| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 18, June 2022, pages 109-116
Bioactivities of Calocybe indica protein combined with cell-free supernatant of Lactobacilli from a fermented cereal against free radicals and microorganisms
Taiwo Scholes Adewolea, Clement Olusola Ogidib, *, Adenike Kukua, c
aDepartment of Chemical Sciences, Biochemistry Unit, Kings University, PMB 555, Odeomu, Nigeria
bDepartment of Food Science and Technology, School of Agriculture, Food and Natural Resources, Olusegun Agagu University of Science and Technology, PMB 353, Okitipupa, Nigeria
cDepartment of Biochemistry and Molecular Biology, Obafemi Awolowo University PMB 13, Ile-Ife, Nigeria
*Corresponding author: Clement Olusola Ogidi, Department of Food Science and Technology, School of Agriculture, Food and Natural Resources, Olusegun Agagu University of Science and Technology, PMB 353, Okitipupa, Nigeria., E-mail: clementogidi@yahoo.com
DOI: 10.31665/JFB.2022.18314
Received: April 26, 2022
Revised received & accepted: June 29, 2022
| Abstract | ▴Top |
Edible fungi and lactic acid bacteria (LAB) are emerging as reservoirs of diverse bio-functional products. This study investigated antioxidant and antimicrobial activities of a mushroom; Calocybe indica protein (CIP) and cell-free supernatant (CFS) of Lactococcus lactis, Lactobacillus fermentum, and Lactobacillus brevis isolated from a fermented cereal food-Kati against free radicals and microorganisms associated with fruits spoilage. Protein extract from C. indica was subjected to ammonium sulfate precipitation (55% saturation). Synergistic radical scavenging activities of CIP and CFSs of LAB against 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+), 1,1-diphenyl-2-picrylhydrazyl (DPPH•), hydroxyl radicals (OH•) and their Fe2+ ion chelation activity were within 70.39–84.77%, 62.51–87.63%, 70.58–88.56%%, and 62.83–78.76%, respectively. Combinatory effects of CIP and CFSs showed pronounced zones of inhibition of 6.5 mm to 12.1 mm against tested microorganisms. The study established that, bioactivities of C. indica and Lactobacilli can be attributed to their inherent bioactive proteins, suggesting their potential exploitation as natural bio-preservatives.
Keywords: Edible mushrooms; Lactic acid bacteria; Antioxidant; antimicrobial; Bio-preservatives; Bioactive protein
| 1. Introduction | ▴Top |
The fungi kingdom encompasses several macrofungi of high medicinal and nutritional values, which contain myriads of intrinsic natural bioactive compounds including proteins eliciting diverse biological activities (Niego et al., 2021). Owing to the inherent bio-functional properties of medicinal mushrooms like anti-viral, neuro-modulatory, ribosome-inactivating, antimicrobial and antioxidant activities, their proteins have now found a place as potential therapeutics in the management of many human aliments (Wani et al., 2020). Edible mushrooms are a rich source of dietary proteins and are being recognized as functional foods and nutraceuticals for health-promoting purposes (González et al., 2020). Medicinal exploitation of proteins is mostly attributed to their, bioactivities, specificity, mechanism of action, ability to target different tissues, and flexibility in undergoing unique structural modifications (Rondon et al., 2021). Calocybe indica P&C is a highly nutritious edible mushroom, rich in high-quality protein and other bioactive compounds, which have been associated with vital therapeutic and prophylactic functions (Bandura et al., 2021). The medicinal uses of the edible mushroom have been encouraged, especially for the development of functional food products (Nguyen et al., 2021).
Several endogenous physiological reactions in humans constantly generate different reactive oxygen species (ROS) like hydroxyl radical, superoxide anions, ferric ion, nitric oxide, singlet oxygen, and nitric oxide, among others. These in excess have the potential to disrupt the bio-system balance, causing alterations in cellular functions (Matsumoto et al., 2021; Hernansanz-Agustín and Enríquez, 2021). The unpredictable consequences of these entities include the inactivation of enzymes, and irreversible organ and tissue damage resulting in inflammatory injuries and diseases (Sies et al., 2022). Likewise, in the food industry, free radical-induced food spoilage is becoming an issue of concern, and health-related risks regarding synthetic antioxidants used in mitigating this detrimental occurrence are recently being raised. Hence, the search for natural alternatives, especially those of protein origin (Kimatu et al., 2017).
