| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Article
Volume 2, June 2018, pages 104-111
Identification of food-derived peptides in human blood after ingestion of corn and wheat gluten hydrolysates
Akika Ejimaa, Megumi Nakamurab, Yasushi A. Suzukib, Kenji Satoa, *
aDivision of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawaoiwake-cho, Sakyo-ku, Kyoto 606-8502, Japan
bSaraya Co., Ltd., Biochemical Laboratory. 24-12 Tamate-cho, Kashiwara-city, Osaka 582-0028, Japan
*Corresponding author: Kenji Sato, Division of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawaoiwake-cho, Sakyo-ku, Kyoto 606-8502, Japan
DOI: 10.31665/JFB.2018.2145
Received: March 2, 2018
Revised received & accepted: May 20, 2018
| Abstract | ▴Top |
Bioactive peptides in the body after ingestion of plant protein hydrolysates have been speculated but not identified. We aimed to establish an approach to identify small amounts of food-derived peptides in humans after ingestion of non-extracellular matrix protein hydrolysates. Corn and wheat gluten hydrolysates were digested using pancreatin and leucine aminopeptidase; the resultant peptides were identified via size-exclusion chromatography and reverse-phase HPLC-tandem mass spectrometry (MS/MS). Structures of indigestible peptides were confirmed via LC-MS/MS in multi-reaction monitoring mode. All indigestible peptides in the exopeptidase digest were diprolyl and di- and tripyroglutamyl peptides. Blood collected from healthy volunteers (n = 4) before and after ingestion of 9 g of the hydrolysates was assessed for the indigestible peptides via LC-MS/MS. Six peptides (Pro-Ala, Pro-Gly, Pro-Gln, pyroGlu-Pro, pyroGlu-Leu-Pro, and pyroGlu-Gln-Pro) significantly increased in human plasma up to 10–100 nM compared to the baseline. This may hence be a powerful tool for identifying food-derived peptides in blood.
Keywords: Bioactive peptide; Protein hydrolysate; Blood; Liquid chromatography tandem mass-spectrometry; Food; Pyroglutamyl peptide
| 1. Introduction | ▴Top |
Enzymatic hydrolysates of food proteins, such as soy, corn, wheat, milk, and meat proteins, exert beneficial effects on health beyond those of conventional amino acid sources (Möller et al., 2008; Malaguti et al., 2014). In most cases, thermostable bacterial proteases having high endoproteinase activity but low exopeptidase activity are used to obtain hydrolysates of food proteins. Peptides in the endoproteinase digest are degraded by exopeptideases in digestive tract and resultant amino acids and di- and tripeptides penetrate enterocytes via amino acids transporters as well as peptide transporter 1 (PepT1) (Adibi, 1997; Miner-Williams et al., 2014). Some longer peptides can penetrate enterocytes via paracellular diffusion (Shimizu et al., 1997). Most di- and tripeptides are further degraded to amino acids by cytosolic and blood exopeptidases (Hübl et al., 1989; Satake et al., 2002; Miner-Williams et al, 2014). Therefore, the majority of food-derived peptides were thought to be degraded into amino acids during digestion and absorption.
This concept was, however, dramatically altered owing to the detection of approximately 10–30 μM hydroxyproline (Hyp)-containing peptides in human plasma 1 hour after ingestion of 10 g of a collagen hydrolysate (Iwai et al., 2005). To isolate food-derived collagen peptides in human plasma, a series of size-exclusion chromatography (SEC) and reverse-phase (RP)-HPLC have previously been performed (Iwai et al., 2005; Ohara et al. 2007). To improve the resolution and detection of hydrophilic food-derived peptides in human plasma, pre-column derivatization with phenyl isothiocyanate (PITC) has been performed previously (Aito-Inoue et al., 2006; Shigemura et al., 2011; 2017). By comparing chromatograms before and after ingestion of collagen hydrolysates, seven Hyp-containing peptides; Pro-Hyp, Pro-Hyp-Gly, Ile-Hyp, Leu-Hyp, Hyp-Gly, Glu-Hyp, Ala-Hyp, and Pro-Gly have been identified (Shigemura et al., 2017). In most cases, Pro-Hyp accounts for more than 50% of total collagen peptides in human plasma. Pro-Hyp enhances fibroblast growth on a collagen gel (Shigemura et al., 2009; 2011) and production of glycosaminoglycans by fibroblasts and chondrocytes (Nakatani et al., 2009; Ohara et al., 2010), which partly explain the beneficial effects of collagen hydrolysates; improvement of wound healing (Sugihara et al., 2015) and skin (Proksch et al., 2014a,b) and joint conditions (Zdzieblik et al., 2017). However, collagen hydrolysates ingested comprised collagen peptides with an average molecular weight of 5000 Da and did not contain Pro-Hyp (Iwai et al., 2005). These findings indicate that biological activities of peptides in the body rather than in food are well associated with the biological response on ingestion of protein hydrolysates.
