Anthocidaris crassispina, and green sea urchin Hemicentrotus pulcherrimus (Powell et al., 2014; Sahara et al., 2002).
4.1.3.5. Crinoidea
The class Crinoidea is the most primitive form of existing echinoderms. Crinoids include sea lilies and feather stars, which have three major body sections: the calyx, the stem, and the arms. The most important organs, digestive and reproductive organs, are present in the globular or cup-shaped capsule called calyx (Gomes et al., 2014). Sea lilies are sessile and widely present in the deep sea (>100 m), while feather stars are abundant in the coral reefs of the deep sea and intertidal oceans. Crinoids possess both chemical and physical mechanisms to escape from fish predators (Kamyab et al., 2020). For instance, some crinoids use spike-like pinnules as the physical defense and oxidized quinones and polyketide derivatives as toxic chemical substances for their defense as well as for their colorful appearance (Goto et al., 2015; Feng et al., 2017).
Although around 700 species from 16 different genera of crinoids have so far been identified, only about 25 species were examined for natural bioactive compounds (Feng et al., 2017). Inagaki et al. (2007) discovered ganglioside and cerebrosides from Comanthina schlegelii. naphthopyrones comaparvin with anti-inflammatory properties and gymnochrome D (anthraquinoid pigment) with antiviral activities were isolated from Comanthus parvicirrus (Chovolou et al., 2011; Chen et al., 2014), and Gymnocrinus richeri (Laille et al., 1998), respectively.
4.1.4. Mollusca
Mollusca is one of the largest Metazoan phyla next to arthropods in the number of taxonomic diversities. This phylum consists of approximately 850,000 known existing species and 35,000 known fossil species (Zhang, 2013). The classes of the phylum Mollusca include Gastropoda, Bivalvia, Cephalopoda, Scaphopoda (tusk shells), Monoplacophora (circular shape body with a single, cap-like shell), Aplacophora (spicule-bearing, simple worms), and Polyplacophora (Chitons - flattened, ovoid, shell plate-bearing). Among all Mollusca species, nearly 95% belong to either gastropods (80%) or bivalves (15%), the most studied groups of molluscs, while the other classes are not much prevalent (Haszprunar, 2020). Figure 18 shows examples of species belong to the classes Gastropoda, Bivalvia, and Cephalopoda.
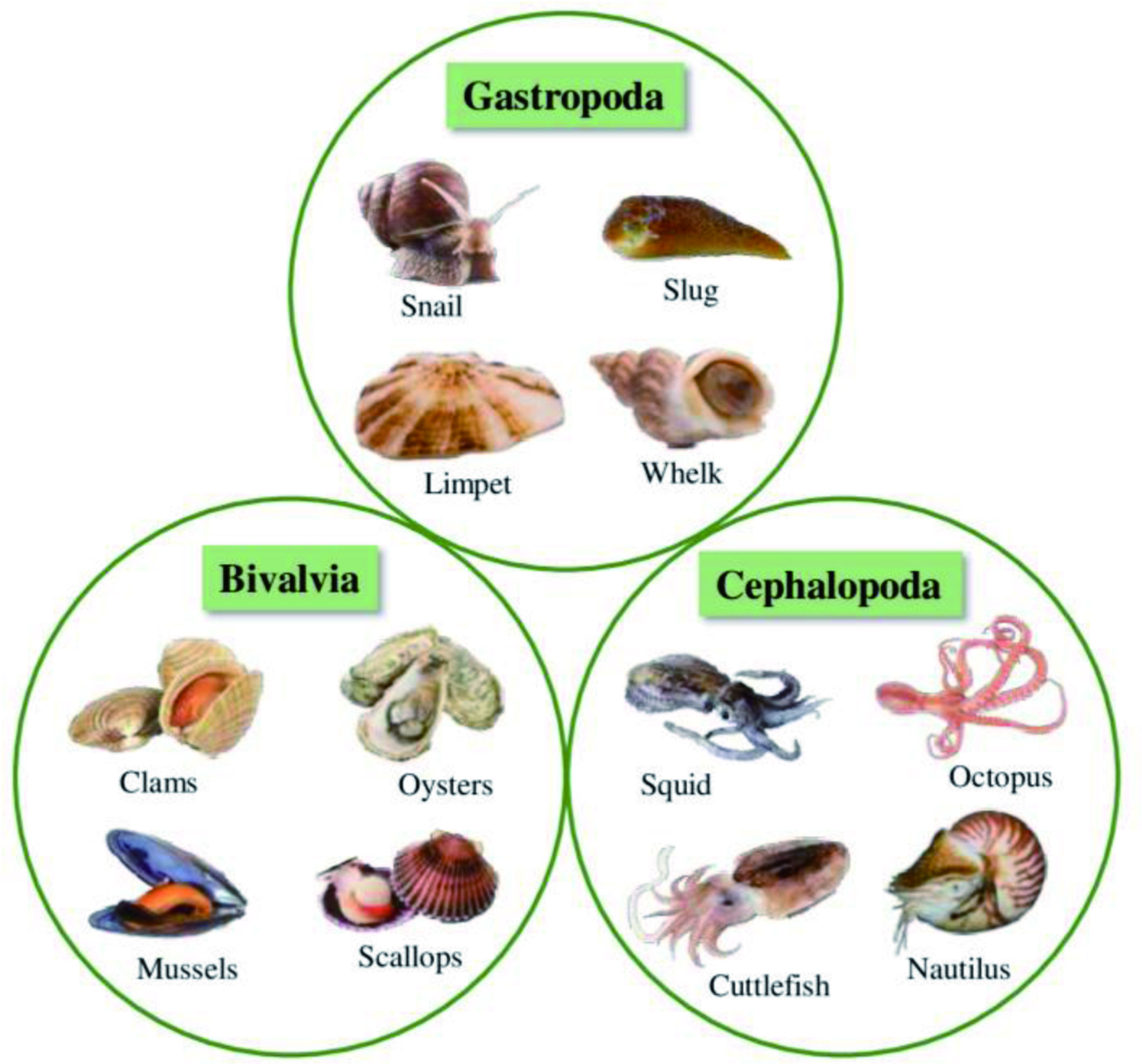 Click for large image | Figure 18. Examples of species belong to the classes Gastropoda, Bivalvia, and Cephalopoda. |
The size of the body of molluscs ranges from 0.4 mm (omalogyrid gastropods) to 7 m (giant squids without their arms), and they are characterized by different contrasting body plans (Haszprunar, 2020). The phylum Mollusca live in various habitats such as marine basins, freshwater, and land. Most of them either have a shell (mechanical defense) or an effective swimming mechanism to protect them from predators. Besides, some species, particularly the soft-bodied molluscs, produce defensive chemicals to deter predators (Avila, 2006). Molluscs play a significant role in recycling nutrients, water filtration, and soil generation and act as bioindicators of the quality of their aquatic habitats (Àvila et al., 2000). The immune systems of the human body were found to be enhanced by the range of nutrients present in molluscs (Khan and Liu, 2019).
Several marine molluscs are used in traditional medicine as a source of therapeutically essential components by many cultures worldwide (Khan and Liu, 2019), which proves the potential of the molluscs-based compounds to be used in pharmaceutical, functional foods, and nutraceutical applications. The isolation of biologically active secondary metabolites from molluscs has increased, with over 1,145 compounds discovered in the past few decades (Benkendorff et al., 2015). Only a tiny proportion (less than 1%) of the known molluscan species have been analyzed for the occurrence of natural bioactive compounds. At the same time, about 52% of the secondary metabolites isolated from molluscs were not investigated for the presence of biological activities (Ahmad et al., 2018). Most of the bioactive compounds were extracted mainly from the class Gastropoda (848 compounds), followed by Bivalvia (190 compounds) and Cephalopoda (24 compounds) (Avila, 2006; Benkendorff, 2010; Chakraborty and Joy, 2020).
4.1.4.1. Class gastropoda
The class Gastropoda contains approximately 70,000 species, including snails, slugs, limpets, and whelks. Gastropods have tremendous commercial potential, gaining significant attention worldwide. Type of organism, body compartments, reproductive cycle, collection sites, seasonal changes, temporal variations, and spatial changes determine the nutritional profile of gastropods (Smoothey, 2013). The protein contents of the predatory gastropods are higher than that of herbivorous ones, while the lipid content is higher in herbivorous gastropods compared to predatory ones (Chakraborty and Joy, 2020). Besides, gastropods are rich in other nutrients such as vitamins, minerals, PUFAs (DHA and EPA), and essential amino acids. Salas et al. (2018) studied the nutritional values of low-valued gastropod species C. ramosus, the branched murex from the Gulf of Mannar. They found that these species contain a lower sodium/potassium ratio and higher contents of calcium, phosphorous, and selenium (antioxidative mineral). Furthermore, it was suggested that C. ramosus could be used as a functional food to increase bone mineralization and reduce the risk of CVD and hypertension due to the presence of essential nutritional components (Salas et al., 2018).
An abundance of secondary metabolites with biological activities has been isolated from the phylum Gastropoda. The major chemical classes of compounds identified from gastropods are dominated by the terpenes (55%), followed by polypropionates, polypeptides, alkaloids, fatty acid derivatives, sterols, and macrolides (Figure 19). Recently, cembrane-type diterpenoids with antioxidative and anti-inflammatory activities were identified from the organic extracts of muricid gastropod C. ramosus (Chakraborty et al., 2020), also the same species produced two unusual Δ5 sterols with anti-inflammatory properties (Salas and Chakraborty, 2018b). The organic extract of Babylonidae gastropod B. spirata contains a 16-membered polyether macrocyclic lactone exhibiting antioxidative and anti-lipoxygenase activities (Salas and Chakraborty, 2018a).
 Click for large image | Figure 19. Relative proportion of the various chemical classes of secondary metabolites isolated from gastropods since 2000. |
In 1989, kabiramide B with antifungal activity was discovered from Pacific nudibranch Hexabranchuss anguineus (Matsunaga et al., 1989b). Antimicrobial compounds manoalide and secomanoalide were identified from Chromodoris willani (Uddin et al., 2009). Chemical investigation of Australian Scutus antipodes yielded epimers scutinin A and B exhibiting antibacterial and antifungal activities (Chand and Karuso, 2017). Anti-inflammatory malyngamide S and 6-bromoisatin, along with its analogs, were isolated from B. leachii and Dicathais orbita, respectively (Appleton et al., 2002; Ahmad et al., 2017). Hawaiian H. sanguineus was found to produce ulapualides, C–E, and Actinocyclus papillatus contains a diacylguanidine actinofide with moderate antiproliferative activity (Parrish et al., 2017; Carbone et al., 2017). C. ramosus yielded drimane-type sesquiterpenoid, ramosane with antioxidant properties attenuating the 5-lipoxygenase and carbolytic enzymes (Chakraborty and Salas, 2019). A lactonic steroid with 1, 10-8, 9-disecoergostane framework, extracted from B. spirata, showed carbolytic enzyme inhibitory and anti-inflammatory activities (Chakraborty et al., 2019b).
Monodontins A and B, terahydropyrans purified from the snail Monodonta labio, showed weak cytotoxic activities (Huong et al., 2017). Lamellarin N (alkaloid) isolated from Lamellaria sp. and hectochlorin and deacetyl derivatives obtained from B. leachii exhibited cytotoxic effects (Pla et al., 2006; Suntornchashwej et al., 2005). Pleurobranchus albiguttatus and P. forskalii were reported to produce cytotoxic haterumaimides L and M along with 3β-hydroxychlorolissoclimide (Fu et al., 2004). Pulmonate T. peruvianus was found to contain labdane diterpenes and cytotoxic polyhydroxylated steroids (Dıaz-Marrero et al., 2003). Auriculol, a cytotoxic squalene metabolite, was identified from a sea hare Dolabella auricularia (Kigoshi et al., 2001). Aplyronine A and sesquiterpene derivatives with antitumor properties were purified from A. kurodai and A. dactylomela, respectively (Yamada et al., 1993; Dias et al., 2005). Table 5 summarizes some of the important gastropod metabolites discovered since 2000 apart from the compounds mentioned above.
 Click to view | Table 5. Some of the important gastropod metabolites discovered since 2000 |
4.1.4.2. Class bivalvia
The class Bivalvia is also known as Pelecypoda or Lamellibranchia containing around 20,000 species that include clams, oysters, mussels, and scallops. Bivalves occupy the highest percentage of the total edible molluscs, and they have the nutritional content of proteins, amino acids, vitamins, and minerals along with ω3 PUFAs (EPA/DHA) (Astorga-Espana et al., 2007). For instance, P. malabarica and V. cyprinoides were found to contain higher levels of the following components; vitamins A (greater than 35 IU) and D3 (greater than 160 IU), calcium and phosphorous (greater than 530 mg/100 g wet tissue), selenium (greater than 25 μg/100 g wet tissue) along with ω3 PUFAs (higher than 15% of total fatty acids) (Joy and Chakraborty, 2017a).