Microorganisms contribute immensely to spoilage of spoilage; posing severe threats to food security and consumer safety. This has created the search for natural microbial inhibitors as well as unique microflora such as the lactic acid bacteria (LAB), which could be exploited via green technologies for the production of natural bio-preservatives (Djukic-Vuković et al., 2021). LAB are disparate groups of industrially remarkable bacteria generally considered safe. These organisms produce key biochemical metabolites, with broad and narrow-spectrum antimicrobial activities against pathogenic and spoilage microbes; making these microbes of unique importance, especially as probiotics, bio-preservatives, and bio-control agents (Ağagündüz et al., 2022). Aside from their antimicrobial activities, LAB has also been reported to elicit antioxidant potentiating activities, owing to inherent cell-free extracts, encouraging their application in food and beverage preservation (Nasrollahzadeh et al., 2022). This study therefore, investigated antioxidant and antimicrobial activities of C. indica protein combined with cell-free supernatant obtained from Lactococcus lactis, Lactobacillus fermentum, and Lactobacillus brevis, with the aim of exploiting these products as natural bio-preservatives.
| 2. Materials and methods | ▴Top |
2.1. Chemicals and reagents
β-mercaptoethanol was purchased from England (BDH Chemical Limited, Poole). Tris base, butylated hydroxytoluene (BHT), Trichloroacetic acid (TCA), and ammonium sulfate are products of Sigma-Aldrich, St Louis, Missouri, USA. Mueller Hinton Agar and Potato Dextrose Agar are products of Lab M Ltd, Lancashire, United Kingdom. All other chemicals are of analytical grade.
2.2. Source of C. indica and selected microorganisms
C. indica was obtained from a farmland in Ibadan, South-West Nigeria. The identity of an edible mushroom was confirmed with Odeyemi and Adeniyi (2015). Test microorganisms namely: Bacillus cereus, Serratia marcescens, Pseudomonas aeruginosa, Geotrichum candidum, Pichia fermentans, Aspergillus niger, Aspergillus flavus, Fusarium solani were isolated from different fruits; tomatoes, apple, pineapple, pawpaw, and mango. Lactococcus lactis, Lactobacillus fermentum, and Lactobacillus brevis were isolated from Kati; a fermented cereal food using standard microbiological methods (Cheesbrough, 2006) and identified using Cowan and Steel (1993). The molecular identity of the LAB isolated from fermented cereal-Kati has been reported by Gabriel et al. (2021).
2.3. Isolation and precipitation C. indica crude protein
C. indica crude protein extract was prepared as described by Li et al. (2011) with slight modifications. Briefly, fresh fruiting bodies of C. indica were homogenized in 25 mM Tris-HCl buffer (1:3 w/v), pH 8.0 containing 0.1% (v/v) β-mercaptoethanol for 24 h at 4 °C. The homogenate was centrifuged (12,000 g, 4 °C) for 30 mins, and supernatant (crude protein) was collected. Anhydrous ammonium sulfate (55% saturation) was added slowly with gentle stirring to precipitate the crude protein and kept overnight at 4 °C. Protein precipitate was collected by centrifugation (12,000 g, 4 °C) and re-suspended protein dialyzed for 48 h using a 3 kDa dialysis tubing against extraction buffer and distilled water at 4 °C. Obtained dialysate which constituted the CIP used in this study was lyophilized and kept frozen (−20 °C) until use.
2.4. Production and collection of LAB cell-free supernatants
L. lactis, L. fermentum, and L. brevis were grown in De Man, Rogosa, Sharpe (MRS-Oxoid, UK) broth, incubated at 37 °C for 48 h, and their cell-free supernatant (CFS) was obtained after centrifugation at 8,000×g for 10 min. The CSF was filter-sterilized with a 0.45 μm Millipore membrane filter and kept frozen at −20 °C until use.
2.5. Protein concentration assay
Assay method of Lowry et al. (1951) was employed in estimating the protein concentration of C. indica and Lactobacilli cell-free supernatants. Standard protein used was bovine serum albumin (1 mg/ml).
2.6. Antioxidant assays
2.6.1. ABTS radical scavenging assay
Assay method described by Tovar-Pérez et al. (2017) was employed. Briefly, ABTS (2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid) stock solution (7 mmol/l) was reacted with potassium persulfate (2.45 mmol/l) and left placidly for 12 h in the dark to generate the ABTS radical cation. Obtained radical solution was diluted (1:35) in phosphate-buffered saline (0.15 M, pH 7.4) to obtain an absorbance of 0.70 at 734 nm. Resulting solution (3 ml) was mixed with an aliquot (0.15 ml) of the respective samples (CIP and CFS), and absorbance was up to 7 min under dark condition. Deionized water and PBS were used as control and blank, respectively. Percentage inhibition was calculated as follows:
