| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 18, June 2022, pages 90-97
Improving the oxidation stability of high-oleic sunflower oil with composite antioxidants
Xue-Chen Peia, Yu-Xin Liua, b, c, Hui-Lin Liua, b, c, De-Yang Lia, b, c, Fa-Wen Yina, b, c, Zi-Xuan Wua, Yong-Fu Wangd, Da-Yong Zhoua, b, c, *
aSchool of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, PR China
bNational Engineering Research Center of Seafood, Dalian 116034, PR China
cCollaborative Innovation Center of Seafood Deep Processing, Dalian 116034, PR China
dQingdao Seawit Life Science Co., Ltd, Qingdao 266200, PR China
*Corresponding author: Da-Yong Zhou, School of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, PR China. Tel: +86-411-86323453; Fax: 86-411-86323262; E-mail: zdyzf1@163.com
DOI: 10.31665/JFB.2022.18312
Received: May 5, 2022
Revised received & accepted: June 29, 2022
| Abstract | ▴Top |
This study aimed to search for the optimum antioxidant mixture for improvement of the stability of high-oleic sunflower oil. Seven antioxidants including vitamin E, phytic acid, antioxidant of bamboo leaves, rosemary extract, tea polyphenols, ascorbyl palmitate (AP) as well as tea polyphenol palmitate (TPP) were used. Peroxide value, free radicals and Rancimat induction time results all proved that TPP was the best single antioxidant. Therefore, TPP was selected to mix with other antioxidants to form composite antioxidants. The combination of TPP and AP revealed the best antioxidant efficiency among the composite antioxidants comprised of two and three constituents in the oil. By optimizing the mixture proportions, the best antioxidant activity could be achieved when combining 480 mg/kg TPP with 40 mg/kg AP. The high-oleic sunflower oil sample (containing 480 mg/kg TPP and 40 mg/kg AP) had 1.96-fold longer shelf life (prediction by Rancimat method) compared with the blank sample.
Keywords: Composite antioxidant; High-oleic sunflower oil; Oxidative stability; Accelerated oxidation; Rancimat method
| 1. Introduction | ▴Top |
High-oleic sunflower oil is extracted from high-oleic sunflower seed which is obtained through induced mutation and selected breeding during seed development (Rauf et al., 2017). High-oleic sunflower oil has a high content of oleic acid (60–85% of total fatty acids), which can decrease the risk of coronary heart disease and be more suitable for deep frying and cooking compared with regular sunflower oil (Rauf et al., 2017). However, because of its large levels of unsaturated fatty acids (UFAs), high-oleic sunflower oil is prone to lipid oxidation, which causes the generation of multiple toxic compounds, bad flavors and odors, thus decreasing the nutritional quality and then causing negative health effects (Villeneuve et al., 2021).
In order to inhibit edible vegetable oil oxidation effectively, choosing tailored antioxidants and appropriate food packaging materials can be used (Hu et al., 2020; da Silva et al., 2021). Antioxidants are substances that be able to delay, retard or prevent oxidation processes, which are widely used considering their good applicability and convenience (da Silva et al., 2021). However, due to the possible toxicity of synthetic antioxidants during long-term intake, it seems logical to replace them with antioxidants of natural plant origins (da Silva et al., 2021). Previous study indicated that natural antioxidants play a role in inhibiting the oxidation of sunflower oil. For instance, Elsayed et al. (2020) reported that plum leaves extract could exert an effect on retarding the oxidation process of sunflower oil. Saeed et al. (2022) found that potato peel extract exhibited very strong antioxidant activity, almost equal to that of butylated hydroxyanisole (BHA) in sunflower oil.
Meanwhile, to further enhance antioxidant efficiency, the method of mixing various antioxidants together has also been applied in vegetable oils due to the synergistic effects. For instance, Yin et al. (2021) revealed that an antioxidant blend comprised of vitamin E (VE), rosemary extract (RE) and ascorbyl palmitate (AP) could effectually enhance the oxidation stability of microalgal docosahexaenoic acid (DHA)-rich oil. Farhoosh and Nyström (2018) proved that combining gallic acid with methyl gallate could inhibit the autoxidation of triacylglycerols more effectively in sunflower oil. However, for all we know, studies on the combination of natural antioxidants and their derivatives to enhance the antioxidant effect of high-oleic sunflower oil have not yet been reported.