Bivalves were not widely studied for biomedical and pharmaceutical properties; however, some previous reports revealed the presence of several bioactive secondary metabolites in Bivalvia (Benkendorff, 2010; Chakraborty et al., 2014a). The predominant chemical class of compounds identified from bivalves is sterols (41%), followed by polypropionates, terpenes, alkaloids, polypeptides, fatty acid derivatives, and macrolides (Figure 20). For the first time, 1,1′-dimethyl- [2, 2′]-bipyridyldiium salt was isolated as a natural derivative from the clam Callista chione (Vagias et al., 2000). The Japanese Ark clam, Scapharca broughtonii was found to contain N-methyl-D-glutamic acid, which was the first time to extract it from a natural source (Tarui et al., 2003). The major carotenoids in bivalves include lutein, peridinin, peridininol-5, pyrrhoxanthin 5,8-furanoxide, 8-furanoxide, 8-furanoxide, 7,8-didehydro-β-cryptoxanthin and pyrrhoxanthinol-5. In addition, they contain a few carotenoids such as corbiculaxanthin-3′-acetate, 6-epiheteroxanthin, and corbiculaxanthin that were not identified from other shellfishes earlier (Maoka et al., 2005a, 2005b). Figure 21 illustrates the structure of the main carotenoids present in bivalves.
 Click for large image | Figure 20. Relative proportion of the various chemical classes of secondary metabolites isolated from bivalves since 2000. |
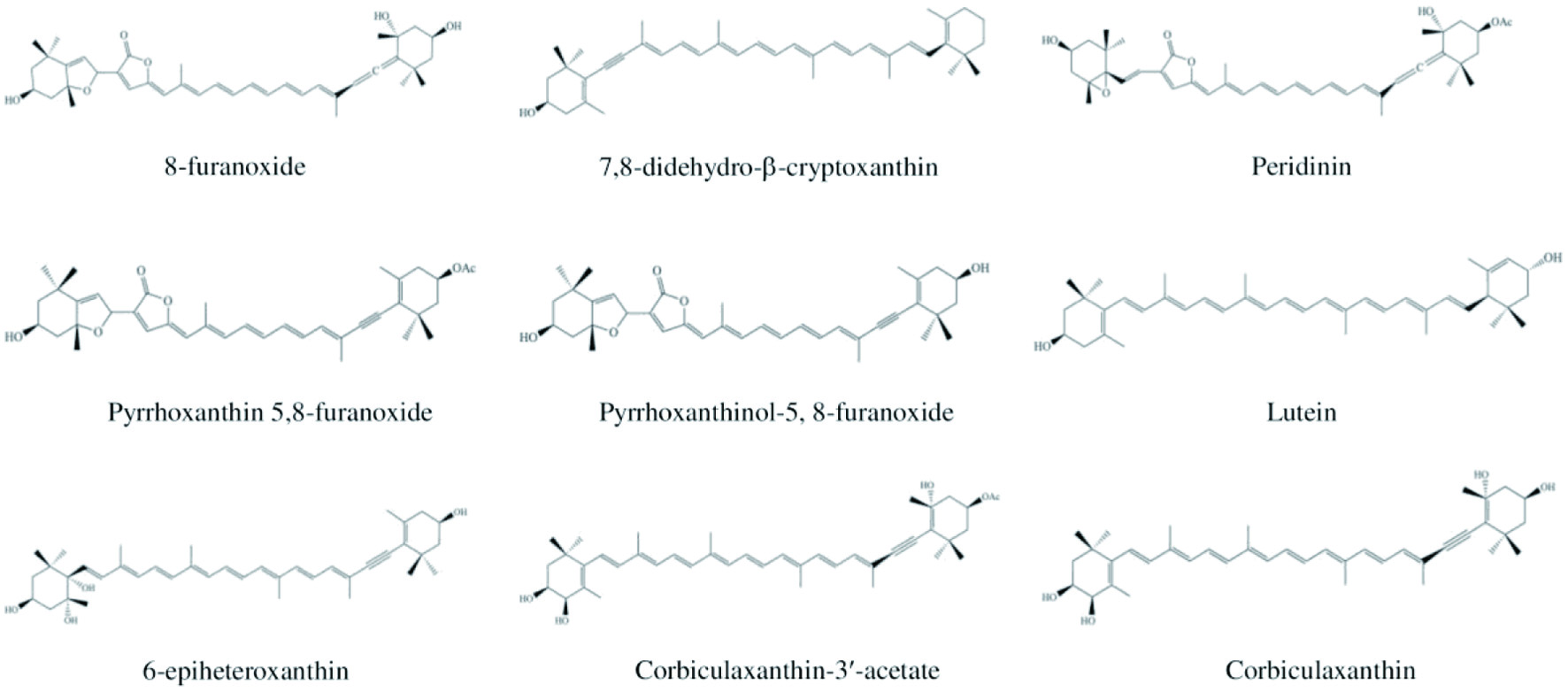 Click for large image | Figure 21. Chemical structures of the main carotenoids present in bivalves. |
Bivalves contain anti-inflammatory agents, resolvins D and E, responsible for combating the inflammatory prostanoids biosynthesis (Chakraborty et al., 2014b). The chemical investigation of V. cyprinoides yielded pyranoids, isochromenyl group of compounds, O-spirocyclic ether derivatives (oxygenated heterocyclics), and an irregular meroterpenoid derivative exhibiting antioxidative and anti-inflammatory properties (Joy and Chakraborty, 2018b, 2018c). P. canaliculus, New Zealand green-lipped mussel, contains anti-inflammatory 7, 11, 14, 17-eicosatetraenoic acid as well as a homologous series of ω3 PUFAs (Treschow et al., 2007). Moreover, anti-inflammatory and antioxidative aryl polyketides were purified from P. malabarica, which are useful for preventing the inflammatory ailments induced by oxidative stress and substituting the commercial antioxidants in food applications (Joy and Chakraborty, 2017c).
Pacific edible oyster, C. gigas, was found to possess a polyphenolic compound named 3,5- dihydroxy-4-methoxybenzyl alcohol with potential antioxidative properties (Watanabe et al., 2012). Two anti-inflammatory benzo[h]naphtho[1,2-c]chromene derivatives with chromene-16-carbaldehyde and chromen-12-one functionalities, two dodecahydro-phenanthrenone derivatives, and two sterol analogs were extracted from Asian green mussel P. viridis (Chakraborty et al., 2019a). The extract of C. madrasensis was found to have two phenylacetyloxy-trimethylpicene-23-carboxylate derivatives displaying antioxidant and anti-inflammatory activities (Chakraborty and Joy, 2019).
Scallops (family Pectinidae) contains the metabolites of diatoxanthin and alloxanthin, namely pectenolone, pectenol, hydroxyalloxanthin, and 4-ketoalloxanthin (Faulkner, 2000a). Antimicrobial peptides, spirolides B–C and mytilin-A were isolated from M. edilis and M. edulis, respectively (Charlet et al., 1996; Seo et al., 2005). The analogs of peptide spirolides B–C displayed antimicrobial activities against marine yeast, fungi, and Vibrio (Charlet et al., 1996). Antitumor compounds bathymodiolamides A and B were extracted from Bathymodiolus thermophiles (a deep-sea mussel) (Andrianasolo et al., 2011). Table 6 shows some of the important metabolites produced by bivalves since 2000 besides the above-described compounds.
 Click to view | Table 6. Some of the important metabolites produced by bivalves since 2000 |
4.1.4.3. Class cephalopoda
The class Cephalopoda includes 900 existing and about 40,000 fossil species. The examples of species included in this class are squids, octopuses, cuttlefish, and nautiluses. Cephalopods are a rich source of protein, minerals (antioxidative selenium), vitamins, and essential amino acids (Zlatanos et al., 2006). Most species of cephalopods contain high levels of lipids (greater than 2 mg/100 g), particularly rich in PUFAs such as EPA and DHA, that are considered the key biomarkers of cephalopods (Chakraborty et al., 2016; Simopoulos, 2009).
In addition, significant amounts of bioactive secondary metabolites have been extracted from cephalopods; however, only a limited number of publications are available. Figure 22 shows the critical metabolites identified from cephalopods. These include cyclophosphamine, a popular chemotherapeutic drug, isolated from squid ink (Zhong et al., 2009), cytotoxic tyrosinase from the cuttlefish Sepia officinalis ink (Russo et al., 2003), and cardiovascular peptide from the brain of Octopus vulgaris (Kanda and Minakata, 2006), among others. The biochemical analysis of U. duvauceli yielded C20 diterpenoid (22a), irregular C15 sesquiterpenoid (22b), and C19 furanonorditerpenoid (22c), which are antioxidative oxygenated terpenoids with anti-inflammatory activities (Chakraborty et al., 2021). Sterol derivative (22d) and octahydroazulenopyrandione (22e) with antioxidant and anti-inflammatory activities were purified from A. marginatus (Chakraborty and Joy, 2019).
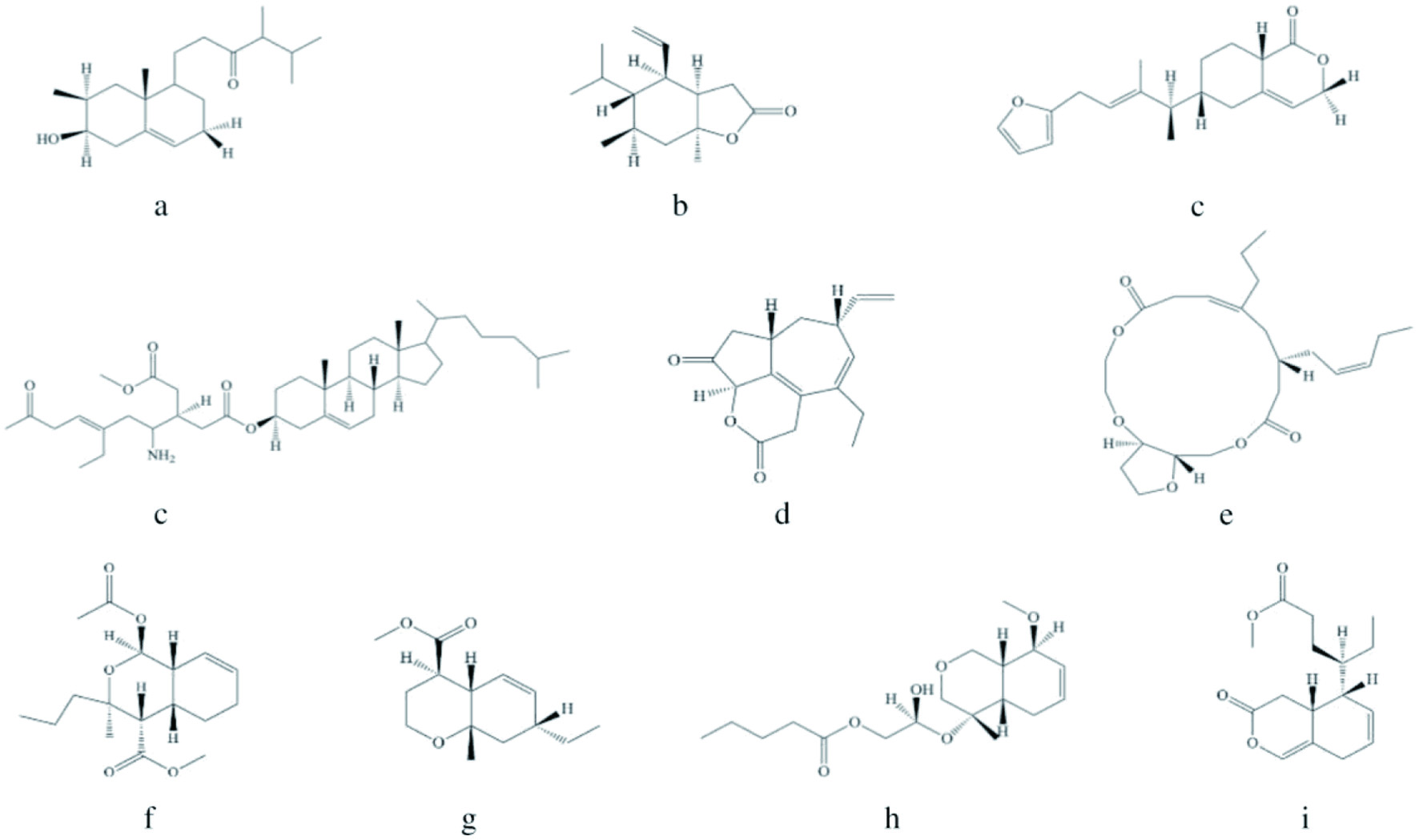 Click for large image | Figure 22. Important metabolites identified from cephalopods. [a - C20 diterpenoid; b - irregular C15 sesquiterpenoid; c - C19 furano norditerpenoid; d – Sterol derivative; e - octahydroazulenopyrandione; f - 14-((Z)-pent-14b-en-14a-yl)-16-propyl-octahydro-furo [1,4,8] trioxacyclohexadecine-12,19-dione; g - methyl 1- acetoxy-hexahydro-3-methyl-3-propyl-1H-isochromene-4-carboxylate; h - methyl 7-ethyl-hexahydro-8a-methyl-2H-chromene-4-carboxylate; i - 11-(hexahydro-8-methoxy-4-methyl-1H-isochromen-4-yloxy)- 11- hydroxyethyl pentanoate; j - methyl 9-(tetrahydro-3-oxo-3H-isochromen-5-yl) hexanoate]. |
Moreover, four macrocyclic lactones exhibiting antihypertensive properties were identified from Octopodidae cephalopod A. neglectus. Out of these compounds, a 16-membered cyclic bislactone derivative, 14-((Z)-pent-14b-en-14a-yl)-16-propyl-octahydro-furo[1,4,8] trioxacyclohexadecine-12,19-dione (22f) showed protecting effect against angiotensin-II stimulated cardiac hypertrophy on the H9C2 cell lines (Chakraborty et al., 2019c). Recently, Krishnan et al. (2020) investigated spineless cuttlefish S. inermis and identified four chromenyl derivatives, including methyl 1-acetoxy-hexahydro-3-methyl-3-propyl-1H-isochromene-4-carboxylate (22g) and methyl 7-ethyl-hexahydro-8a-methyl-2H-chromene-4-carboxylate (22h) exhibiting anti-inflammatory and antioxidant activities in vitro. The same species yielded compounds with antihyperglycemic and antioxidative properties such as 11-(hexahydro-8-methoxy-4-methyl-1H-isochromen-4-yloxy)-11-hydroxyethyl pentanoate (22i) and methyl 9-(tetrahydro-3-oxo-3H-isochromen-5-yl) hexanoate (22j) (Krishnan et al., 2019).