2.6.2. DPPH radical scavenging assay
Assay method of Chen et al. (2012) was employed. Typically, equal volume (0.5 ml) of the sample (CIP and CSF) was mixed in 0.1 M DPPH radical in methanol solution with vigorous agitation. Resulting solution was incubated in the dark for 30 min, and absorbance measured at 515 nm. Methanol and deionized water were used as blank and control respectively, in place of DPPH radical and sample. Percentage radical inhibition was calculated as follows:
2.6.3. Hydroxyl radical scavenging assay
The hydroxyl radical scavenging activity of protein fractions was carried out as described by Halliwell and Gutteridge (2015). Briefly, the assay was performed by adding 0.1 ml of EDTA, 0.01 ml of FeCl3, 0.1 ml of H2O2, 0.36 ml of deoxyribose, 1.0 ml of sample (CIP and CSF), 0.33 ml of phosphate buffer (50 mM, pH 7.4) and 0.1 ml of ascorbic acid in sequence. The mixture was then incubated at 37 °C for 1 h. About 1.0 ml of 10% TCA and 1.0 ml of 0.5% TBA to develop the pink chromogen measured at 532 nm. The standard was BHT:
2.6.4. Fe2+ chelating activity
Metal chelating activity was determined according to the assay method described by Cai et al. (2015) with slight modification. Briefly, aliquot (1 ml) of the sample (CIP and CSF) was mixed with distilled water (4.7 ml), and resulting solution added to 2 mM FeCl2 solution (0.1 ml). Ferrozine (0.2 ml, 5 mM) was used to initiate the reaction. Control contains deionized water in place of sample. Resulting mixture was vigorously agitated and incubated for 20 min at room temperature. Absorbance was measured at 562 nm. Metal chelation activity was calculated as follows:
2.7. Assessment of antimicrobial activity
Agar well diffusion method was used to investigate the antimicrobial activity of CIP and CFS against pathogenic microorganisms (Cheesbrough, 2006). The inoculum size of microorganisms was adjusted to 0.5 McFarland turbidity standards at 600 nm using visible spectrophotometer. A sterile swab stick moistened with the inoculum was spread on Mueller Hinton Agar and Potato Dextrose Agar for bacteria and fungi respectively. Subsequently, well of 6 mm diameter were bored equidistant from each other with a sterile cock borer into the respective agar media and filled with (50 µl) of the respective extracts. Amoxicillin (200 µg/ml) and ketoconazole (100 µg/ml) were used as positive controls against bacteria and fungi, respectively. Petri dishes were incubated at 37 °C for bacteria and 25 °C for 48–72 h for fungi. Zones of inhibition were measured in millimeters (mm).
2.8. Statistical analysis
Data obtained during the experiment were presented as mean ± standard error, and subjected to one-way analysis of variance (ANOVA). Means were separated with Duncan’s New Multiple Range Test (DNMRT) and differences were considered significant at P ≤ 0.05.
| 3. Results | ▴Top |
3.1. Estimation of protein content
Data from this study showed C. indica and the cell-free supernatant from L. lactis, L. fermentum, and L. brevis were rich in protein as shown in Table 1.
 Click to view | Table 1. Protein content of C. indica and Lactobacilli cell-free supernatant |
3.2. Antioxidant and antimicrobial studies
Findings from the radical scavenging activities (ABTS•+, DPPH•, and OH•) and Fe2+ ion chelating activity of C. indica protein (CIP) and cell-free supernatant (CFS) obtained from L. lactis, L. fermentum and L. brevis are presented in Figures 1–4. The results showed that CIP2 (containing 0.6 mg/ml C. indica protein) exhibited potent antioxidant activity, displaying the strongest ABTS+ scavenging activity (70.92 ± 0.66%), DPPH• scavenging activity (72.71 ± 1.15%), OH• scavenging (74.95 ± 0.67%) and Fe2+ ion chelating (71.04 ± 0.82%) activity, relative to the CFS1 (48.51 ± 1.15%, 44.45 ± 0.23%, 47.91 ± 0.65%,, 39.92 ± 0.1% ), CFS2 (45.89 ± 0.72%, 40.38 ± 0.23%, 49.09 ± 0.57%, 42.58 ± 1.04%) and CFS3 (50.45 ± 0.24%, 45.03 ± 0.11%, 46.83 ± 1.06%, 42.35 ± 0.41%) respectively. However, antioxidant activities of combined CIP and CFS were moderately increased, indicating a synergistic effect. Table 2 shows zones of inhibition (mm) displayed by CIP, CFS from Lactobacilli, and their combinatory effects. CIP at 0.2 mg/ml has zones of inhibition ranging from 5.0 to 5.3 mm, and CIP 2 at 0.6 mg/ml displayed zones of inhibition of 5.3 mm to 8.2 mm. The CFS from Lactobacilli (CFS1-3) have zones of inhibition of 5.0 to 7.3 mm against tested microorganisms. Combination of CIP and CFS from LAB inhibited all the microorganisms isolated from fruits with zones of inhibition ranged from 6.5 to 12.1 mm.