Therefore, in order to get the optimum antioxidant blend for high-oleic sunflower oil, seven commonly used natural antioxidants (VE, phytic acid (PA), antioxidant of bamboo leaves (AOB), RE, tea polyphenols (TP), AP and tea polyphenol palmitate (TPP) (a derivative of TP)) were chosen and their functions against lipid oxidation were estimated by determining Rancimat induction time (IT), peroxide value (POV) and free radicals in high-oleic sunflower oil. In addition, Rancimat method was used for the shelf life prediction of high-oleic sunflower oil. This study will be helpful to provide reasonable natural antioxidant mixture to stabilize high-oleic sunflower oil products.
| 2. Materials and methods | ▴Top |
2.1. Oil and antioxidants
The refined high-oleic sunflower oil with no extra antioxidants was obtained from Qingdao Seawit Life Science Co., Ltd (Qingdao, Shandong, PR China). Food grade antioxidants were provided by Kemiou Chemical Reagant Factory (Tianjin, China), encompassing VE (α-, β-, γ-, δ-tocopherol); PA; AOB, a mixture with the main active ingredient was isoorientin; RE, a mixture with the main active ingredients were carnosic acid, rosmarinic acid and carnosol; TP, a mixture of polyhydroxy compounds with the main active ingredient was catechin; AP; and TPP, a mixture of polyphenol palmitate with the main active ingredient was catechin palmitate.
2.2. Fatty acid composition
An Agilent 7890B Gas Chromatography (Palo Alto, CA, USA) was used to analyze the fatty acid methyl ester (FAME) on the basis of the method with a small modification (Czerniak et al., 2015).
2.3. Sample preparation
Twenty seven testing groups (groups 1–27) were built up on the basis of the species and quantity of antioxidants added as seen in Table S1. The blank oil sample with no antioxidants was named as group 1. The oil samples with single antioxidants (VE, PA, AOB, RE, TP, AP or TPP) were named as groups 2–8 (each antioxidant was added to the oil at its maximum legal quantity on the basis of the Chinese Standard GB 2760-2014). The oil samples with binary composite antioxidants (TPP and one of the other six antioxidants) were named as groups 9–14 (each antioxidant was added to the oil at its one half of maximum legal quantity). The oil samples with ternary composite antioxidants (TPP, AP and one of the other five antioxidants) were named as groups 15–19 (each antioxidant was added to the oil at its one third of maximum legal quantity). The oil samples with different proportions of TPP and AP were named as groups 20–27. Among which, TPP was added to the oil at its 1/3, 2/3, 1/4, 3/4, 1/5, 4/5, 2/5 or 3/5 of maximum legal quantity, while AP was added correspondingly at its 2/3, 1/3, 3/4, 1/4, 4/5 or 1/5 of maximum legal quantity.
2.4. Oxidative stability
The IT of high-oleic sunflower oil samples was monitored through using a Rancimat model 892 (Metrohm, Herisau, Switzerland). Briefly, three grams of oil sample were added to each reaction vessel and subjected to constant heating at 130 °C and 140 °C (the rate of airflow: 20 L/h) on the basis of the American Oil Chemists’ Society (AOCS) official method AOCS Cd 12b-92 (2009).
2.5. Peroxide value
All samples were saved under 55 °C for a 6 day’s storage period and collected each 2 days. The POV was determined and recorded as meq/kg oil based on AOCS official method AOCS Cd 8b-90 (2003).
2.6. Free radicals
Electron spin resonance (ESR) spectrometer A200 (Bruker, Karisruhe, Germany) was used for monitoring free radical signals of oil samples according to Chen et al. (2018). Briefly, 2 mL of oil were mixed with 200 μL of 56.4 mmol/L N-tert-butyl-α-phenylnitrone (PBN) in a 10 mL eppendorf tube and then saved under 120 °C for a 12 hour’s storage period, and collected each 6 hours. Next, the mixture was transferred to a nuclear magnetic tube and then inserted in the resonant cavity.
2.7. Shelf life prediction
The ITs of high-oleic sunflower oil samples were measured at 110 °C, 120 °C, 130 °C and 140 °C. The natural logarithms of the ITs versus selected temperatures (110 °C, 120 °C, 130 °C and 140 °C) were plotted and the lines were fitted to the data (Log IT = a(t) + b). The Q10 value which indicates the increase in reaction rate due to a 10 °C rise in temperature was calculated as: IT at t (°C) / IT at t + 10 (°C). The shelf life was calculated by extrapolation of the linear relationship of Log IT and t for a 25 °C temperature according to Rodríguez et al. (2020).
2.8. Statistical analysis
The experiments mentioned above were executed three time and the results were presented as mean ± standard deviation (SD). The data were analyzed by SPSS, then followed by one-way ANOVA analysis of variance (student–Newman–Keuls (S-N-K) test) in the case of significant differences (P < 0.05).
| 3. Results | ▴Top |
3.1. Selection of the most effective single antioxidant
Rancimat method was selected to monitor the whole oxidation stage of high-oleic sunflower oil samples containing single antioxidant (groups 1–8). At 130 °C, the IT values of oil samples (groups 1–8) were 4.23 ± 0.11, 4.24 ± 0.07, 4.32 ± 0.12, 4.27 ± 0.08, 5.07 ± 0.05, 5.53 ± 0.21, 5.58 ± 0.08 and 6.37 ± 0.23 h, respectively (Figure 1a). Compared with group 1 (blank sample), the IT values from groups 2–8 were increased by 1.00-, 1.02-, 1.01-, 1.01-, 1.20-, 1.31-, 1.32- and 1.51-fold, respectively. Similarly, the IT values of the oil samples (groups 1–8) at 140 °C were 2.07 ± 0.08, 2.09 ± 0.07, 2.29 ± 0.19, 2.23 ± 0.06, 2.46 ± 0.07, 2.60 ± 0.08, 2.84 ± 0.04 and 3.81 ± 0.60 h, respectively (Figure 1b). Apparently, the addition of several single antioxidants such as AP (group 7) and TPP (group 8) significantly retarded the oxidation process of high-oleic sunflower oil (P < 0.05). At the same time, TPP (group 8) exerted significantly higher antioxidant effectiveness than other single antioxidants based on the IT (P < 0.05).