Furthermore, ingestion of corn and wheat gluten hyrolysates also exerts beneficial effects on alcohol-induced liver damage and muscle injury after extensive physical exercise (Koikawa et al., 2009; Wu et al., 2014). To identify food-derived peptides after ingestion of corn and wheat gluten hydrolysates, the aforementioned technique was applied in our preliminary experiments. However, it was difficult to directly detect plant protein-derived peptides owing to their lower levels than those of extracellular proteins-derived peptides in blood. The objective of the present study was to establish an approach to identify small amounts of food-derived peptides in human blood after ingestion of non-extracellular matrix proteins to understand molecular mechanism for beneficial effects of protein hydrolysates on ingestion, which has so far been a black box.
| 2. Materials and methods | ▴Top |
2.1. Corn and wheat gluten hydrolysates
Food-grade enzymatic hydrolysates of corn and wheat gluten were provided by Japan Food Peptide Institute (Osaka, Japan). These products were prepared by the method described in Japanese Patent No. 6190999. The corn and wheat gluten were digested by Alcalase and Neutrase (Novozymes, Bagsværd, Denmark). Contents of peptides with molecular weight of 1000 Da or less in the corn gluten and wheat gluten hydrolysates were approximately 90 and 85%, respectively (information from supplier).
2.2. Regents
Acetonitrile (HPLC-grade), methanol, formic acid, ammonium acetate, tris(hydroxymethyl)aminomethane (Tris), and porcine pancreatin with carboxypeptidase A activity were purchased from Nacalai Tesque (Kyoto, Japan). Porcine leucine aminopeptidase was purchased from Sigma-Aldrich (St. Louis, MO, USA). Piperidine, 4-methylmorpholine, N,N-dimethylformamide, t-butyl methyl ether, trifluoroacetic acid, 6 mol/L hydrochloric acid, a standard mixture of amino acid (Type H), and phenyl isothiocyanate (PITC) were purchased from Wako Chemical (Osaka, Japan). L-pyroglutamic acid, 9-fluorenylmethoxycarbonyl (Fmoc) amino acid derivatives, Fmoc amino acid-bound p-alkoxybenzyl alcohol (Alko) resin, proline-bound 2-chlorotrityl chloride (Barlos) resin, 1H-benzotriazol-1-yloxy-tri(pyrrolidino)phosphonium hexafluorophosphate (PyBOP), and 1-hydroxybenzotriazole (HOBt) were purchased from Watanabe Chemical Industries (Hiroshima, Japan).
2.3. Human study
This study was approved by the ethical committee in Saraya and performed in accordance with the tenets of the Declaration of Helsinki under the supervision of medical doctors. Volunteers were informed of the study objectives and the risks of sample ingestion and blood collection. Healthy volunteers (2 male and 2 female, 20–40 years of age) ingested 180 g of jelly beverage containing 9 g of corn gluten hydrolysate or wheat gluten hydrolysate after a 12-h fast. Approximately 2 mL of venous blood was collected from the cubital vein before and after ingestion (0.5, 1, 2, and 4 h). The plasma obtained from the venous blood was deproteinized by addition of an equal volume of ethanol. The ethanol-soluble fraction was harvested by centrifugation at 13,000 g for 10 min at 5 °C and stored at −80 °C until use.