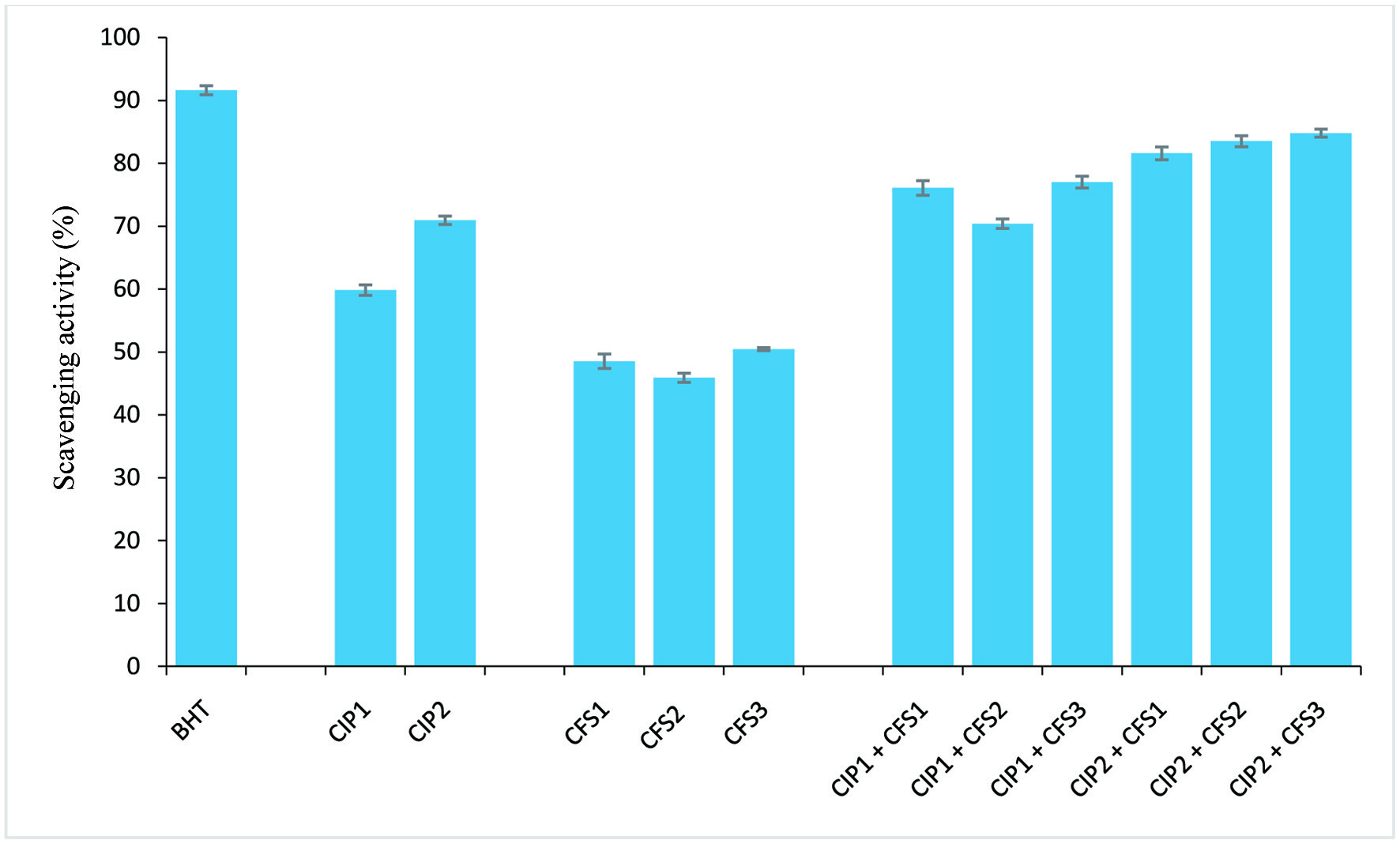 Click for large image | Figure 1. ABTS radical scavenging activity. CIP1: 0.2 mg/ml protein; CIP2: 0.6 mg/ml protein; CFS1: L. lactis CFS; CFS2: L. fermentum CFS; CFS3: L. brevis CFS; BHT: butylated hydroxytoluene (positive control). Error bar is standard error. |
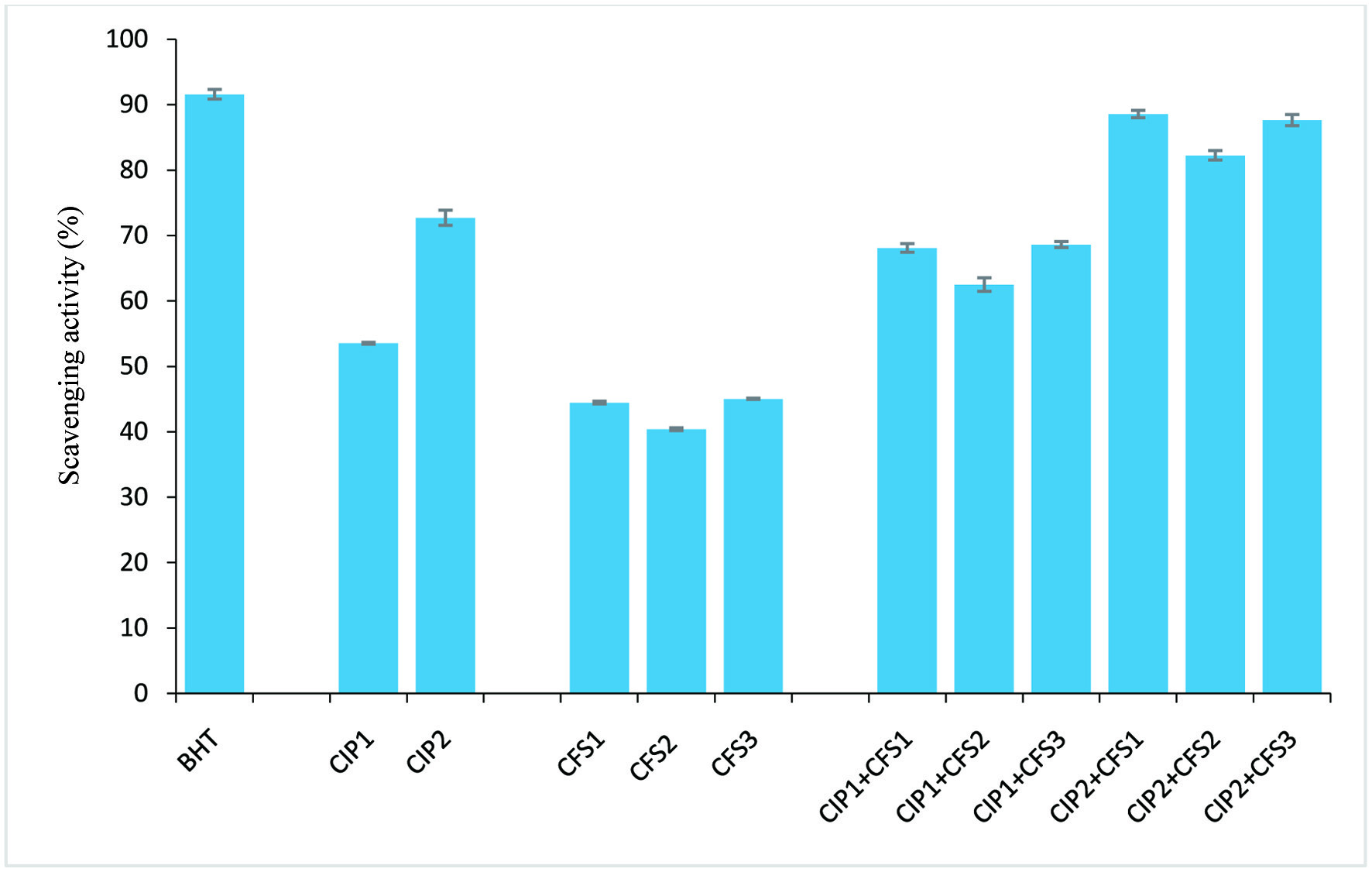 Click for large image | Figure 2. DPPH radical scavenging activity. CIP1: 0.2 mg/ml protein; CIP2: 0.6 mg/ml protein; CFS1: L. lactis CFS; CFS2: L. fermentum CFS; CFS3: L. brevis CFS; BHT: butylated hydroxytoluene (positive control). Error bar is standard error |
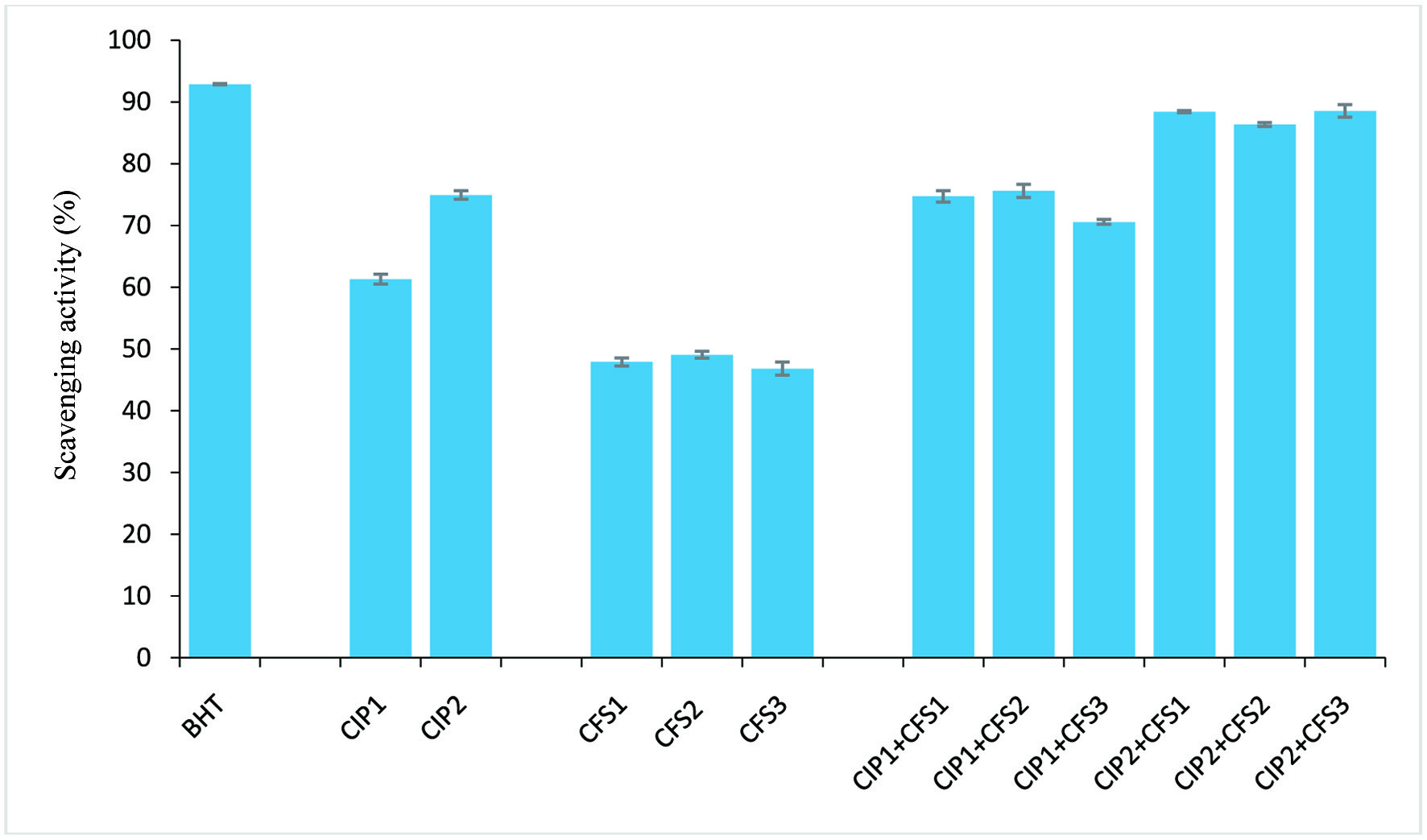 Click for large image | Figure 3. Hydroxy radical scavenging activity. CIP1: 0.2 mg/ml protein; CIP2: 0.6 mg/ml protein; CFS1: L. lactis CFS; CFS2: L. fermentum CFS; CFS3: L. brevis CFS; BHT: butylated hydroxytoluene (positive control). Error bar is standard error |
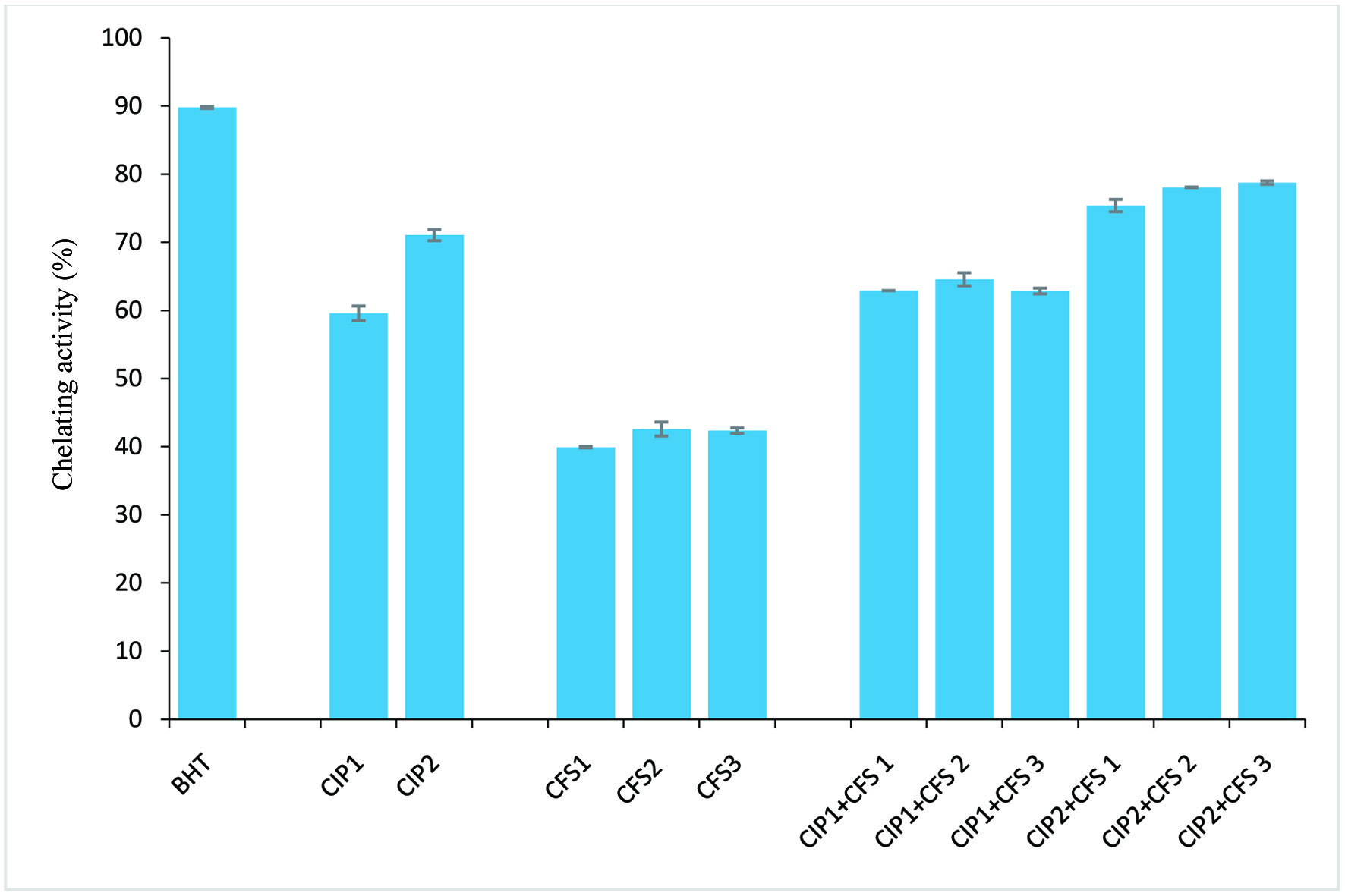 Click for large image | Figure 4. Fe2+ chelating activity. CIP1: 0.2 mg/ml protein; CIP2: 0.6 mg/ml protein; CFS1: L. lactis CFS; CFS2: L. fermentum CFS; CFS3: L. brevis CFS; BHT: butylated hydroxytoluene (positive control). Error bar is standard error |
 Click to view | Table 2. Zones of inhibition (mm) displayed by CIP and CFS of Lactobacilli against microorganisms isolated form fruits |
| 4. Discussion | ▴Top |