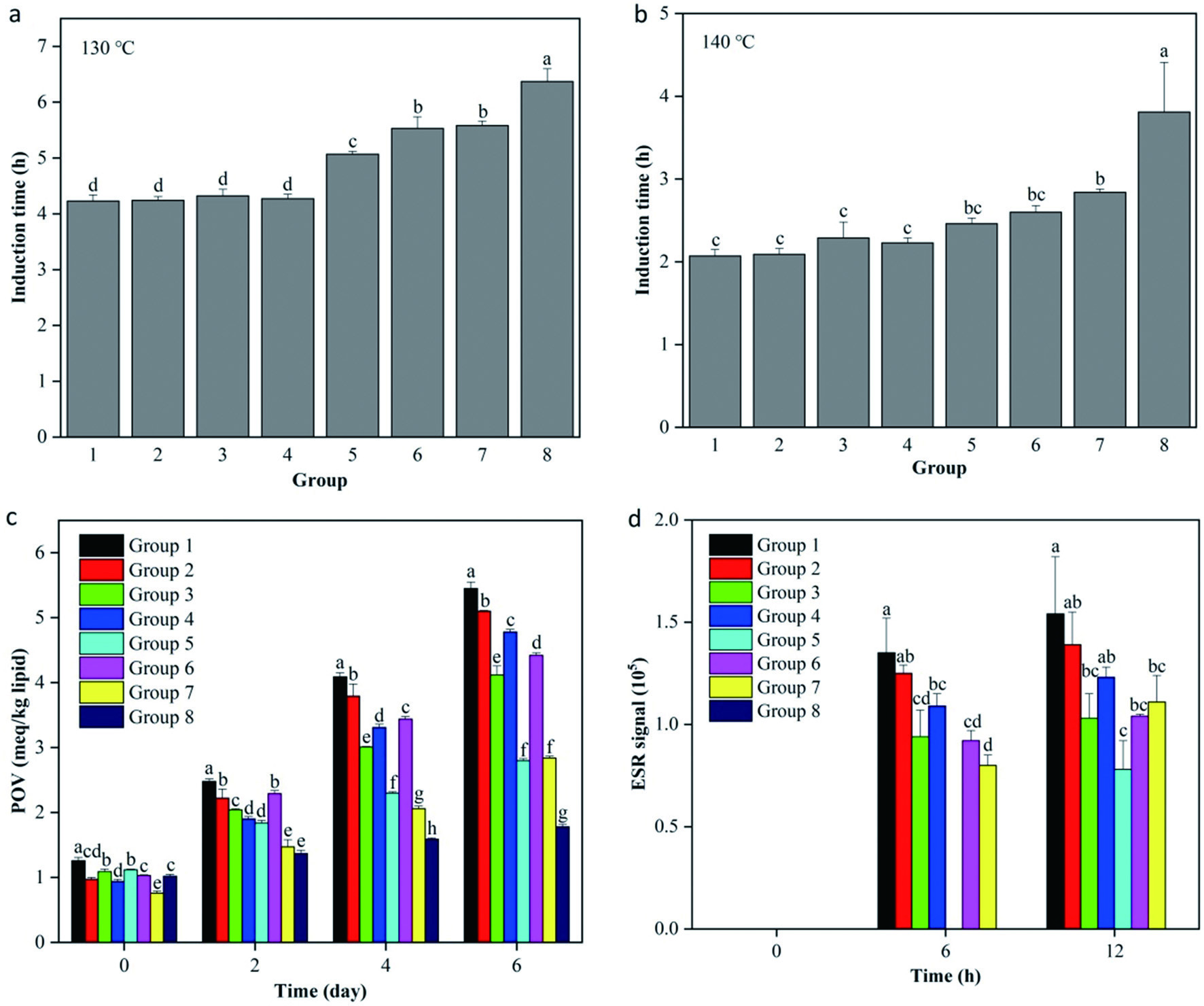 Click for large image | Figure 1. Antioxidant activities of various single antioxidants in high-oleic sunflower oil. A, Rancimat induction time at 130 °C; B, Rancimat induction time at 140 °C; C, Peroxide value (POV) at 55 °C; D, Free radical intensity at 120 °C. Group 1 was the control high-oleic sunflower oil without adding any antioxidants. Groups 2–8 were the high-oleic sunflower oil samples containing vitamin E, phytic acid, antioxidant of bamboo leaves, rosemary extract, tea polyphenols, ascorbyl palmitate and tea polyphenol palmitate, respectively. All experiments were repeated three times. Different letters (a-h) in each panel at same storage time indicate significant differences from each other (P < 0.05). |
The POV was selected to measure the contents of hydroperoxides formed in the early oxidation stage of high-oleic sunflower oil during the storage at 55 °C (Figure 1c). The POV for high-oleic sunflower oil samples from groups 1–8 all went up significantly accompanied by the increase of storage time (P < 0.05), showing that all the oil samples were progressively oxidized. Post to two, four and six days storage, the POV of groups 2–8 were significantly lower than that of group 1, indicating that these antioxidants could significantly retard the primary oxidation of high-oleic sunflower oil (P < 0.05). Meanwhile, TPP (group 8) exerted significantly higher antioxidant effectiveness than others based on the POV (P < 0.05).