4.1.5. Arthropoda (crustaceans)
Arthropods are one of the most ubiquitous animal groups, with more species than other phyla. The subphylum Crustacea is the predominant group of invertebrates among marine arthropods, and they have higher economic importance (Gonçalves and de Oliveira, 2016). The characteristic features of the crustaceans include a segmented body with a pair of jointed appendages on each segment and a hard exoskeleton made up of chitin and calcium. Crustaceans include crabs, shrimps, lobsters, barnacles, krill, and crayfish, which account for around 6.4 million tons (9.7%) of the total aquaculture production (66.6 million) in 2012 (FAO, 2014). The annual production of crustaceans is continually increasing; for instance, global shrimp production has increased by 4.8% from 2016 to 2019 (Gulzar and Benjakul, 2019).
Both edible and inedible parts of the crustaceans are recognized as rich sources of natural bioactive compounds such as bioactive peptides, carotenoids, chitosan and its derivatives, including chitosan oligosaccharide (COS) and glucosamine (Shahidi and Brown, 1998). The primary structural component of the crustacean’s exoskeleton (shell) is chitin, which can be deacetylated to chitosan and its derivatives for many applications (Elieh-Ali-Komi and Hamblin, 2016).
4.1.5.1. Crab
Crabs are a specific group of decapod crustaceans living in diverse habitats such as freshwater, marine, intertidal, terrestrial, and semi-terrestrial. Around 7,000 crab species have been identified to date (John et al., 2018; Ng, 2017). They are one of the most important seafood with healthy and highly nutritious meat. Crabmeat is rich in digestible proteins, free and essential amino acids, unsaturated fatty acids (ω3 fatty acids), vitamins, minerals (iron, calcium, zinc, phosphorus, selenium, and potassium), and glycosaminoglycans (Mandume et al., 2019; Wang et al., 2018c). The legs, claws, and viscera of the crab are also potential sources of high-quality protein, lipids, antioxidants, calcium, enzymes, and pigments with health-promoting properties (Tremblay et al., 2020). Moreover, both meat and by-products of crabs contain bioactive molecules with specified functions (Hamdi et al., 2020; Kang et al., 2019). Furthermore, crab shell contains chitin, chitosan, and COS with varying biological and functional properties (Shahidi et al., 2019). The processing discards of snow crab (Chionoecetes opilio) were reported to contain carotenoids (astaxanthin and its esters) and chitin (Shahidi and Synowiecki, 1991).
Numerous studies have revealed that peptides and various shell extracts of crabs have therapeutic potential with antitumor, antimicrobial, and immunomodulatory activities (Rainey et al., 2021; Long et al., 2021; Narayanasamy et al., 2020; Al-Shammari et al., 2012). For instance, several bioactive components were derived from various tissues and organs of Portunid crabs (Laith et al., 2017). An antimicrobial peptide, dromidin, was identified from the haemolymph extract of sponge crab Dromia dehaani (Anbuchezian et al., 2018). It was reported that haemolymph extract of C. lucifera showed antioxidant activity (Soundarapandian et al., 2014), and haemolymph extracts of seven different marine crabs showed antifungal and antibacterial effects on eight fungal strains and ten bacterial strains, respectively (Anbuchezhian et al., 2009). Moreover, protein hydrolysates of marine crab Portunus sanguinolentus showed antioxidant, antihypertensive, antimicrobial, and anticancer activities (Manjusha et al., 2017).
Recently, Hamdi et al. (2020) analyzed the carotenoid content of blue crab Portunus segnis using different extraction methods. It was revealed that carotenoproteins present in this crab could be used as a natural antimicrobial and antioxidant agent. Carotenoproteins are the important sources of carotenoids, mainly astaxanthin and its esters. Naczk et al. (2004) found that European green crab (Carcinus maenus) meat is a rich source of carotenoids (5.1–19.2%), while the shell discards contained a relatively large amounts of chitin (12.6 - 14.5%) and carotenoids (4.4–9.3%) on a dry weight basis. Bioactive hydrolysates were developed from underutilized green crab by enzymatic proteolysis using Protamex. This hydrolysate exhibited more significant α-glucosidase inhibitory activity with the potential for treating type 2 diabetes (Kang et al., 2020).
Bioactive peptides, carotenoids, chitosan, and their derivatives obtained from crabs showed excellent antioxidant activity. For example, Jiang et al. (2018) investigated the antioxidant activity of protein hydrolysates produced from the crab shell of Portunus trituberculatus with Protamex and Flavourzyme modified with fructose. They found that fructose modification enhanced its antioxidant properties. The hydrolysates produced from the same species with pepsin and Protamex exhibited antioxidant activities in 2,2-azino-bis (3-ethylbenzothiazoline- 6-sulphonic acid) radical scavenging activity (ABTS-RSA), 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity (DPPH-RSA) and reducing power assay (RPA) (Jiang et al., 2017). Besides, astaxanthin isolated from three species of crabs such as Portunus sanguinolentus, Paralithodes brevipes, and Callinectes sapidus exhibited strong antioxidant properties (Suganya and Asheeba, 2015).
4.1.5.2. Shrimp
Shrimp and shrimp products are the most important seafood with high nutritional value consumed all over the world. The global shrimp production was 5.03 million tons in 2020 and is expected to grow up to 7.28 million tons by 2025 (IMARC, 2020). More than 80% of shrimps are produced by Asian countries, particularly Thailand (Mao et al., 2017). Shrimps are processed for storage and export with or without shells, which results in the solid waste of around 50-60% as by-products containing shells, viscera, head, etc. (Senphan and Benjakul, 2012). Shrimps and their by-products have been identified as potential sources of bioactive compounds such as peptides, chitosan and its derivatives, pigments (mainly astaxanthin and its esters), enzymes, lipids, minerals, and vitamins (Nirmal et al., 2020; Shahidi and Synowiecki, 1991).
Biologically active carotenoids have been isolated from by-products of shrimp Penaeus indicus using the enzyme treatment (trypsin, papain, and Alcalase) in the extraction medium of sunflower oil (Sachindra and Mahendrakar, 2011). Shrimp Penaeus japonicus was reported to contain different of carotenoids such as β-carotene, canthaxanthin, astaxanthin, lutein, echinenone, phoenicoxanthin, dihydroxy-pirardixanthin, and zeaxanthin (Shahidi and Brown, 1998). Astaxanthin extracted from shrimp (Penaeus mondon) shell exhibited anticancer activity against ovarian cancer cell line (PA-1) (Srinivasan et al., 2018). Moreover, Pacific white shrimp (Litopenaeus vannamei) waste heads derived astaxanthin showed antioxidant activity (Superoxide and nitric oxide scavenging activity) and anti-inflammatory activity by inhibiting tumor necrosis factor-α in rat alveolar macrophages (Santos et al., 2012). Wang et al. (2012a) reported that astaxanthin extracted from shrimp waste (head and carapace) had a hypoglycemic effect in alloxan induced diabetic mice. Further, methanol extract of astaxanthin from Asian tiger shrimp (Penaeus monodon) shell displayed anti-inflammatory and antibacterial activity against Pseudomanas auriginosa, S. mutans, E. Coli, S. aureus, and S. typhi (Sukmawati et al., 2019). Astaxanthin derived from shrimp (L. vannamei) shell showed tyrosinase inhibitory and antioxidant activity (Chintong et al., 2019).
Protein hydrolysates extracted from Pacific white shrimp cephalothorax using autolysis and Alcalase hydrolysis exhibited varying antioxidant activities (Sinthusamran et al., 2018). The head of the shrimp Penaeus monodon was identified as the potential source of antioxidant rich carotenoprotein (Sowmya et al., 2011). In addition, carotenoprotein obtained from the viscera of the shrimp Serranus scriba showed antioxidant activity such as β-carotene bleaching activity and DPPH radical scavenging activity (Nasri et al., 2015). Northern shrimp (Pandalus borealis) was reported as a source of carotenoprotein with the potential for ACE inhibitory and antihypertensive activities (Gildberg et al., 2011). Ambigaipalan and Shahidi (2017) have identified three peptides with potential ACE inhibitory properties from the shrimp shell discards for the first time. Also, they mentioned that raw shrimp shell discards hydrolysates and isolated shrimp shell protein hydrolysates possess strong antioxidant activities by exhibiting reducing ferrous ion chelating, radical scavenging and reducing power activities.
Ghorbel-Bellaaj et al. (2017) reported that protein hydrolysates produced from shrimp waste, using several proteolytic enzymes, displayed diverse biological activities. Hydrolysates obtained from the by-products of shrimp Litopenaeus vannamei showed ABTS-RSA and metal chelating activity (Djellouli et al., 2020). Salted fermented shrimp produced from Acetes vulgaris and Macrobrachium lanchesteri exhibited antioxidant activity, namely DPPH-RSA, RAP, ABTS-RSA, hydrogen peroxide scavenging activity, singlet oxygen scavenging activities, and metal chelating activity (Pongsetkul et al., 2017). Chitosan obtained from Shrimp (M. Monoceros) shell showed antioxidant, antimicrobial, and antitumor activity against human bladder cell line (RT112) (Younes et al., 2014).
Protein hydrolysates extracted from shrimp wastes were demonstrated to possess antioxidant (Pérez-Santín et al., 2013), ACE-inhibitory (Gao et al., 2014), and antiproliferation activity towards colon and liver cancer cells (Kannan et al., 2011). Kleekayai et al. (2015) demonstrated that Thai traditional fermented shrimp paste produced from Acetes vulgaris and Macrobrachium lanchesteri is a fermented product with high protein content showing ACE-inhibitory activity and antioxidative activities against ABTS radical cation. Besides, shrimp shell oil fed obese rats showed improved insulin response, glucose tolerance, antioxidant capacity, adiponectin and lowered oxidative stress and inflammation, serum insulin, leptin, and hemoglobin A1c. (Nair et al., 2017).
4.1.5.3. Lobster
Lobsters are a group of large marine crustaceans with long bodies and muscular tails that are found in the oceans across the world. They inhabit the crevices or burrows of the sandy, rocky, and muddy sea floor. In 2012, the global production of lobster was around 304,000 tons, including both captures and aquaculture (Sabatini, 2015). The four major commercial lobster species are the American lobsters (Homarus americanus) (54%), Tropical or Spiny lobsters (Panulirus sp.) (38%), European lobster (Homarus gammarus), and Rock lobster (Jasus sp.) and the main producers of lobsters are Canada, America, and Australia. More than half of the global lobster production has been utilized to process different lobster products and around 50–70% of the raw material of the lobster processing accounts for by-products such as shells, heads, eggs, and liver (Nguyen et al., 2017). Both meat and by-products of lobsters have been identified as a source of bioactive molecules with potential biological activities.