2.4. In vitro exopeptidase digestion of peptides in protein hydrolysates
Corn and wheat gluten hydrolysates were dissolved in 1 mL of 50 mM Tris-HCl (pH 8.0) to a final concentration of 0.25% w/v. Pancreatin (100 μg/3 μL of the Tris-HCl buffer) and 2.45 U of leucine aminopeptidase were added to the solution and digestion was carried out at 37 °C for 24 h. Enzymes were extracted via ultrafiltration using an Amicon Ultra 10K device (Merck Millipore, Burlington, MA, USA). The effluent was collected and used as the exopeptidase digest. To separate pyroglutamyl peptides from normal peptides, solid-phase extraction with a strong cation exchanger (AG50W-×8, hydrogen form, 100–200 mesh, Bio-Rad Laboratories, Hercules, CA, USA) was performed as described previously (Higaki-Sato et al., 2006; Kiyono et al., 2013). The resin was thoroughly washed with 50% methanol and packed into a spin column (15 mm × 7 mm i.d., 5.0 μm pore size, Ultrafree-MC, Merck Millipore). Two hundreds microlitters of 0.1% formic acid containing 10% acetonitrile were added onto the column and eluted by centrifugation at 815 g for equilibration (three times). Two hundreds microliters of the exopeptidase digest were loaded onto the column and eluted by centrifugation. The effluent was used as the pyroglutamyl peptide fraction.
2.5. Identification of indigestible peptides in hydrolysate
Peptides in the exopeptidase digest were fractionated via size-exclusion chromatography (SEC) using Superdex Peptide 10/300 GL (GE Healthcare, Buckinghamshire, UK) and equilibrated with 0.1% formic acid containing 10% acetonitrile at a flow rate of 0.5 mL/min. Fractions were collected every 1 min. Each fraction was dried under vacuum and peptides in each fraction were derivatized with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AccQ) (Toronto Research Chemicals, Toronto, ON, Canada). Structures of AccQ and derivatives are shown in Figure 1. The residue was dissolved in 20 μL of 20 mM HCl and reacted with 20 μL of 0.3% AccQ-acetonitrile solution and 60 μL of 50 mM sodium borate buffer (pH 8.8). The reaction was carried out at 50 °C for 10 min and terminated by cooling to 5 °C. The reactant was mixed with 50 μL of 5 mM sodium phosphate buffer (pH 7.5) and clarified via passing through Cosmonice (R) filter (4 mm i.d., 0.45 μm, Nacalai Tesque). The filtrate (20 μL) was injected to liquid chromatography-electrospray ionization-tandem mass spectrometer (LC-MS/MS, LCMS 8040, Shimadzu, Kyoto, Japan) equipped with an Inertsil ODS-3 column (2 mm i.d. × 250 mm, GL Science, Tokyo, Japan). A binary liner gradient was used with 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B) at a flow rate of 0.2 mL/min. The gradient program was as follows: 0–15 min, 0–50% B; 15–20 min, 50–100% B; 20–25 min, 100% B; 25–25.1 min, 100–0% B; 25.1–35 min, 0% B. The column was maintained at 40 °C. AccQ-derivatives were specifically detected by selecting precursor ions, which generated ions of the AccQ-derived product (Figure 1; b1 ion, m/z = 171.1) at positive mode (collision energy −35 V) in the range of m/z = 250–350, 350–450, 450–550, and 550–650 (precursor ion scan). The pyroglutamyl peptide fraction (200 μL) was subjected to SEC. An aliquot of the SEC fraction (30 μL) was subjected to LC-MS with the aforementioned elution conditions. Total ion intensity was monitored at a positive mode in the range of m/z = 180–700 (total ion scan). The m/z of AccQ-peptides and pyroglutamyl peptides were recorded and entered into the program (LabSolutionsLCMS Ver. 5.5, Shimadzu) for product scan mode (collision energy −15 and −35 V) to estimate peptide structure.