The ESR spectroscopy was selected to detect the intensity of free radical signals in high-oleic sunflower oil during the storage at 120 °C (Figure 1d and S1). Before heat inducement, the oil samples all had negligible free radical intensities. While free radical intensities in high-oleic sunflower oil samples from groups 1–7 increased with the time during heat inducement, indicating that free radicals were progressively generating in these oil samples. After 12 h of heat inducement, groups 2–7 all had significantly lower free radical intensities than that of group 1, indicating that all added antioxidants could suppress the production of free radicals (P < 0.05). However, the free radical intensities in group 8 (containing TPP) were always at very low levels (below the detection limit) throughout the whole heat inducement process, indicating that TPP could show the best antioxidant efficiency by scavenging free radicals in inhibiting the oxidation of high-oleic sunflower oil.
3.2. Selection of formulation of the mixed antioxidants
The results above all showed TPP (group 8) exerted the most excellent antioxidant effect in high-oleic sunflower oil among all seven single antioxidants. In order to obtain a better antioxidant effectiveness, one of the other six antioxidants (VE, PA, AOB, RE, TP and AP) was used to mix with TPP to form binary composite antioxidants (groups 9–14). At 130 °C, the IT values of oil samples from groups 8–14 were 6.37 ± 0.23, 6.57 ± 0.21, 9.46 ± 0.48, 7.22 ± 0.34, 8.88 ± 0.16, 9.38 ± 0.19 and 9.70 ± 0.04 h, respectively (Figure 2a), while the corresponding values were 3.81 ± 0.60, 3.54 ± 0.38, 4.70 ± 0.05, 3.84 ± 0.19, 4.35 ± 0.05, 4.66 ± 0.10 and 4.94 ± 0.11 h, respectively, at 140 °C (Figure 2b). Apparently, the high-oleic sunflower oil samples including TPP + PA (group 10), TPP + TP (group 13) and TPP + AP (group 14) showed significantly higher oxidative stability than others (P < 0.05). However, based on the POV assay, group 14 (containing TPP + AP) showed the best antioxidant efficiency in inhibiting the oxidation of high-oleic sunflower oil among groups 8–14 (P < 0.05) (Figure 2e). Therefore, this combination of two antioxidants was used for further study.