C. indica mushroom is a rich source of protein. Supporting our findings, elevated protein yields have also been reported in the Basidiomycota Agaricus bisporus Agaricus semotus, and Pleurotus eryngii (Tehrani et al., 2012; Yuan et al., 2017; Adedoyin et al., 2021). Although, mushroom protein content varies within species, these saprophytic organisms are however a unique reservoir of diverse bioactive/functional proteins such as lectins, laccases, ribosome-inactivating proteins, and storage proteins, which could be exploited in the development of novel pharmaceuticals and bio-preservatives (Karami and Akbari-Adergani, 2019; González et al., 2020; Yu et al., 2021). Research into these mushrooms proteins/peptides has shown they exhibit diverse health-related benefits (Goswami et al., 2021; Khongdetch et al., 2022). In fact, antihypertensive, antioxidant, anti-inflammatory, nematotoxic, and immunomodulatory activities have been reported for mushroom proteins isolated from Pleurotus cystidiosus, Agaricus bisporus, Lignosus rhinocertis, and Pleurotus eryngii (Lau et al., 2012; Plaza et al., 2016; Pushparajah et al., 2016; Kimatu et al., 2017; Yuan et al., 2017). The cell-free supernatants (CFSs) of L. lactis, L. fermentum, and L. brevis are rich in protein content (Table 1). CFS of LAB contain diverse biochemical metabolites including bacteriocin, which are the low molecular weight host defense peptides that have been associated with antioxidant and antimicrobial activities (Prosekov et al., 2017). Members of the genera Lactococcus, Lactobacillus, Carnobacterium, Enterococcus and Pediococcus have been well documented to secret peptides that are rich in lysyl and arginyl residues (Emmanuel et al., 2017).