The by-products of lobsters such as liver, head, and shells are a rich source of protein containing about 41%, 20%, and 25% of protein on a dry basis, respectively. A good proportion of all essential amino acids are present in lobster proteins, particularly in lobster head and shell proteins (Nguyen et al., 2017). Lobsters contain astaxanthin either in free form or in complex with protein, one of the first pigments extracted from them. A high proportion (16%) of carotenoproteins, a complex protein fortified with its natural astaxanthin as a potent antioxidant, is present in lobster shells (Ya et al., 1991). Cephalothorax, one of by-products of lobster processing, is significantly rich in lipid content (mainly long chain ω3 PUFAs), however, less than 2% of lipids is present in lobster body (Shahidi, 2006).
Moreover, lobster shells contain about 16–23% of chitin in the form of α-chitin (Rinaudo, 2006). Water soluble chitosan acid salts of lobsters are the chitosan derivatives that can be used in drug delivery (Cervera et al., 2011). It was found that lobster shell chitosan is safe to utilize in therapeutic applications since Lagarto et al. (2015) have observed no fatalities or changes in the common behaviour of the rats in both the repeated- and acute-dose toxicity studies at 2,000 mg/kg oral doses of lobster shell chitosan. In addition, chitosan nanoparticles with high absorptivity, antimicrobial, and antioxidant properties could also be used as therapeutic ingredients. It was revealed that halitosis could be prevented and treated by the lobster shell chitosan nanoparticles (Safitri et al., 2014).
4.1.5.4. Crayfish
Most crayfish species live in freshwater, and a few species exist in the marine environment. More than half of the crayfish species are found in North America. Generally, crayfish proteins are considered critical active components with essential amino acids and potential antioxidant activity. Crayfish (Procambarus clarkii) was identified as an excellent source of chitin, protein, and pigments, particularly astaxanthin (Guillou et al., 1995). Felix et al. (2017) have reported the strong antioxidant activity (using ABTS-RSA, DPPH-RSA, and Folin-Ciocalteu assays) of protein gels like system produced from crayfish (P. clarkii) hydrolysates and concentrate using pancreatic trypsin. Furthermore, Elkhodary et al. (2017) analyzed the carotenoid, total phenolic and total flavonoid content, and total antioxidant capacity of P. clarkii. They found that these bioactive molecules extracted from the crayfish exhibit potent antioxidant and antimicrobial properties.
4.1.5.5. Krill
Krill, also referred to as euphausiids, consists of more than 80 species widely distributed in all the world’s oceans. Most of their species are planktonic, with specific importance in certain marine ecosystems (Everson, 2008). Their appearance is almost similar to shrimp with small semi-transparent bodies. Krill oil, protein, astaxanthin, vitamins, and flavonoids are biologically active compounds isolated from them. Krill is commonly utilized in the manufacture of fish feeds, however, their application in the food industry is limited due to the poor solubility of krill oil and proteins. Antarctic krill contains 16.31% of crude protein of high biological value with 25.88% of essential amino acids (particularly, high content of lysine) of the total amino acids (Chen et al., 2009; Wang et al., 2022). With more than 30% of long-chain PUFAs, such as EPA and DHA, krill oil is used as a dietary supplement possessing various biological activities including reducing the risk of coronary heart diseases (Gigliotti et al., 2011).
Farooqui (2009) has mentioned that the antioxidant potency of krill oil is 48 times greater than fish oil because krill oil contains astaxanthin, canthaxanthin, and vitamin E, D, and A, which are powerful antioxidants contributing to its antioxidant capacity. Moreover, as around 30–65% of krill oil fatty acids are in the form of phospholipids, the bioavailability of krill oil is better than fish oil (Ulven et al., 2011; Schuchardt et al., 2011). Yoon et al. (2011) have investigated and compared the cholinesterase inhibitory and antioxidant (using DPPH-RSA, ABTS-RSA, and FRAP assays) activities of acetone, methanol, and pretanol extracts of Euphausia superba. They found that acetone extracts of Euphausia superba showed the highest cholinesterase inhibitory and antioxidant activities. Low molecular weight fractions of krill protein hydrolysates produced from Euphausia superba using pepsin exhibited potent antioxidant (DPPH-TSA, ABTS-RSA, ORAC, and ion reducing power) and ACE inhibitory activities (Park et al., 2016).
4.1.6. Chordata
Phylum Chordata comprises all vertebrate animals, including mammals. Ascidians are marine invertebrates belonging to the phylum Chordata and class Ascidiacea. Ascidians have a specific evolutionary position between vertebrates and invertebrates as the larval forms show vertebrate-like tadpole characteristics, and adult forms exist as filter-feeding invertebrates (Delsuc et al., 2006). Figure 23 shows the adult form of ascidians. They exhibit functions that do not evolve in vertebrates and contain genes that are not found in invertebrates. Therefore, ascidians are closely related to humans, and they are the highly developed group of animals studied for marine natural products (Menna et al., 2011). Ascidians are capable of biosynthesizing cellulose, which makes them a unique organism in the animal kingdom (Bhattachan and Dong, 2017; Nakashima et al., 2004). Ascidians are also commonly called sea squirts or tunicates. Nearly 3,000 extant species have so far been reported with varying shapes and sizes (Shenkar and Swalla, 2011), and they are mostly attached to rocks and high current fields due to their external covering.
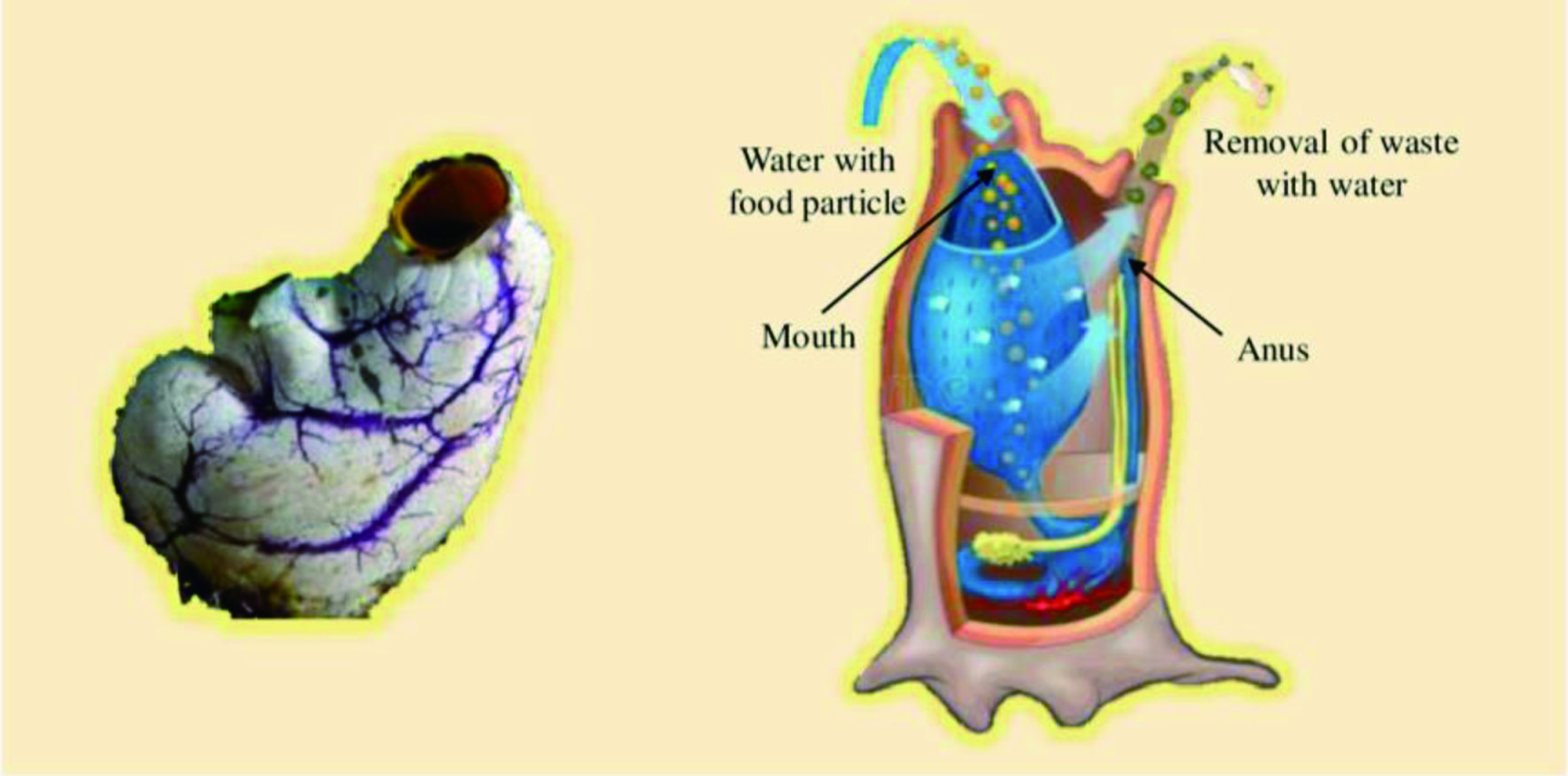 Click for large image | Figure 23. The adult form of ascidians. |
Ascidians are mostly colonial (60%) or solitary sessile marine invertebrates with a soft body and a life span of about two months to one year. They exist in varying body colors of translucent to yellow, green, blue, red, and brown (Holland, 2016; Shenkar and Swalla, 2011). Ascidians have been recognized as one of the rich sources of marine natural products with promising biological activities and therapeutic potential (Carroll et al., 2022). Ascidians synthesize secondary metabolites to protect themselves from fish predators, prevent the growth and settlement of microorganisms and other fouling species, and compete for spaces. They do not have well-developed physical defense mechanisms due to their soft body. Therefore, they largely depend on chemical substances to escape from predators (Palanisamy et al., 2017). The microbial symbionts (such as bacteria, cyanobacteria, actinobacteria, and fungi) of the ascidians are also responsible for producing natural products, contributing about 8% of the bioactive compounds derived from ascidians (Chen et al., 2018).
In 1967, geranyl hydroquinone was extracted from Aplidium sp., the first natural compound isolated from Ascidians. This compound exhibited cytotoxicity against leukemia (Rudali and Menetrier, 1967). Subsequently, several ascidians were investigated for bioactive compounds, and around 1,200 different compounds have been recorded from ascidians thus far (Blunt et al., 2018). Remarkably, over 300 novel secondary metabolites have been isolated from ascidians after 2015 (Watters, 2018). The major compounds isolated from ascidians are alkaloids, peptides, quinones, polyketides, and steroids exhibiting biological activities such as antiviral, antitumor, anti-inflammatory, and immune-suppressive, among others (Figure 24). It was observed that over 80% of the molecules derived from ascidians have nitrogen, and around 70% of nitrogen-containing compounds are alkaloids. Table 7 describes some important groups of compounds extracted from ascidian and their biological activities.
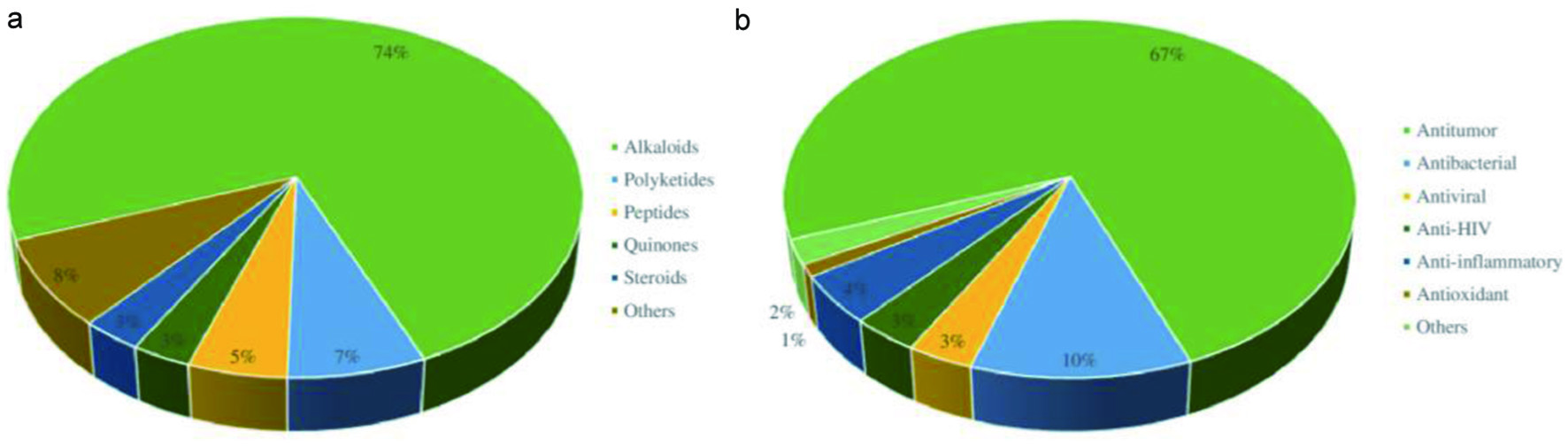 Click for large image | Figure 24. (a) Distribution of bioactive compounds identified from ascidians; (b) Distribution of biological activities exhibited by metabolites extracted from ascidians. |
 Click to view | Table 7. Some important groups of compounds extracted from ascidian and their biological activities |
4.2. Fish
Fish is a cold-blooded vertebrate animal living in either the marine environment or freshwater. Most fish have limbs in the form of fins, and their body is covered with scales. Fish contain a vertical tail, a lateral line to detect water pressure changes, and gills to obtain oxygen for respiration. According to FAO (2018b), global fish production was around 175 million tons in 2017, and it is estimated to increase up to 194 million tons by 2026. Earlier, fish were considered a single group of animals; however, they could be categorized into several classes such as cartilaginous fish, bony fish, lobe-finned fish, jawless fish, and placoderms.