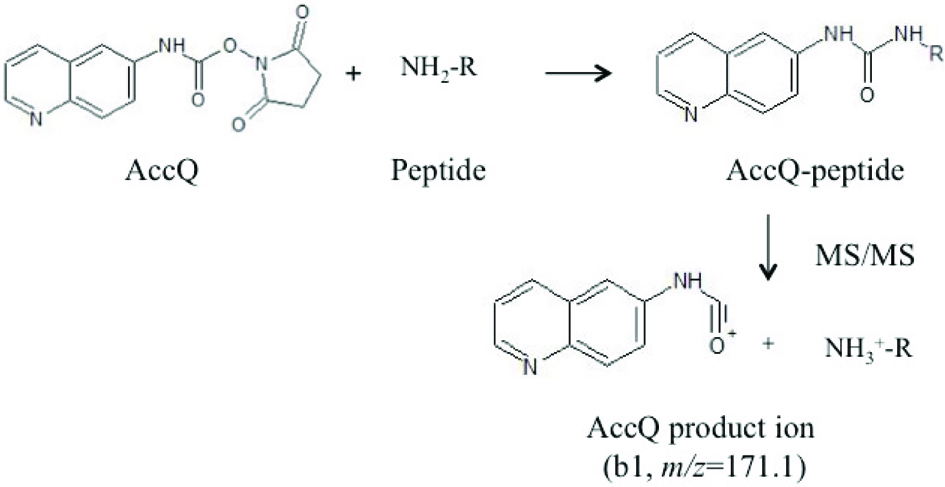 Click for large image | Figure 1. Derivatization of peptides with AccQ and generation of AccQ-derived product ions (b1, m/z = 171.1) via tandem mass spectrometry. |
2.6. Peptide synthesis
Peptides were synthesized per Fmoc strategy using a PSSM-8 peptide synthesizer (Shimadzu). Synthetic peptides were purified via RP-HPLC using a Cosmosil 5C18-MS-II column (10 mm i.d. × 250 mm, Nacalai Tesque). A binary liner gradient was used with 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B) at a flow rate of 2 mL/min. The gradient program was as follows: 0–20 min, 0–50% B; 20–30 min, 50–100% B; 30–35 min, 100% B; 35–35.1 min, 100–0% B; 35.1–45 min, 0% B. The column was maintained at 40 °C. Elution of peptides was monitored spectrophotometrically at 214 and 254 nm. Peptide purity of the peptides was confirmed via LC-MS. Contents of the peptides were evaluated via amino acid analysis of HCl hydrolysates (Bidlingmeyer et al., 1984; Sato et al., 1992).
2.7. Peptide determination
Five microlitters of the exopeptidase digest and a 100 µL of the ethanol-soluble fraction of human plasma were dried under vacuum and then derivatized with AccQ as described above. Contents of the AccQ-peptides and pyroglutamyl peptides in the reaction mixture were determined via LC-MS/MS in multiple-reaction monitoring (MRM) mode. The elution condition was the same as that described above. The synthetic peptides were used for optimization of the MRM condition using LabSolutionsLCMS Ver. 5.5 (Shimadzu).
2.8. Statistical analysis
The statistical analysis was performed using software GraphPad PRISM version 6.04 (USACO Corporation, Tokyo, Japan). Difference between the plasma peptide content before and after ingestion of the hydrolysates was analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test for post-hoc analysis. A P-value less than 0.05 was considered as statically significant.
| 3. Results | ▴Top |
3.1. Identification of exopeptidase-resistant peptides
The LC-MS/MS chromatograms of AccQ-derivatives in the SEC fractions in precursor ion scan mode at the scanning range of m/z = 250–300 and 350–450 are shown in Figure 2a and b, respectively; LC-MS chromatograms in the SEC fractions of pyroglutamyl peptide fraction, Figure 2c. The m/z of the peaks marked with uppercase or lowercase and asterisks were recorded. The peaks marked with uppercase were identified as amino acids designated with one-letter abbreviations on the basis of their retention times and m/z. The m/z of the peaks marked with asterisks could not be assigned to peptides with any combination of amino acids. Compounds in the peaks marked with lowercase were subjected to MS/MS analysis. Product ion patterns are shown in the supplementary data. Table 1 summarizes the estimated structure of peptides based on m/z of precursor and product ions. All estimated peptides were synthesized. In addition, all dipeptides with reversed sequence except for pyroglutamyl peptides were synthesized and could be resolved via RP-HPLC. Presence of the 26 and 28 indigestible peptides including those with reversed sequence (Pro-Ala, Pro-Ser, Pro-Val, Pro-Leu, Pro-Phe, and Pro-Ile from the corn hydrolysate and Pro-Ser, Pro-Gln, Pro-Val, Pro-Gly, Pro-Phe, and Pro-Leu, Pro-Ile from the wheat gluten hydrolysate) were confirmed in the exopeptidase digests of corn and wheat gluten hydrolysates on the basis of retention time and MRM detection. All indigestible peptides from the hydrolysates were diprolyl and di- and tripyroglutamyl peptides. The contents of the indigestible peptides in the exopeptidase digests are shown in Figure 3. PyroGlu-Pro, pyroGlu-Gln, pyroGlu-Leu-Pro, and pyroGlu-Gln-Pro were the primary constituents in both digests. For prolyl dipeptides, the peptides with C-terminal prolyl residues were directly detected via the precursor ion scan targeting product ions from AccQ, while those with N-terminal prolyl residues were not (Figure 2). However, MRM using the peptides with reversed sequence as standards detected dipeptides with N-terminal prolyl residues (Figure 3). The sum of all indigestible peptides per corn and wheat gluten hydrolysates was 5.4 and 10.4% (w/w), respectively. Therefore, approximately 90% of peptides were degraded into amino acids.