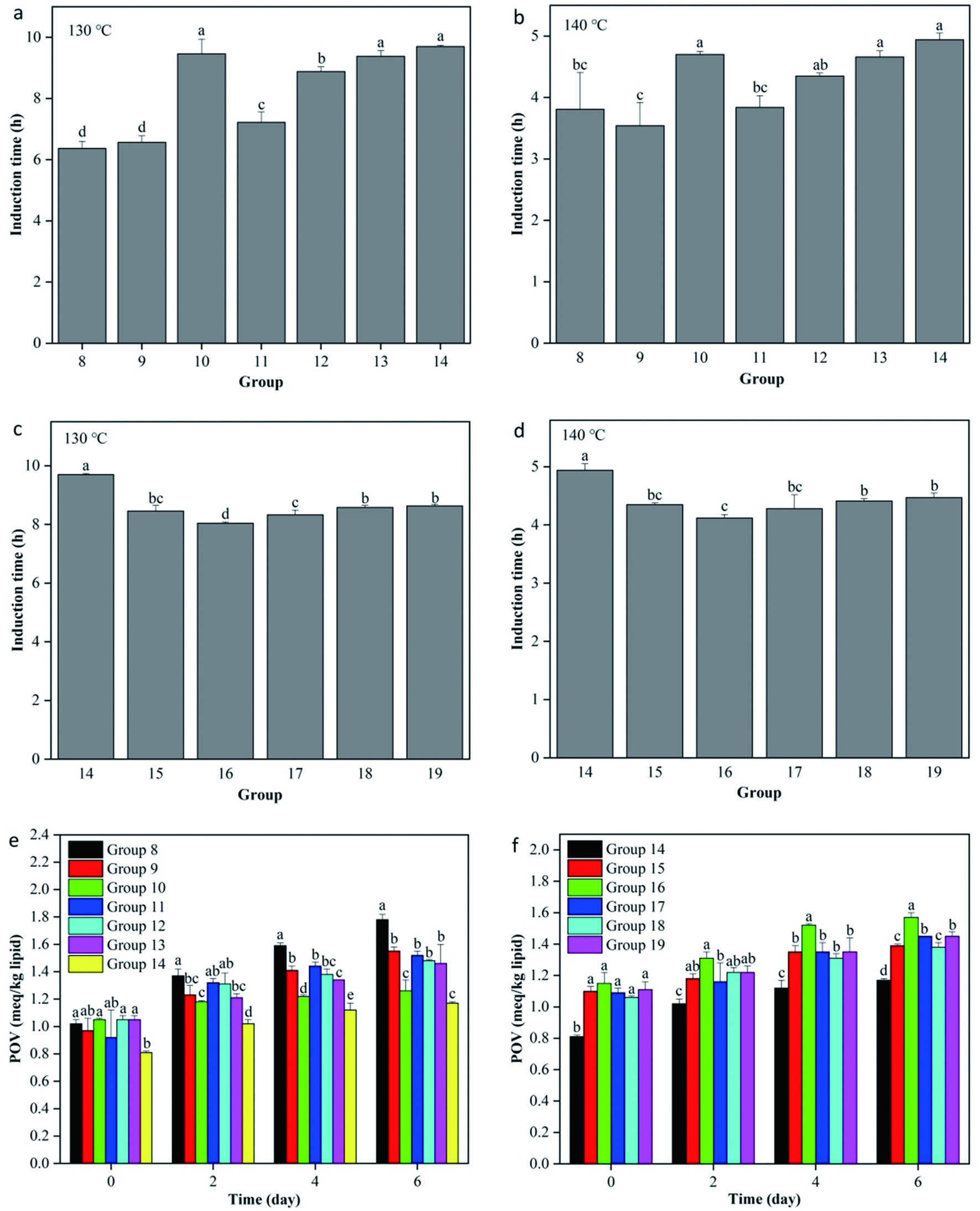 Click for large image | Figure 2. Antioxidant activities of binary and ternary composite antioxidants in high-oleic sunflower oil. A-D, Rancimat induction time (130 °C and 140 °C); E and F, peroxide value (POV) at 55 °C. Group 8 was the high-oleic sunflower oil sample containing tea polyphenol palmitate (TPP). Groups 9–14 were the high-oleic sunflower oil samples containing TPP and one of the other six antioxidants (vitamin E (VE), phytic acid (PA), antioxidant of bamboo leaves (AOB), rosemary extract (RE), tea polyphenols (TP), and ascorbyl palmitate (AP)). Groups 15–19 were the high-oleic sunflower oil samples containing TPP, AP and one of the other five antioxidants (VE, PA, AOB, RE, and TP). All experiments were repeated three times. Different letters (a-e) in each panel at same storage time indicate significant differences from each other (P < 0.05). |
The optimum mixture (TPP + AP) was combined with one of the other five antioxidants (VE, PA, AOB, RE and TP) to form ternary composite antioxidants to enhance the antioxidant effectiveness further. At 130 °C, the IT values of oil samples from groups 14–19 were 9.70 ± 0.04, 8.46 ± 0.19, 8.04 ± 0.05, 8.33 ± 0.16, 8.58 ± 0.07 and 8.63 ± 0.06 h, respectively (Figure 2c). Meanwhile, the IT values at 140 °C for groups 14–19 were 4.94 ± 0.11, 4.35 ± 0.03, 4.12 ± 0.06, 4.28 ± 0.24, 4.41 ± 0.04 and 4.47 ± 0.08 h, respectively (Figure 2d). By contrast, the high-oleic sunflower oil sample containing the combination of TPP + AP (group 14) showed significantly higher oxidative stability than all the oil samples containing the combinations of three antioxidants at 130 °C and 140 °C (P < 0.05). As shown in Figure 2f, group 14 also showed the highest oxidation stability among groups 14–19 based on the POV at 55 °C (P < 0.05). Therefore, the optimum combination of selected antioxidants for high-oleic sunflower oil was TPP + AP.