In humans, biological processes are fueled by oxidative processes during normal cellular metabolism, resulting in the continuous in vivo generation of free radicals and reactive species, which in elevated unchecked levels can cause severe detrimental effects (Zhou et al., 2020). Checkmating and/or removal of these reactive species are coordinated by highly efficient endogenous enzymatic and non-enzymatic antioxidant systems, which could be overwhelmed under certain conditions resulting in severe pathologies (Bechaux et al., 2019; Zhou et al., 2020). Four major in vitro assays were employed in this study to assess the antioxidant activities of C. indica protein and CFS of the tested Lactobacilli strains due to the reactivity mechanism of free radicals. C. indica protein (CIP) showed remarkable antioxidant activity against ABTS•+, DPPH•, OH• and chelating Fe2+ ion (Figures 1–4). Similar results have been reported for proteins isolated from A. bisporus and P. ostreatus against free radicals (Kimatu et al., 2017; González et al., 2021). These authors suggested that protein eliciting antioxidant properties possess unique amino acid residues with electron-donating potentials capable of stabilizing or sequestering radicals. Natural products such as peptides and proteins have been implicated to play potent roles in mitigating oxidative processes as dietary antioxidants, by reacting with chelating catalytic metals, free radicals, and scavenging oxygen (Esfandi et al., 2019). Antioxidant activities of proteins have been attributed to free radical proton-donating potential by inherent aromatic amino acids (tryptophan, phenylalanine, and