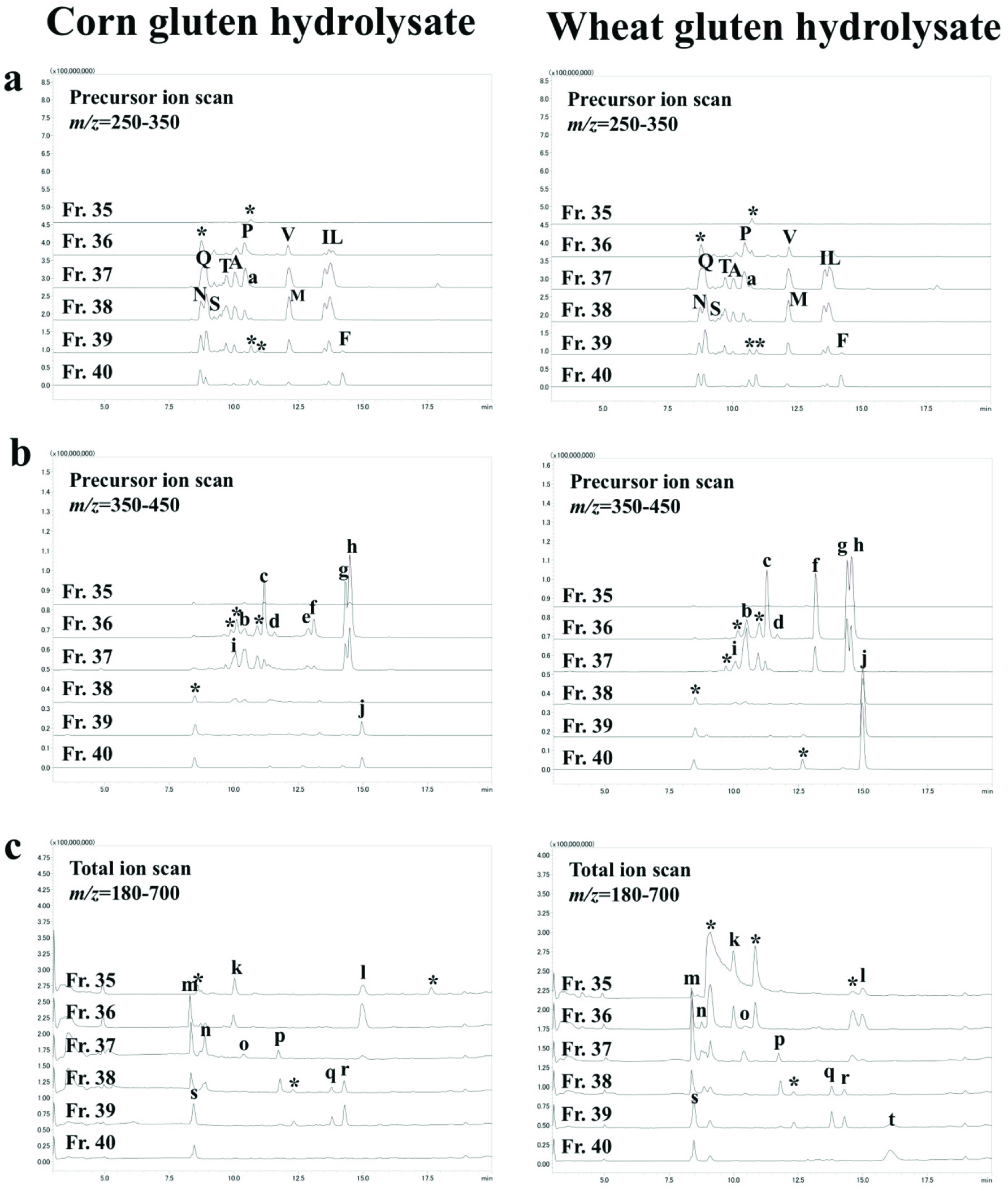 Click for large image | Figure 2. Mass spectrometry chromatograms of AccQ-derivatives (a, b) and pyroglutamyl peptides (c) in SEC fractions 35-40. Peaks marked with uppercase represent amino acid derivatives; lowercase, AccQ-peptides (a-j) and pyroglutamyl peptides (k-t). Peaks marked with asterisks could not be assigned to peptides with any combination of amino acids. |
 Click to view | Table 1. Estimated sequences of peptides in size-exclusion chromatographic fractions of exopeptidase digests of corn (C) and wheat (W) gluten hydrolysates |
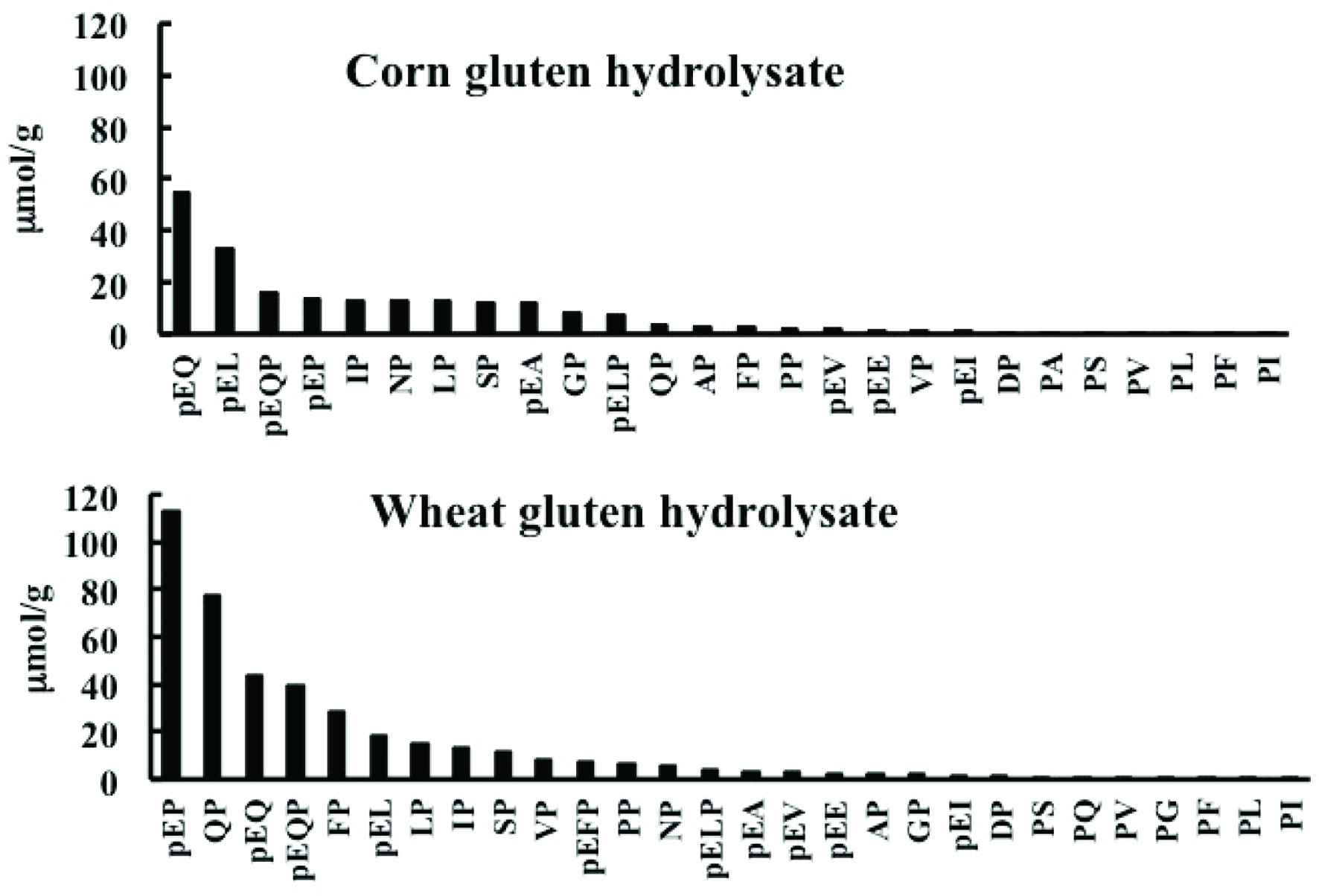 Click for large image | Figure 3. Contents of exopeptidase-resistant peptides from corn and wheat gluten hydrolysates. |
3.2. Contents of food-derived peptides in human blood
Most of the indigestible peptides shown in Figure 3 were detected in human plasma before and after ingestion of the hydrolysates. As shown in Figure 4, three pyroglutamyl peptides, pyroGlu-Pro, pyroGlu-Leu-Pro, and pyroGlu-Gln-Pro, were significantly increased after ingestion of corn and wheat gluten hydrolysates. Maximum levels of these peptides were approximately 10–100 nM. Unexpectedly, only the dipeptides with N-terminal prolyl residues (Pro-Ala, Pro-Gly, and Pro-Gln) significantly increased to 20–50 nM after ingestion, while those of peptides in the hydrolysates were much lower than those with C-terminal prolyl residues (Figure 3), which did not increase significantly. The di- and tripeptides approached maximum levels 1 and 4 h after ingestion, respectively.