3.3. Optimization of proportion of the mixed antioxidants
Based on the above results, the mixture proportions of TPP + AP were further optimized to improve the antioxidant effect. The IT values of high-oleic sunflower oil samples from group 14 and groups 20–27 were 9.70 ± 0.04, 8.91 ± 0.16, 10.83 ± 0.03, 8.41 ± 0.10, 11.04 ± 0.42, 8.04 ± 0.13, 11.18 ± 0.29, 9.53 ± 0.05 and 10.70 ± 0.05 h, respectively, at 130 °C (Figure 3a). While the corresponding values were 4.94 ± 0.11, 4.57 ± 0.16, 5.59 ± 0.16, 4.53 ± 0.23, 5.64 ± 0.40, 3.98 ± 0.15, 5.81 ± 0.32, 4.87 ± 0.30 and 5.34 ± 0.13 h, respectively, at 140 °C (Figure 3b). In contrast, the high-oleic sunflower oil samples containing 400 mg/kg TPP + 66.67 mg/kg AP (group 21), 450 mg/kg TPP + 50 mg/kg AP (group 23) and 480 mg/kg TPP + 40 mg/kg AP (group 25) had significantly longer IT values than others (P < 0.05). However, the POV for oil samples from group 14 and groups 20–27 increased by 1.67-, 1.65-, 1.34-, 1.82-, 1.36-, 1.92-, 1.33-, 1.63- and 1.57-fold, respectively, after 6 days of accelerated storage at 55 °C (Figure 3c). Hence, the high-oleic sunflower oil sample containing 480 mg/kg TPP + 40 mg/kg AP (group 25) showed the best antioxidant effectiveness among the nine groups mentioned above based on the POV assay (P < 0.05). As a result, the optimum composite antioxidant for high-oleic sunflower oil was the blend of 480 mg/kg TPP + 40 mg/kg AP.
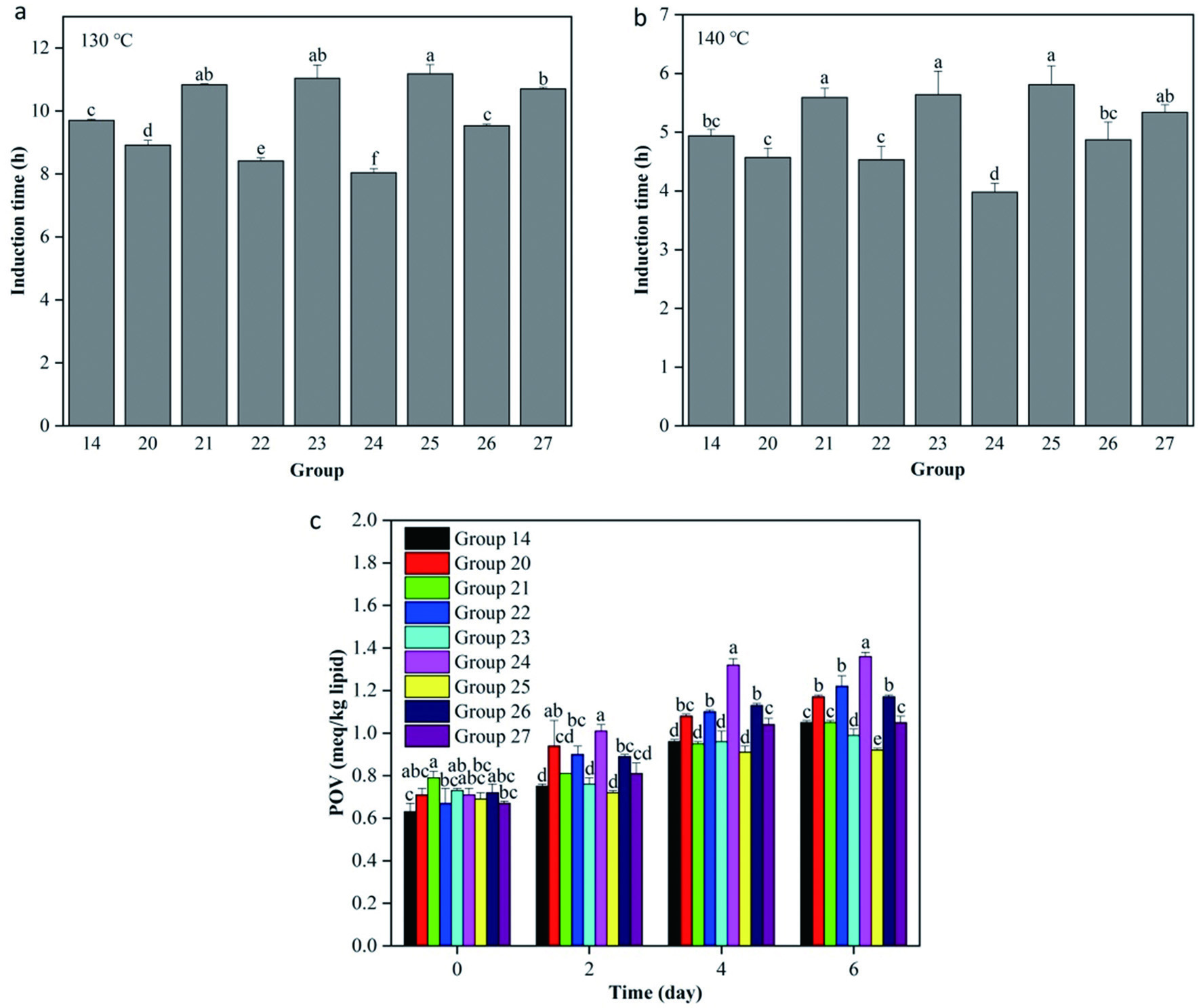 Click for large image | Figure 3. Antioxidant activities of the optimum antioxidants combination containing tea polyphenol palmitate and ascorbyl palmitate with different proportions in high-oleic sunflower oil. A, Rancimat induction time at 130 °C; B, Rancimat induction time at 140 °C; C, peroxide value (POV) at 55 °C. All experiments were repeated three times. Different letters (a-f) in each panel at same storage time indicate significant differences from each other (P < 0.05). |
3.4. Shelf life prediction
The IT values of the blank high-oleic sunflower oil sample and the oil sample containing the best antioxidant blend (containing 480 mg/kg TPP + 40 mg/kg AP) under different temperatures (110 °C, 120 °C, 130 °C and 140 °C, respectively) were consistent with the Q10 values, indicating that the reaction rate doubled for every 10 °C of temperature increased (Table 1). Based on the fitting results (Figure 4 and Table 1), it is not hard to see that the oil samples obeyed the linear relationship between the natural logarithm of the IT (Log IT) and the temperature (t) (R2 > 0.99). The shelf life was calculated by extrapolation of the linear relationship of Log IT and t for an expected temperature according to Rodríguez et al. (2020). The shelf lives for the two high-oleic sunflower oil samples mentioned above under 25 °C could be predicted to be 342.36 and 669.56 days, respectively. By contrast, the high-oleic sunflower oil containing the optimum composite antioxidants had 1.96-fold lengthier shelf life than that of the blank high-oleic sunflower oil under 25 °C.