tyrosine) and metal chelating properties by charged amino acids like lysine, arginine, aspartic acid, and glutamic acid (Hou et al., 2001; Choo and Yong, 2011; Ramakrishna et al., 2012). In investigating the antioxidant efficacy of hydrogen-donating and chain-breaking compounds, the ABTS and DPPH assays are mostly employed (Binsan et al., 2008; Klompong et al., 2009). Findings of Nalinanon et al. (2011) indicated that, hydrophilicity is usually associated with compounds exhibiting high ABTS radical scavenging activity. Hence this study is suggesting there is abundance of polar amino acids in CIP. Scavenging of DPPH radical by CIP might be due to its structural conformation and/or the presence of amino acids capable of donating electrons to halt the radical chain reaction of ROS by forming stable products (Elias et al., 2008; Nalinanon et al., 2011; Jin et al., 2016). In a similar vein, the Fe2+ ion chelating activity of CIP may be attributed to its constituent amino acids as reported for A. bisporus protein (Kimatu et al., 2017). In fact, effective chelating properties of protein have been linked to the additive effects of its amino acids (He et al., 2013). CFS of the tested Lactobacilli species exhibited impressive antioxidant activity, scavenged DPPH•, ABTS•+, OH•, and exhibited Fe2+ ion chelating ability, which reveals their probiotic potentials. Similar antioxidant properties have been reported for CFS obtained from L. acidophilus (Bing et al., 1998), L. rhamnosus (Wang et al., 2012), and Bifidobacterium animalis 01 (Shen et al., 2011).