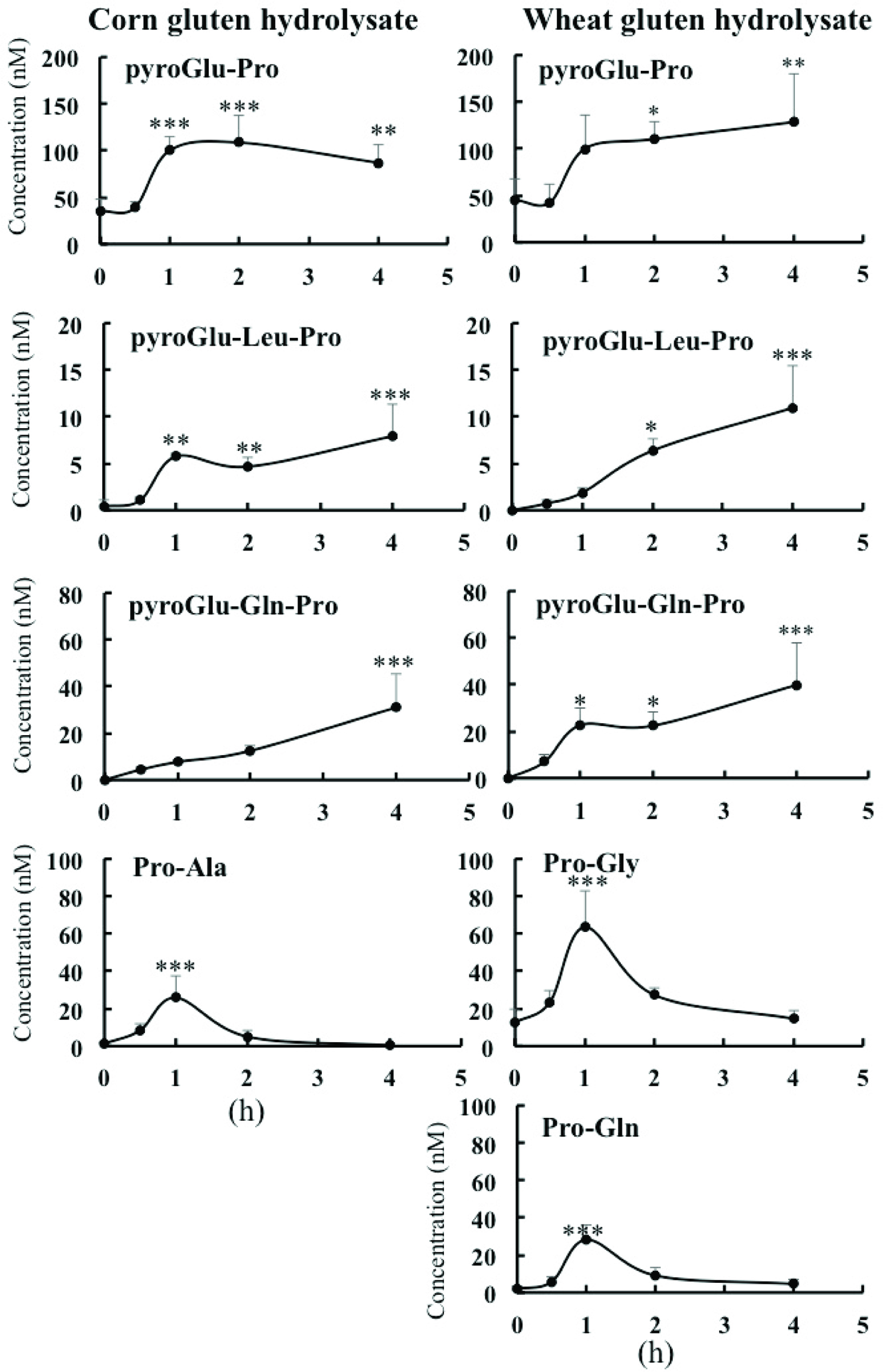 Click for large image | Figure 4. Contents of the exopeptidase-resistant peptides in human blood plasma after ingestion of corn or wheat gluten hydrolysates. |
| 4. Discussion | ▴Top |
Resolution of short-chain peptides, especially hydrophilic peptides, via RP-HPLC is generally inadequate owing to poor retention in the column. To overcome this issue, pre-column labeling using PITC has previously been used (Aito-Inoue et al., 2006; Shigemura et al., 2011; 2012; 2017). The derivatives, phenyl thiocarbamyl (PTC)-peptides, can be separated via RP-HPLC in higher resolution before the derivatization and directly sequenced via Edman’s degradation method. However, ionization efficiency of PTC-peptides in MS analysis was low. In the present study, AccQ was used for derivatization of amino groups, as AccQ-derivatives are ionized with high efficiency and generate product ion of m/z = 171.1 derived from AccQ in MS/MS analysis, which enables specific detection of the components with amino groups by selecting product ion of m/z = 171.1 via MS/MS in precursor ion scan mode. This method allows for identification of major indigestible peptides in the exopeptidase digests of corn and wheat gluten hydrolysates (Table 1). It may also be applied directly to detect food-derived peptides in human plasma after the ingestion of corn and wheat gluten hydrolysates. However, despite this method, it was difficult to detect the increased peptides after ingestion owing to the presence of numerous amino compounds (data not shown). In the present study, indigestible peptides against carboxypeptidase A in pancreatin and leucine amino peptidase were first identified. It can be assumed that at least part of these indigestible peptides resist exopeptidase digestion in the digestive tract and blood, and are absorbed in the blood. In fact, six indigestible peptides increased in human plasma after ingestion of corn and wheat gluten hydrolysates. The maximum levels of these peptides in plasma were approximately 10–100 nM, which were considerably lower than those of food-derived collagen and elastin peptides (10–50 μM) (Iwai et al., 2005, Ohara et al., 2007, Shigemura et al., 2012; 2017). Therefore, it was difficult