Fish is one of the essential parts of the human diet worldwide due to its high nutritional value. Since ancient times, fish has been considered an affordable source of protein as it contains a significant amount of high-quality protein with all essential amino acids. Based on FAO (2022a) data, the average proximate composition of 62 fish species includes about 59.1-87.8% water, 9–24% proteins, 0.8–23.5% lipids, and 1.6–6.2% minerals (Ashraf et al., 2020)–In addition, fish also contains water-soluble (vitamins A, B3, B6, and B12) and fat-soluble (vitamins A and D) vitamins. The mineral content includes calcium, iron, zinc, phosphorous, selenium, and iodine (Marques et al., 2019; Singh and Ranjan, 2016). However, the nutrition composition of fish greatly varies among different species due to their age, feeding habits, sex, hatching, adaptation, temperature, and season (Pal et al., 2018).
Research on developing novel functional foods, nutraceuticals, and pharmaceutical products from fish sources continuously increase due to several bioactive components in fish and the awareness of the association between fish consumption and improved health (Chiesa et al., 2016). The bioactive compounds in fish include lipids, proteins, minerals, and vitamins. The primary therapeutic potential of fish ingestion is attributed to the high content of long-chain ω3 PUFAs, particularly EPA and DHA, which results in a low incidence of coronary artery diseases and positive effects on health among fish-consuming populations (Tilami and Sampels, 2018). According to American Heart Association (2018) and Food and Agriculture Organization (FAO, 2022b), fish is recommended for regular consumption, providing nearly 200-500 mg of EPA and DHA (for example, from salmon or trout).
On the other hand, fish by-products are also considered important sources of bioactive components with therapeutic potential. Based on FAO (2018b) data, the growth of fish processing industries creates vast amounts of fish by-products and residuals, such as heads, skins, frame (bones), viscera, and fillet cut-offs. Approximately 70% of the fish used in the processing industries accounts for fish by-products. For instance, it was estimated that only 40% of the fish is utilized for food production (fillets), and the remaining 60% is accounted for by-products and residuals in the cod processing industries, as shown in Figure 25 (Välimaa et al., 2019).
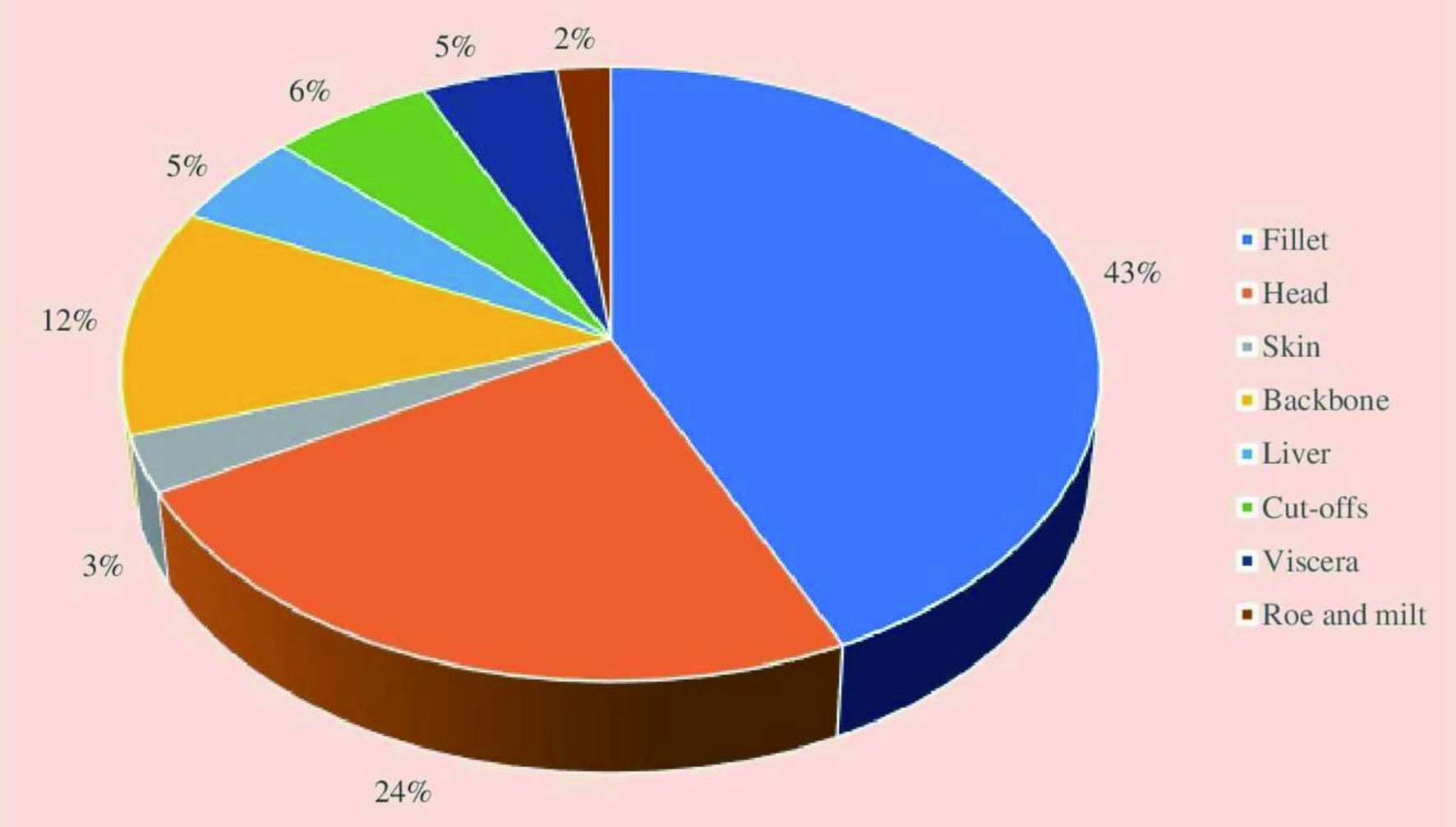 Click for large image | Figure 25. Percentages of food products (fillets) and different by-products from fish processing industries. |
Earlier, these fish residuals, low-value fish catches, and under-utilized fish species were used to manufacture pet foods, feedstock for farm animals, aquaculture, fertilizer, and energy. However, utilization of these fish wastes in the development of nutraceuticals, pharmaceuticals, and cosmetic production has received a significant attention in the last two decades as they contain valuable bioactive components (FAO, 2018b). For instance, fishbone is an excellent source of calcium, bioactive peptides can be extracted from the skin, and fish frame proteins and fish intestine are good sources of crude enzymes (Barrow and Shahidi, 2007). Recent studies have revealed that low-value fish catches and under-utilized fish species are wonderful sources of bioactive components, including fish oil, collagen, protein and peptide, gelatin, chitin, minerals, and enzymes (Kim and Mendis, 2006). These bioactive components from fish waste products are recovered using three primary techniques: microbial method, physical or chemical method, and enzymatic method, which is considered the best technique for all fish species (Wang et al., 2019a).
All valuable ingredients and fractions of fish and their by-products have countless health benefits for humans as they serve as excellent sources of good quality fat, easily digestible protein, and essential micronutrients that help overcome the global prevalence of malnutrition and associated syndromes (Yao et al., 2006). Fish-based nutraceuticals contain bioactive components such as ω3 long chain PUFAs (EPA and DHA), fish oil, amino acids, protein hydrolysates, peptides, vitamins (mainly fat-soluble), fishbone minerals (calcium), collagen and gelatin (Kundam et al., 2018). These nutraceuticals from fish and fish waste products reduce complications of several disorders, namely cardiovascular diseases, dermatologic problems, viral infections, cancer, parasitic infections, and hypertension, particularly during pregnancy. Besides, they also exhibit antioxidant, anti-inflammatory, and anticoagulant activities (Sen et al., 2016). Different bioactive components isolated from fish species are described in subsequent sections.
4.2.1. Lipids (fish oil)
The fatty acid composition and content of fish oils vary according to the species, age, nutrition of the fish, the body part, living habitat, and season (Usydus et al., 2007). The fat content of lean fish (e.g., pike, perch, and pike perch) is 1%, medium-fatty fish (e.g., Baltic herring, European whitefish, and vendace) is 2–10%, and fatty fish (e.g., salmon and rainbow trout) exceeds 10%. Most fish’s belly and tail muscles contain a high-fat content (Välimaa et al., 2019). Fatty fish like anchovies, mackerel, sardines, and some salmon species are excellent sources of EPA and DHA. However, these fatty acids are not synthesized by the fish, and they are derived from their food (Tasbozan and Gökçe, 2017). Besides, the oil extracted from fish by-products could be used to develop dietary fish oil supplements rich in ω3 fatty acids. The average EPA and DHA contents of oil from viscera are 6.4 and 6.0%, from spine is 8.7 and 7.3%, and from heads is 7.9 and 6.3%, respectively (Välimaa et al., 2019).
4.2.2. Proteins, peptides, and amino acids
Fish proteins are more digestible and richer in bioactive peptides and a balanced composition of essential amino acids, particularly methionine and lysine (Njinkoue et al., 2016). Therefore, it is an important source of animal protein with high biological value, and it supplies more than 30% of animal protein to around 60% of people living in developing countries (Balami et al., 2019). The protein content of most fish species ranges from 15 to 20%. The body regulatory factors are balanced by fish proteins. For instance, it has been reported that a sardine protein diet improves hyperglycemia and reduces tumor necrosis factor (TNF)-α, insulin resistance, and oxidative stress in adipose tissue of rats with induced metabolic syndrome (Tilami and Sampels, 2018).
Fish is also a good source of bioactive peptides which could be helpful in preventing several chronic disorders. Bioactive peptides extracted from fish exhibit antimicrobial, antioxidative, antitumor, hypertensive, antidiabetic, antiviral, anticoagulative, analgesic, immunomodulating, cholesterol-lowering, and anxiolytic properties (Cheung et al., 2015; Khora, 2013; Kim and Wijesekara, 2010). The antioxidative peptides obtained from fish proteins typically comprise 2 to 16 amino acid residues. They are beneficial in extending the shelf life of foods containing free iron or hemoglobin that leads to oxidation (Chalamaiah et al., 2012). For instance, Shahidi et al. (1995) have stated that incorporation of around 3% capelin (Mallotus villosus) protein hydrolysates in meat model systems inhibited the oxidation by 17.7–60.4% when determined by the 2-thiobarbituric acid test. The antioxidant activity of capelin protein hydrolysates was attributed to their chelation effect.
Paradaxin was the first natural antimicrobial peptide isolated from fish (Primor and Tu, 1980); later, it was utilized for commercial applications. The fish-derived antimicrobial peptides are mainly from the peptide families of defensin, cathelicidin, piscidin, hepcidin, and histone. They exhibit extensive antimicrobial activities against pathogenic bacteria, viruses, moulds, yeast, and parasites (Masso-Silva and Diamond, 2014). Numerous antimicrobial peptides have been identified from different marine fish species’ body parts, including salmon liver, Atlantic cod mucus, Atlantic mackerel, and its by-products, tilapia by-products, and rainbow trout by-products (Välimaa et al., 2019).
Sardines were reported to contain ACE inhibitory peptides responsible for antihypertensive properties. Subsequently, ACE inhibitory peptides have been isolated from several fish species such as tuna, salmon, shellfish, and bonito. There are some fish protein hydrolysate products capable of reducing blood pressure available on the market, namely, Vasotensin® and PeptACE™ derived from Bonito (Sarda orientalis) (Chalamaiah et al., 2012). In addition to these peptides, some fish bioactive peptides can inhibit the development of cancer and activate the immune system (Undeland et al., 2009). Dark tuna muscle hydrolysates and tuna cooking juice hydrolysates are identified as the sources of antiproliferative peptides (Hsu et al., 2011; Hung et al., 2014). Furthermore, a peptide purified from the gelatin hydrolysates of Alaska pollack skin was identified as a natural antioxidant exhibiting potent antioxidant activity when measured using thiobarbituric acid method and it protected Donryn rat liver cells from t-BHT induced oxidative injury in vitro (Kim et al., 2001). Also, a peptide fraction of capelin protein hydrolysates showed a notable antioxidant activity in a β-carotene-lineolate model system (Amarowicz and Shahidi, 1997).