 Click to view | Table 1. Shelf life (induction time at 25 °C, IT25) and Q10 (increase of reaction rate due to a 10 °C temperature rise) of high-oleic sunflower oil |
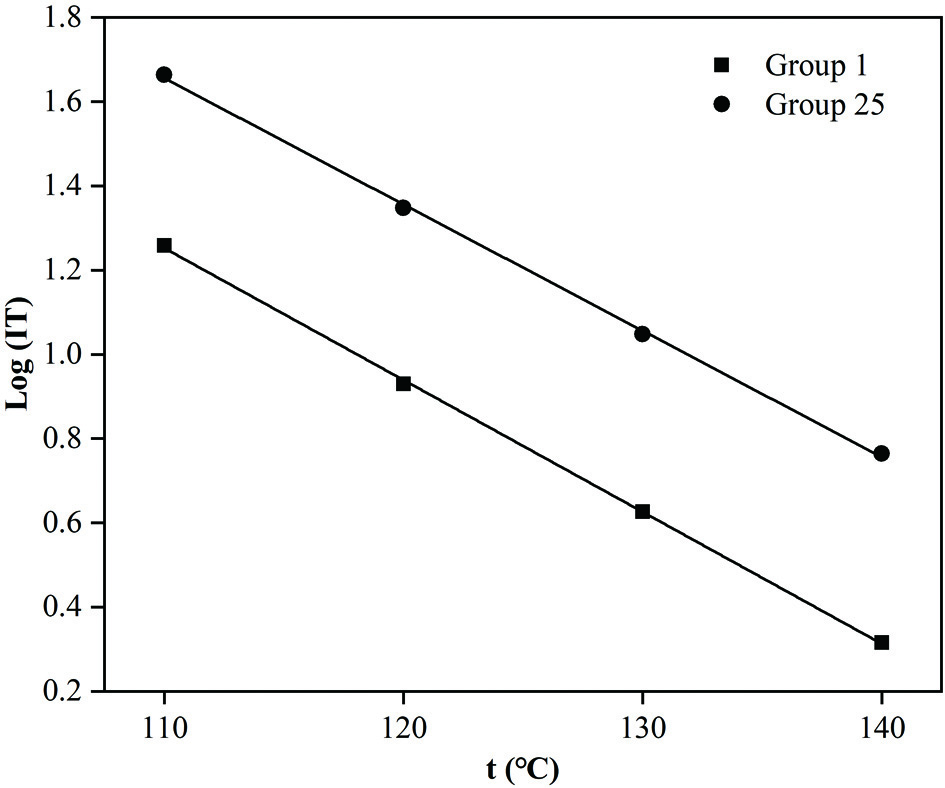 Click for large image | Figure 4. The linear relationship between the natural logarithm of the Rancimat induction time (IT) and the temperature (t). Group 1 was the control high-oleic sunflower oil without adding any antioxidants. Group 25 was the high-oleic sunflower oil sample containing 480 mg/kg tea polyphenol palmitate and 40 mg/kg ascorbyl palmitate. |
| 4. Discussion | ▴Top |
Our results indicated the fresh high-oleic sunflower oil used in this research had a high proportion of oleic acid (C18:1 n-9c, 77.62 ± 2.05 %), followed by linoleic acid (C18:2 n-6c, 9.21 ± 0.25 %) and palmitic acid (C16:0, 4.33 ± 0.14 %) (Table S2). Oleic acid was the most prevalent fatty acid in high-oleic sunflower oil, a result close to 80.2% reported by Guinda et al. (2003) and within the range of 77–85% reported by Belingheri et al. (2015). The high unsaturation degree of high-oleic sunflower oil makes it susceptible to oxidation under aerobic conditions, which usually leads to the decrease of sensory and nutritional quality, and will influence consumer acceptance (Villeneuve et al., 2021). Unsaturated fatty acids reacted with oxygen through a free-radical chain mechanism, which includes initiation, propagation and termination stages. In this process, lipid hydroperoxides (ROOH) are vital primary oxidation products of lipid oxidation (Miyashita, 2019). In this study, POV was selected as an indicator to monitor primary oxidation products in high-oleic sunflower oils.