to directly detect these peptides in blood via LC-MS/MS in precursor ion scan mode. Pre-identification of indigestible peptides against exopeptidases enabled specific detection of the indigestible peptides in blood via LC-MS/MS in MRM mode. The present exopeptidase digestion can degrade approximately 90% of peptides and provide candidate for the food-derived peptides in the body.
The contents of indigestible dipeptides with C-terminal prolyl residues in the exopeptidase digests of corn and wheat gluten hydrolysates were significantly higher than those with N-terminal prolyl residues (Figure 3). Nevertheless, dipeptides with C-terminal prolyl residues did not increase in plasma after ingestion of corn and wheat gluten hydrolysates. The dipeptides with C-terminal prolyl residue can be degraded by intracellular and plasma prolidase (Cunningham et al., 1997; Lupi et al., 2008), which can explain the inconsistency between in vitro and in vivo results in the present study. For better prediction of the structure of food-derived peptides in the body, other exopeptidases such as prolidase and aminopeptidase P, among others, could be used in addition to leucine aminopeptidase and carboxypeptidase A in pancreatin.
| 5. Conclusion | ▴Top |
The present study shows that 6 prolyl and pyroglutamyl peptides significantly increase in human plasma after ingestion of 9 g of corn and wheat gluten hydrolysates. Maximum levels of these peptides were approximately 10–100 nM. Among them, Pro-Gly attenuated hypertension-induced damage to endothelial cells in arteries of spontaneously hypertensive rats (Takemori et al., 2015). In addition, short-chain pyroglutamyl peptides attenuated hepatitis in rat (Sato et al., 2013) and colitis-induced dysbiosis of mice (Wada et al., 2013; Kiyono et al., 2016) at relatively low doses. The activities of food-derived peptides in plasma might be associated with their beneficial effects on ingestion of corn and wheat gluten hydrolysates, which warrants further confirmation via further in vitro and in vivo studies using these peptides. The present method would be useful to identify food-derived peptides with potential bioactivity in the blood after ingestion of other protein hydrolysates.
| References | ▴Top |