4.2.3. Polysaccharides
Chitin and chitosan are the major polysaccharides present in the bony fish scales of marine fishes. For instance, chitin was extracted from the scales of Nile tilapia (Boarin-Alcalde and Graciano-Fonseca, 2016) and common carp fish (Zaku et al., 2011). In addition to chitin and chitosan, another polysaccharide, glycosaminoglycan, was also extracted from fish. For instance, sulfated glycosaminoglycans were extracted from various body parts of Baltic herring and mackerel using enzymatic methods (Raghuraman, 2013). Glycosaminoglycans comprise three significant components of glucosamine sulfate, chondroitin sulfate, and hyaluronic acid. Glucosamine is a crystalline component found in connective tissue (chitin), and chondroitin sulfate is a structural component of cartilage providing resistance to compression (Hochberg, 2010). The enzyme activities of phospholipase A2 and collagenase, which are responsible for the cartilage resorption, are inhibited by glucosamine. Therefore, osteoarthritis and arthritis pain can be minimized by using a combination of glucosamine and chondroitin sulfate (Vasudeva et al., 2017). Ingestion of chondroitin sulfate (1,200 mg/d), glucosamine sulfate (20 mg/kg body weight/day), and hyaluronic acid (50–100 mg/d) as nutraceuticals are reported to exhibit antioxidant (redox balance), anti-inflammatory and anabolic properties (Castrogiovanni et al., 2016).
4.2.4. Carotenoids and squalene
Carotenoids are pigments that impart different colors to fish. The common carotenoids present in fish species are β-carotene (orange), lutein (greenish-yellow), zeaxanthin (yellow-orange), astaxanthin (yellow), α, β-doradexanthins (yellow), and canthaxanthin (orange-red). Astaxanthin is very common in red fish species, especially the pink coloration of salmon, rainbow trout and Arctic charr (Ashraf et al., 2020). Carotenoids are essential to human health as they are potent antioxidants, thus reducing the risk of neurological disorders, cancer, and cardiovascular diseases (de Carvalho and Caramujo, 2017).
Squalene is an isoprenoid hydrocarbon compound, a precursor for the biosynthesis of secondary metabolites such as sterols, vitamins, or hormones. The deep-sea shark liver has been identified as a rich source of squalene. Usually, shark liver oil yields around 2.3–8.4 g/100 g of squalene. Squalene is used in the food processing and cosmetics industries. Also, it has positive effects on human health as it prevents coronary heart diseases, inhibits cancer (breast, lung, colon, and ovarian cancers), lowers skin damage by UV radiation, boosts immunity, and has antioxidant and antimicrobial effects, enhances stamina, and treats gastritis, among others (Ibrahim et al., 2020; Popa et al., 2014). Besides, the side effects of drugs are reduced by the detoxifying effect of squalene (Du Preez, 2007), and it is advisable to include squalene in the human diet due to its broad spectrum of biological activities without causing a health risk (Ibrahim et al., 2020).
4.3. Seaweeds
Seaweeds are chlorophyll-containing autotrophic organisms with complex tissue and simple structure showing little or no cellular differentiation. Seaweeds are talophytes and are also known as edible marine algae or marine macroalgae (Kharkwal et al., 2012; Pangestuti and Kim, 2011). Seaweeds are commonly found in coastal habitats or attached to a solid substrate such as rock, shells, pebbles, dead corals, and other plant materials. They can grow in shallow coastal sea areas and deep-sea up to a depth of 180 m (Pal et al., 2014). Seaweeds can be classified into different groups based on the factors such as pigmentation, chemical properties of photosynthetic storage products, components and organization of photosynthetic membrane, and other morphological features (Kılınç et al., 2013). Generally, seaweeds are subdivided into three major groups of red algae (Rhodophyta), brown algae (Phylum Ochrophyta, Class Phaeophyceae), and green algae (Chlorophyta), as shown in Figure 26.
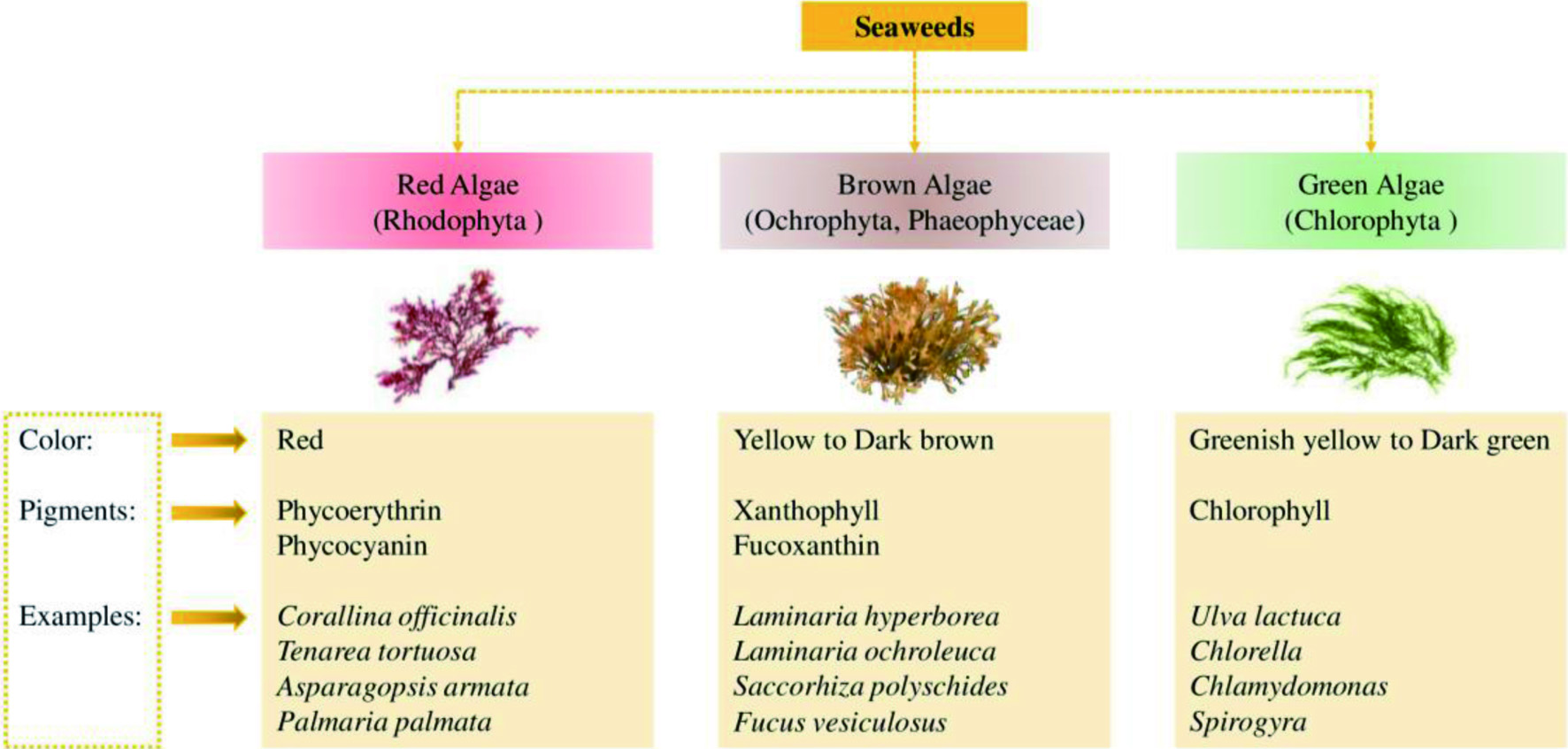 Click for large image | Figure 26. Major groups of seaweeds (red, brown, and green seaweeds). |
At present, seaweeds are classified into four domains: Bacteria, Protozoa, Plantae, and Chromista. They are characterized by their unique sizes and morphology, and they exist as unicellular or multicellular microalgae or colony-forming seaweeds and macrophytes (Khalid et al., 2018). Traditionally, seaweeds have been consumed as a food in Japan, China, and Korea, where nearly 66% of algal species are used as a common ingredient in their meals, and in some Latin American countries (Mexico). At present, seaweed is consumed in many counties due to the migration of people from the above counties to all over the world (Peñalver et al., 2020). In recent years, the demand for seafood, either as human food or as an ingredient in different industrial processing, has increased. For instance, the global seaweed production was 30.4 million tons in 2015 (FAO, 2018a). The most important algae utilized for human consumption are Laminaria/Saccharina spp., Porphyra/Pyropia spp., and Undaria spp. (Peñalver et al., 2020).
Seaweeds have been recognized as a potential source of bioactive compounds with high nutritional value and promising biological activities. Recently, studies on seaweeds have gained much attention and become an important field of research due to their potential to develop novel functional foods, nutraceuticals, and pharmaceuticals (Choudhary et al., 2021). In Asian countries, edible seaweeds are used as alternative medicine and a nutrition-rich food source (Ali et al., 2000), while in western countries, specific components of seaweeds are extracted and utilized in food, pharmaceuticals, and cosmetics industries (Suleria et al., 2016; Gómez-Ordóñez et al., 2010). Algae is considered a vital source of active ingredients due to its universal availability, natural abundance, and diverse origin (Domínguez, 2013). The nutritional and bioactive composition greatly varies in seaweeds depending on the species, maturity, habitat, period of harvesting, and environmental conditions (Tanna and Mishra, 2019).
Since the seventeenth century, seaweeds have long been used in biomedical applications due to their diverse range of potential phytochemical components. The bioactive constituents derived from seaweeds include polysaccharides and dietary fibers, proteins, polyunsaturated fatty acids, minerals, vitamins, natural pigments, and polyphenols. These compounds exhibit antimicrobial, anti-inflammatory, antioxidant, antitumor, antihypertensive, anticoagulant, antidiabetic, and hypocholesterolemic activities (Tanna and Mishra, 2019; Frestedt et al., 2009). Table 8 describe the bioactivities of various natural products extracted from seaweeds. These bioactive components are categorized into two groups depending on their mechanisms of action: (i) low molecular weight substances that are absorbed in the small intestine and directly influence the homeostasis of the human body, and (ii) high molecular weight substances that are not absorbed (Murata and Nakazoe, 2001).
 Click to view | Table 8. Biological activities of various natural products extracted from seaweeds |
4.3.1. Polysaccharides
Seaweeds have been recognized as an excellent source of novel and different types of polysaccharides with numerous applications. Seaweed polysaccharides are mainly present in the cell wall structure, as well as storage polysaccharides and mucopolysaccharides (Kumar et al., 2008; Murata and Nakazoe, 2001). Although homopolysaccharides are the major component of the cell wall structure of most seaweeds, some heteropolysaccharides such as ulvan (Chlorophyta) and carrageenan (Rhodophyta) are also present (Stiger-Pouvreau et al., 2016). The cell walls of seaweeds could be distinguished from that of terrestrial plants as they commonly contain sulfated polysaccharides, which are not found in the cell walls of terrestrial plants (Rupérez et al., 2002). The composition of cell walls and storage polysaccharides are species-specific (Kraan, 2012). Polysaccharides content of most seaweeds ranges from 4 to 76%. A high content of polysaccharides is present in green seaweeds like Ulva, up to 65% of dry weight. In addition, other seaweeds such as Porphyra, Ascophyllum, and Palmaria contain a high quantity of polysaccharides (Holdt and Kraan, 2011).
According to nutritional perspectives, seaweed polysaccharides are important due to their low caloric value and high content of dietary fiber, although they are not absorbed by the human body (Pereira, 2011). The seaweed cell wall comprises cellulose (2–10% of dry weight), hemicellulose (9% of dry weight), and neutral polysaccharides. Red seaweeds or rhodophyta and sargassan consist of carrageenans, water-soluble sulfated galactan, floridean starch (amylopectin like sugar), and porhyran (mucopolysaccharide) in the intracellular spaces. Brown seaweeds or Phaeophyta contain sulfated fucose or fucoidan, alginic acid, and laminarian or β-1, 3 glucans, while green seaweeds or Chlorophyta contain sulphated galactans and sulphuric acid polysaccharide (Kumar et al., 2008; Murata and Nakazoe, 2001).
Among these polysaccharides, the most significant are fucoidan, galactans, alginates, and laminarin (Ferreira et al., 2012). Polysaccharides can promote growth and improve health as well as acting as prebiotics which stimulate the growth of numerous beneficial bacteria in the digestive tract (Vidanarachchi et al., 2009). Moreover, sulfated polysaccharides help in ionic regulation and inhibit the growth of several species of bacteria and viruses (Leonard et al., 2010). The commercial applications of seaweed-derived polysaccharides include their utilization as stabilizers, thickeners, emulsifiers, food sources, and beverages (Tseng, 2001).