Findings from our investigation showed the ABTS and DPPH radical scavenging activities of the CFS from the three Lactobacilli strains were around 50%, with CFS3 from L. brevis showing the strongest ABTS and DPPH radical scavenging activities (50.45%, 45.03%), followed by CFS1 from L. lactis (48.51%, 44.45%) and CFS2 from L. fermentum (45.89%, 40.38%) respectively. Hydroxyl radicals have been implicated as the most harmful reactive oxygen species (Lin et al., 2018). Hydroxyl radical scavenging activity and Fe2+ ion chelation activity of the obtained CFSs ranged between 39–47%, with CFS2 showing the strongest inhibition (49.09%, 42.58%) relative to the control (Figures 1–4). However, there was an increase in antioxidant capacity when CIP was combined with the CSFs of the three strains, suggesting a synergistic effect, as the antioxidant activities was potentiated at levels comparable to the positive standard (butylated hydroxyl toluene).
Results from our investigations showed that CIP inhibited the growth of Bacillus cereus, Serratia marcescens, Geotrichum candidum, Pichia fermentans, and Aspergillus spp. The findings of Tehrani et al. (2012) revealed the antibacterial effect of total protein extract of A. bisporus mushroom against Bacilus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus (MRSA) and E. coli. Proteins from Ganoderma resinaceum, Russula fragilis, and Inocybe grammata inhibited B. cereus, E. coli, Candida krusei, A. fumigatus (Hearst et al., 2010). The bacteriostatic and bactericidal properties of edible and medicinal mushrooms; species of Agaricus, Boletus, Calocybe, Canthraellus, Clitocybe, Cortinarius, Ganoderma, Pycnoporus, Hygrophorous. Hypholoma, Lactarius, Tricholoma, and Lentinus against multidrug-resistant bacteria namely; Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. have been attributed to the presence of protein, peptides and other bioactive compounds (Rezaeian, and Pourianfar, 2016; Ramos et al., 2019 and Ogidi et al., 2020).
Obtained cell-free supernatant inhibited indicator microorganisms. Findings of Koohestani et al. (2018) revealed that cell-free supernatant of L. acidophilus LA5 and L. casei 431 inhibited the growth of S. aureus. Findings from our study showed the cell-free supernatants obtained from L. lactis, L. fermentum, and L. brevis in combination with CIP inhibited the growth of tested microorganisms in a synergistic manner. Corroborative, whey protein isolates supplemented with CFS from L. rhamnosus and L. sakei reportedly inhibited the growth of E. coli, L. monocytogenes, S. aureus, or S. Typhimurium (Beristain-Bauza et al., 2016; del Carmen Beristain-Bauza et al., 2017). Kim et al., (2020) reported the antimicrobial activities of LAB cell-free supernatants are mediated by inherent bioactive peptides and proteins, which further support and establish our findings, as high protein content was detected in the CFS of the Lactobacilli strains used in this study. These researchers reported that L. plantarum CFS showed broad-spectrum activity against Salmonella Gallinarum, Edwardsiella tarda, Pasteurella multocida, and Streptococcus iniae.
| 5. Conclusion | ▴Top |
C. indica protein combined with CFS of lactic acid bacteria scavenged free radicals and inhibited microorganisms isolated from fruits. The pronounced antioxidant and antimicrobial activities displayed by macrofungus; C. indica protein and CFS of LAB can be attributed to the presence of biologically active proteins. These bioactive molecules can be extracted and exploited for use as bio-preservatives and as biopharmaceutical agents. However, further study is ongoing on the purification and characterization of these proteins towards full exploitation.
Conflict of interest
None.
| References | ▴Top |