4.3.1.1. Dietary fibers
Seaweeds contain around 33–62% of total fiber (mainly soluble fiber) on a dry weight basis, which is greater than that of terrestrial plants. The composition, physiochemical property, chemical structure, the biological impact on the human and animal body, and the ability to ferment the gut microbiota greatly vary among dietary seaweed fibers (Lahaye and Kaeffer 1997). Dietary fibers have been recognized as beneficial for human health due to their ability to create a better intestinal environment. Seaweed derived dietary fibers are divided into water-soluble fibers, namely furonan, alginic acid, laminarin, porphyrin, and agar, and water-insoluble fibers such as cellulose, xylan, and mannans. The biological properties of dietary seaweed fibers include antiviral, antitumor, anticoagulant, and antiherpetic activities. In addition, they decrease LDL cholesterol and prevent obesity, diabetes, and colon cancer (Ghosh et al., 2009; Amano et al., 2005; Murata and Nakazoe, 2001). Moreover, major dietary fibers are from undigested polysaccharides of seaweeds, which can alter the digestibility of protein and minerals (Urbano and Goñi, 2002). Therefore, dietary fibers of seaweeds are increasingly used to develop functional foods and nutraceuticals.
4.3.1.2. Fucoidans
Fucoidan, a sulfated polysaccharide, is present in the cell walls of pheophyta (brown seaweeds). Fucoidans are composed of sulfated L-fucose as the main sugar unit, where sulfate ester groups are linked to the fucose via α-1,3-glycosidic bonds (Li et al., 2008a). It was estimated that fucoidans generally contribute to nearly 10% of the dry weight of seaweeds, which could be extracted using either an acid solution or hot water (Chojnacka et al., 2012). However, Fucus vesiculosus constitutes the highest content of fucoidans, about 20% of the dry seaweed weight (Morrissey et al., 2001). The biological activities, absorption, and bioavailability of fucoidans are greatly influenced by their molecular weight. The bioavailability and bioactivities of fucoidans with low molecular weights are higher than that of high molecular weight (Zuo et al., 2015). Fucoidans are known to exhibit several biological activities with positive impacts on human health, such as anticoagulative, antioxidative, anti-inflammatory, antitumor, and antimicrobial activities (Song et al., 2012; Berteau and Mulloy, 2003).
4.3.1.3. Alginates
Alginates are primarily extracted from brown seaweeds, commonly absent in terrestrial plants (Kumar et al., 2008). Alginates are available in acid (known as alginic acid) and salt forms. Alginic acid is a linear polymer containing two hexuronic acid monomers, namely β-D-mannuronic acid and α-L-guluronic acid, linked via 1–4 bonds (Andriamanantoanina and Rinaudo, 2010). The salt form is a significant component of brown algal cell walls, contributing to about 40–47% of the dry weight of algae (Rasmussen and Morrissey, 2007; Holdt and Kraan, 2011). Several industries, including pharmaceuticals, food processing, and cosmetics, utilize alginates commonly extracted from Undaria and Saccharina due to their biological activities, thickening, stabilizing, and colloidal properties (Holdt and Kraan, 2011; Aliste et al., 2000). The biological activities of alginates include strong antibacterial and anti-inflammatory properties as well as possessing cholesterol-lowering and antihypertensive effects. Besides, they can chelate metal ions and inhibit the absorption of toxic substances. They also play a crucial role as dietary fiber, which is beneficial for human and animal health, including protection against carcinogens and protecting the surface of the digestive system (Kim and Lee, 2008; Nishide and Uchida, 2003; Murata and Nakazoe, 2001).
4.3.1.4. Laminarin
Laminarin, one of the major polysaccharides, constitutes β-(1-3)-linked glucose as the main chain and random β-(1-6)-linked side chains, is commonly extracted from brown seaweeds (O’Doherty et al., 2010). Laminarin is largely present in Laminaria/Saccharina and to some extent in Undaria, Fucus, and Ascophyllum species. The usual content of laminarin is around 10% of the dry seaweed weight; however, it could increase up to 32% of dry weight seasonally (Holdt and Kraan, 2011). Laminarin is a dietary fiber that acts as a prebiotic having a notable commercial application (Devillé et al., 2004). However, it also possesses potential antibacterial, antiviral, antitumor, and anticoagulant activities, and its antioxidant property depends on its molecular weight and chemical structure (Li and Kim, 2011). Around 60 species of brown algae have been reported to show blood anticoagulant activities. The anticoagulant property of laminarin can be enhanced by increasing the degree of sulfation (Shanmugam and Mody, 2000). Besides, it has been shown that laminarins could reduce the systolic blood pressure, boosts the immune system via increasing the B cells and helper T cells, and lower the levels of cholesterol in serum, and triacylglycerol, phospholipid, free cholesterol, and total cholesterol in the liver (Hoffmane et al., 1995; Holdt and Kraan, 2011).
4.3.2. Proteins
The biological properties and structure of proteins obtained from seaweeds are not yet well studied. The protein content and composition of seaweeds are determined by the species, habitat, and season (Pangestuti and Kim, 2015). Red and green seaweeds contain the highest and moderate levels of proteins, respectively, while the least content is in brown seaweeds (Ganesan et al., 2019). Most species of seaweeds encompass all the essential amino acids, particularly rich in acidic amino acids (glutamic acid and aspartic acid) (Fleurence, 2004). For instance, the amino acid content of proteins present in Enteromorpha spp. is comparable to those found in soybean proteins (Aguilera-Morales et al., 2005). Furthermore, seaweeds are rich sources of bioactive peptides, including carnosine, glutathione, kahalalide F, SECMA 1, and galaxamide, among others (Holdt and Kraan, 2011).
The most important bioactive protein obtained from seaweeds is lectin, commonly found in macroalgal species such as Eucheuma spp., Gracilaria sp., and Ulva sp. (Bleakley and Hayes, 2017). Bioactive lectins are low molecular weight carbohydrate binding proteins that interact with membrane-bound and soluble glycoconjugates. This protein-carbohydrate interaction is the reason for the participation of lectins in several biological processes such as anti-inflammation, cancer metastasis, induction of apoptosis, antibacterial, and antiviral activities (Holdt and Kraan, 2011; Chojnacka et al., 2012). Besides, seaweed-derived proteins also exhibit antioxidant, antidiabetic, and antihypertensive (ACE inhibition) properties (Admassu et al., 2018).
4.3.3. Lipids
The lipid content of the seaweeds is comparably lower than other marine organisms, in the range of 1–5% on a dry basis. The major groups of lipids present in seaweeds are glycolipids, phospholipids, and neutral lipids (Narayan et al., 2005; Khotimchenko, 2005). The content of essential fatty acids is higher in seaweeds since they can biosynthesize long chain polyunsaturated fatty acids, notably ω3 fatty acids EPA and DHA (Lopez-Huertas, 2010). For example, Enteromorpha spp. was found to contain 10.38% of ω3 long chain PUFA of the total fatty acids (Aguilera-Morales et al., 2005). Green algae have high levels of DHA, linoleic, α-linolenic, oleic, and palmitic acids, whereas linoleic, α-linolenic, and oleic acids concentrations are higher in brown algae, and red algae contain elevated amounts of EPA, oleic, palmitic, and arachidonic acids (Sánchez-Machado et al., 2004). Both red and brown seaweeds are identified as a balanced source of ω3 and ω6 fatty acids (Holdt and Kraan, 2011).
The lipid content and fatty acid composition of the seaweeds are affected by environmental conditions. The accumulation of PUFAs increases with the decrease of environmental temperature. Hence, marine species living in temperate regions have higher contents of PUFAs compared to those living in tropical areas (Narayan et al., 2006). In addition to fatty acids, seaweeds also constitute unsaponifiable matters such as sterols, tocopherols, and carotenoids. The sterols present in the red seaweeds include fucosterol, cholesterol, desmosterol, chalinasterol, and sitosterol. Brown seaweeds contain cholesterol, brassicasterol, and fucosterol, while green seaweeds have β-sitosterol, cholesterol, 24-methylene-cholesterol, and 28-isofucocholesterol (Sánchez-Machado et al., 2004; Whittaker et al., 2000).
The phospholipid content of red seaweed is around 10–21% of the total lipids, containing phosphatidylcholine and phosphatidylglycerol as major phospholipids with phophatidylethanolamine and diphosphatidylglycerol as minor ones (Holdt and Kraan, 2011). They possess several biological activities such as absorption of cholesterol, fatty acids, and other lipophilic components, ease of digestion, and are natural emulsifiers. Compared to fish oils, marine-based phospholipids are more beneficial in several ways. For example, they contain higher levels of EPA and DHA, and other fatty acids with better bioavailability and more resistance to oxidative rancidity (Sørensen, 2009). The important glycolipids present in the seaweeds are sulphaquinovosyldiacylglycerol, monoglycosyldiacylglycerol, and diglycosyldiacylglycerol (Holdt and Kraan, 2011).
4.3.4. Polyphenols
Seaweeds produce polyphenols to protect them from herbivores and external stress factors (Li et al., 2011b). Seaweed polyphenols include phlorotannins, bromophenols, flavonols, and catechins, generally derived from polymerized phloroglucinol units (1,3,5-trihydroxybenzene) (Gómez-Guzmán et al., 2018; Generalić Mekinić et al., 2019). Seaweed phlorotannins exhibit a broad spectrum of biological activities since they contain different molecules with varying chemical structures and degree of polymerization (Li et al., 2017c). The polyphenol content, particularly phlorotannins (5–15% of dry weight), is higher in brown seaweeds compared to red and green seaweeds (Holdt and Kraan, 2011). The major phenolic compounds found in red and green seaweeds are bromophenols, flavonoids, and phenolic acids (Wells et al., 2017). Table 9 shows few examples of bioactive phenolic compounds extracted from seaweeds.
 Click to view | Table 9. Examples of bioactive phenolic compounds isolated from seaweeds |
4.3.5. Pigments
Seaweeds are an excellent source of unique natural pigments, mainly three groups of pigments; chlorophylls, carotenoids, and phycobiliproteins (Pereira et al., 2014). These pigments are lipid-soluble and involved in various biological functions (Li and Kim, 2011). Red seaweeds contain α- and β-carotene, zeaxanthin, and lutein, whereas brown seaweeds contain β-carotene, fucoxanthin, and violaxanthin. Green seaweeds include β-carotene, zeaxanthin, lutein, neoxanthin, and violaxanthin (Haugan and Liaaen-Jensen, 1994). Chlorophyll could prevent cancer due to its antimutagenic effect (Ferruzzi and Blakeslee, 2007). Red seaweeds contain a high concentration of phycobiliproteins, which possess anti-inflammatory, antioxidant, antiviral, and neuroprotective activities (Holdt and Kraan, 2011).
4.4. Marine Microorganisms
Marine microorganisms, particularly bacteria, fungi, and microalgae, are recognized as a potential source of physiologically and structurally unique chemical compounds with a wide range of biotechnological applications. Marine microorganisms possess varying adaptation mechanisms to survive in the extreme conditions of the oceans, which include production of specific bioactive components (Ameen et al., 2021). Furthermore, they synthesize specific bioactive molecules that are not found in terrestrial environments through their unique biochemical pathways (Xie et al., 2018). These chemical compounds exhibit biological activities against another microorganism or some physiological disorders of the human body (Bhatnagar and Kim, 2010). Besides, microorganisms are considered a promising source of bioactive natural products compared to marine macroorganisms since marine microbes are renewable and more easily cultured on a large scale at a reasonable cost (Blunt et al., 2015). The recovery and utilization of natural compounds from marine microorganisms would also prevent extreme exploitation of other marine resources (Romano et al., 2017).
Soil microorganisms have been extensively studied worldwide over the past five decades due to the ease of isolation of microbes from the lithosphere compared to the ocean. In 1949, the first antibiotic cephalosporin C was extracted from a marine fungus.The scientific studies on isolating molecules from marine microorganisms were comparatively low until the 1980s. Afterward, over 15,000 novel natural products exhibiting diverse biological activities have been identified from marine microorganisms (Al-Dhabi et al., 2019; Blunt et al., 2009). In addition, the symbiotic microbial community of marine organisms is also recognized as a potential source of bioactive compounds with functional foods and pharmaceutical applications (Bhatnagar and Kim, 2010).
4.4.1. Bacteria
Although different species of bacteria belonging to the genera Achromobacter sp., Pseudomonas sp., Flavobacterium sp., Vibrio sp., and Micrococcus sp. are found in the marine environment (Baharum et al., 2010), the genus Streptomyces has been reported as the important producer of bioactive molecules (Blunt et al., 2018). Marine bacteria possess unique biochemical, physiological, and molecular properties as opposed to their terrestrial counterparts (Siddharth and Vittal, 2018). Moreover, the leading marine bacterial species identified as potential sources of natural products could be classified under two forms: eubacteria and archaea. Eubacteria include gram-positive actinomycetes and bacteria (Kiuru et al., 2014). The cell membrane of eubacteria is stabilized by catenoid or hopanoid, while glycerol tetraethers are responsible for the stability of archaean cell membranes. Most archaea are creatures or extremophiles living in extreme environments like low temperature, deep oceans, high salinity, etc. Extremophiles are able to biosynthesize stable enzymes with therapeutic and industrial applications (Ghosh et al., 2022).
Recently, certain actinobacteria living in diverse environments such as volcanic locations, extreme elevations, and the sea have attracted the scientific community because they could synthesize thousands of biologically active chemical compounds with numerous activities as they inhabit harsh environments (Dhakal et al., 2017). The bioactive products produced by actinobacteria include Ectoine, Violacein, Bryostatins, Solonamide, Pentabromo-pseudilin, Thiomarinol, Vibriobactin, and Bromoaltero-chromide, along with some antibiotics (Buijs et al., 2019; Speitling et al., 2007).
Cyanobacteria are the only photosynthetic prokaryotes that can absorb CO2 and produce oxygen and energy. Examples of cyanobacteria that produce bioactive secondary metabolites are Anabaena, Oscillatoria, Microcystis, and Nostoc. Certain compounds, such as cryptophycins, dolastatin, and curacin A, identified from cyanobacteria, are under preclinical or clinical trials to treat cancer (Kiuru et al., 2014). On the other hand, gram-negative proteobacteria have gained less attention in terms of isolating bioactive chemicals (Buijs et al., 2019).
Most of the metabolites derived from marine bacteria exhibit antibacterial properties with the potential to develop natural antibacterial drugs (Butler et al., 2013). For instance, Bacillus safensis, B. altitudinis, B. aerius, B. boroniphilus, B. oryzicola, and Virgibacillus senegalensis discovered in the Mexican marine environment showed antimicrobial effects on foodborne poisoning strains Vibrio parahaemolyticus and Staphylococcus aureus (Galaviz-Silva et al., 2018). It was found that Streptomyces chumphonensis (an actinomycete) separated from the mollusc Drupa granulata showed an antagonistic effect on prochloraz-resistant strains of Penicillium digitatum, which is a reason for green mould growth in citrus fruits (Hu et al., 2019). Besides, two bacterial species Pseudomonas stutzeri and Janibacter melonis were isolated from marine soil samples with the ability to produce natural antioxidants. However, the compounds responsible for antioxidant activities and the possibility for industrial utilization are not yet identified (Shivale et al., 2018). Table 10 shows the bioactivities of compounds derived from marine bacteria.
 Click to view | Table 10. Biological activities of certain compounds derived from marine bacteria |
4.4.2. Fungi
Generally, fungi grow in symbiotic relationships with animals and plants in the terrestrial ecosystem and play a vital role in nutrient cycling. Marine fungi are classified as obligate or facultative fungi based on their ability to grow in marine habitats (Borse et al., 2012). Obligate marine fungi grow only in marine or estuarine habitats, while the facultative species adapted to the marine environment that are initially from terrestrial habitats. Until now, nearly 1,500 marine fungal species have been identified, and most of them belong to the phylum Ascomycota (Jones et al., 2015). Certain marine fungi grow as parasites in phytoplankton, fish, crustaceans, dolphins, and seals, whereas others are linked to corals, algae, and detritus of marine macrophytes (Ghosh et al., 2022).
Compared to their terrestrial counterparts, marine fungi possess unique metabolic routes to produce specific bioactive compounds due to the extreme chemical and physical conditions in the marine environment (Abdel-Lateff, 2008). Therefore, a wide range of novel natural products with numerous biological activities have been isolated from marine fungi over the past few decades. Nevertheless, only a few marine fungi derived compounds are utilized by the pharmacological industries to develop drugs (Kiuru et al., 2014). Table 11 describes the biological activities of a few natural products derived from marine fungi.
 Click to view | Table 11. Biological activities of a few natural products derived from marine fungi |
| 5. Techniques for isolation of marine bioactive compounds | ▴Top |
The active compounds in marine organisms are isolated using either traditional methods or novel technologies (Figure 27). Pre-treatments include washing, drying, grinding or milling, and pre-extraction. Washing is suggested to remove sand, stones, epiphytes, and other impurities. The marine source samples could be used as fresh, air dried or freeze dried, which is a better method since it maintains the integrity of the biomolecules (Pádua et al., 2015). The pre-treatment process of grinding or milling is recommended to increase the extraction yield by reducing the particle size and increasing the contact area between the sample and the extraction solvent (Michalak, 2018). Pre-extraction is done to prevent the extraction of other molecules together with the target compound. For instance, before the extraction of phlorotannins from brown seaweed Fucus vesiculosus, a pre-extraction was done using acetone mixture (Koivikko et al., 2005). In order to obtain the anticipated bioactive compounds with all potential activities, the process conditions must be regulated in all methods (Cikoš et al., 2018).
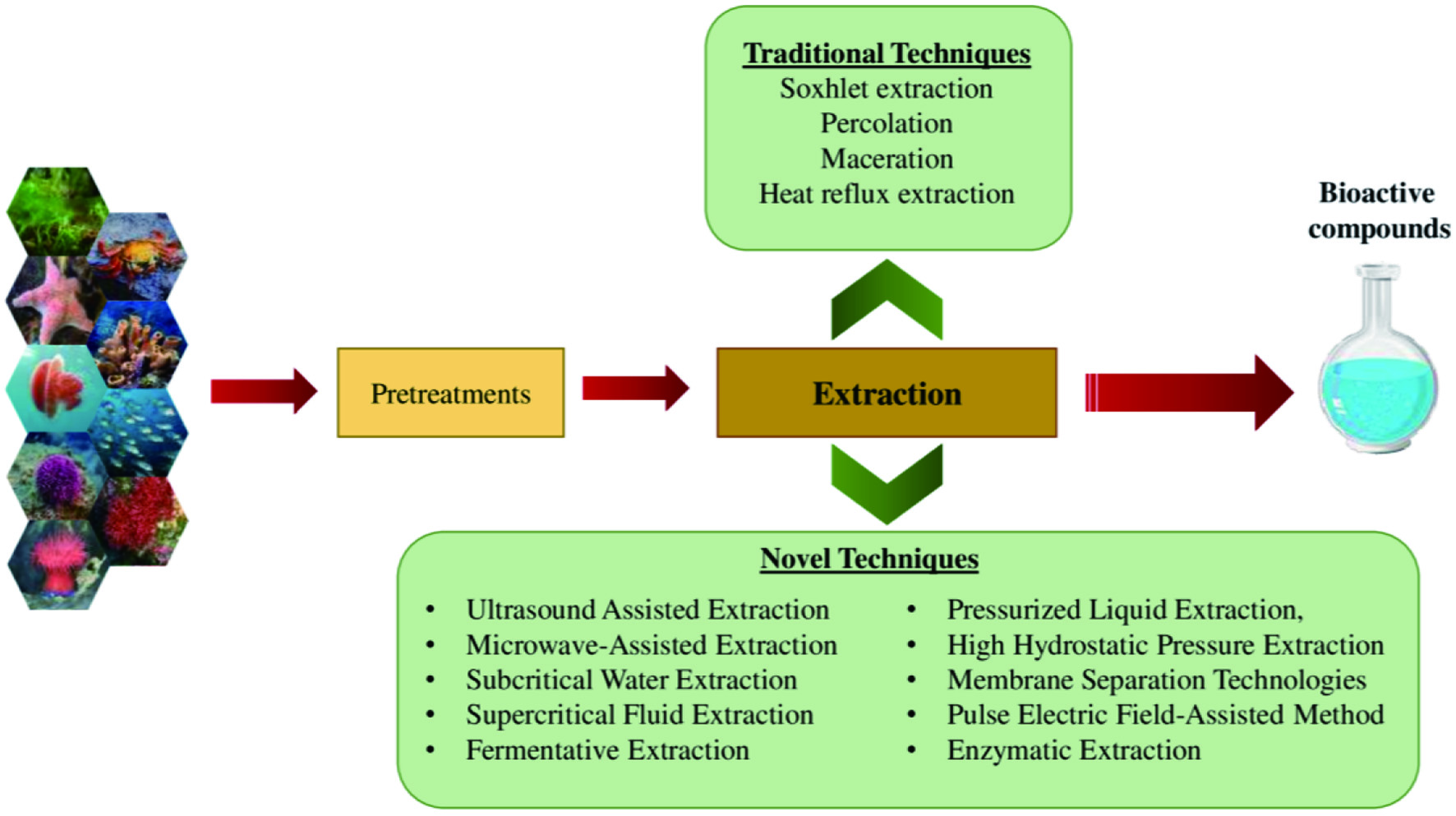 Click for large image | Figure 27. Different traditional and novel techniques used to extract bioactive compounds from marine sources. |
In recent years, modern technologies have commonly been used due to their advantages over traditional methods. Novel techniques include ultrasound-assisted extraction, microwave-assisted extraction, subcritical water extraction, supercritical fluid extraction, pulse electric field-assisted method, pressurized liquid extraction, high hydrostatic pressure extraction, membrane separation technologies, enzymatic extraction, and fermentative extraction (Ghosh et al., 2022). Table 12 summarizes the principle of these novel techniques. Traditional methods are commonly used worldwide to extract active components from natural resources. The common traditional isolation methods are Soxhlet, percolation, maceration, and heat reflux extraction using hot water, ethanol, methanol, ethyl acetate, and acetone as common solvents (Ghosh et al., 2022). The type of solvent used is determined by the polarity of the target bioactive molecules. For instance, to extract hydrophilic phenolic compounds, hydroalcoholic mixtures are used. The efficiency of extraction of specific molecules could be increased by combining acids (hydrochloric, citric, or tartaric acids) with the solvent. The Soxhlet method is more appropriate for extracting the lipophilic metabolites (Vieira et al., 2018; Santos-Buelga et al., 2012).
 Click to view | Table 12. Principles of novel techniques for extracting bioactive compounds from marine sources |
Grosso et al. (2015) stated that although novel techniques are less time and solvent consuming in comparison to traditional methods, they are less efficient in the recovery of bioactive compounds than exhaustive maceration or Soxhlet extraction. The selection of a particular extraction technique depends on the characteristics of target bioactive molecules, degree of recovery, selectivity, volume of solvent needed, extraction time, cost, and greenness of the method. Isolation, characterization, and quantification of the target bioactive compound should be carried out following the extraction process. Based on the type of the compound, several methodologies could be applied for this purpose such as colorimetric methods (Folin-Ciocalteu assay), chromatographic techniques (thin-layer chromatography (TLC), high-pressure liquid chromatography (HPLC) or column chromatography), liquid or gas chromatography with mass spectrometry, or quantitative nuclear magnet resonance (qNMR), among others.
| 6. Conclusion | ▴Top |
Diet-related chronic diseases have become a severe problem for the human population in developed and developing countries. These conditions could be alleviated by developing functional foods and nutraceuticals by incorporating health-promoting natural bioactive compounds. Since ancient times, terrestrial sources have been extensively utilized to extract natural components; however, there is a threat of the extinction of terrestrial species. Therefore, researchers have more recently targeted unexplored and underutilized marine sources for natural products since around 71% of the earth’s surface area is occupied by oceans, and nearly half of the global biodiversity is represented by them. The discovery of bioactive compounds from marine resources has been tremendously growing over the past few decades. Most marine species, mainly fishes, seaweeds, microorganisms, and marine invertebrates, including sponges, cnidarians, echinoderms, molluscs, crustaceans, and ascidians have the potential to produce metabolites with varying chemical structures and promising biological activities. Marine-derived constituents such as polysaccharides (chitin and chitosan derivative, fucoidans), proteins (bioactive peptides, protein hydrolysates, and amino acids), lipids (PUFAs), polyphenols (phlorotannins, bromophenol, etc.), and pigments (carotenoids, chlorophyll, and phycobiliproteins) have received an incredibly increasing attention in the functional foods, nutraceuticals, and pharmaceutical applications due to their wide range of health benefits. The biological activities exhibited by marine-based natural products include antioxidant, anticancer, antihypertensive, cardioprotective, anti-inflammatory, antidiabetic, anti-obesity, antimicrobial, and neuroprotective effects. However, there is a great possibility of discovering more bioactive molecules from marine resources using novel technologies. Furthermore, only a limited number of marine natural products have reached the pre-clinical and human clinical studies and are approved for use as drugs. Therefore, further studies are required to establish a robust relationship between the structure and activity of bioactive compounds to better understand their mechanisms of action and to verify their bioavailability, side effects, and safety prior to their utilization in the functional foods, nutraceuticals, and pharmaceutical applications.
| References | ▴Top |