The primary oxidation products are instable, and they decompose rapidly to give rise to secondary oxidation products, for instance, alkanes, alcohols, carbonyl compounds and so on when a source of energy (heat or light) and/or catalytic compounds such as transition metals exist (Miyashita, 2019). In this study, Rancimat method was applied to assess the oxidative stability of high-oleic sunflower oils by monitoring the production of secondary oxidation products. Rancimat method is generally applied industrially due to its reproducibility, simple, convenient determination without periodic analytical measurement and the use of organic solvents (Aktar & Adal, 2019). In this method, the oil sample is placed into a vessel, subjected to a constant high temperature while constant air is flowed through it and then the volatile oxidation compounds (mostly volatile acids) generated in the oxidative process are collected into ultrapure water, contributing to continuous rise in conductivity (Aktar & Adal, 2019). The IT is the time taken until there is a sudden rise in conductivity, which is determined by the intersection of the baseline with the tangent to the conductivity curve (Aktar & Adal, 2019).
For purpose of lengthening the shelf life of edible oils, use of antioxidants is an efficient, convenient and economical method (da Silva et al., 2021). Antioxidants can inhibit the generation of free radicals, donate H to free radical oxygen species or chelate metals to slow oxidation (Brewer, 2011). In this study, seven commonly used natural antioxidants (VE, PA, AOB, RE, TP, AP and TPP) were opted to protect high-oleic sunflower oil from oxidation. The IT, POV and free radical results all revealed that TPP showed the best antioxidant efficiency among all seven single antioxidants in inhibiting the oxidation of high-oleic sunflower oil. As a polyphenolic compound, TPP’s antioxidant mechanism is identical to other polyphenolic antioxidants. However, it has a better effect to inhibit lipid oxidation in oils due to its better liposolubility (Liu et al., 2014).
Therefore, TPP was selected to mix with one or two of the other six antioxidants (VE, PA, AOB, RE, TP and AP) to enhance the antioxidant effect further. The results of IT and POV both revealed that the antioxidant blend of TPP + AP had more excellent antioxidant capacity than that of the other binary composite antioxidants and all the single antioxidants. Wang et al. (2020) found that the binary composite antioxidants formed by RE and TPP exerted better antioxidant activity than that of RE or TPP in sunflower oil. Chu and Hsu (1999) also found that the mixture of catechin and RE indicated stronger antioxidant effectiveness than the corresponding individual antioxidant effects in peanut oil. TPP can exert antioxidant activity by scavenging free radicals, and AP can prevent lipid oxidation by quenching reactive oxygen species (Brewer, 2011; Let et al., 2007). Therefore, the two antioxidants (TPP + AP) with different antioxidant mechanisms probably provide a synergistic antioxidant effect in high-oleic sunflower oil.
The results of IT and POV also indicated that the mixture of TPP + AP exerted higher antioxidant capacity compared with the antioxidant combinations of three components containing TPP, AP and one of the other five antioxidants (VE, PA, AOB, RE and TP). Thus, for antioxidant mixtures, the greater variety of antioxidants in the combination do not always come in the better antioxidant effectiveness. For instance, Lu et al. (2021) found that the antioxidant ability of the mixture of myristyl gallate and TPP is better than the mixture of myristyl gallate, TPP and AOB in flaxseed oil. Likewise, Shen et al. (2020) revealed that the combination of VE + PA + AP has higher antioxidant activity compared with the combination of VE + PA + AP + AOB in DHA algae oil.
Furthermore, our study also demonstrated that the mixture proportion of TPP and AP made a significant difference to the antioxidant effectiveness in high-oleic sunflower oil. Many previous reports also proved that the mixture proportions of the composite antioxidants had a significant influence on the antioxidant effectiveness in oils. Sarkar et al. (2015) revealed that when the proportion of BHA to BHT was not same, the antioxidant effectiveness was not same in soybean oil. Similarly, Lu et al. (2020) reported that when four-component composite antioxidants formed by tocopherol, AP, PA and TPP had different proportions, their antioxidant effects were also distinct in flaxseed oil.
Rancimat method is applied for the shelf life prediction of food as a rapid automated method (Presa-Owens et al., 1995). In this study, we predicted the shelf life of high-oleic sunflower oils with or without antioxidants using Rancimat method. Our results (Table S3) revealed that the Q10 values of high-oleic sunflower oil (1.92–2.13) were consistent with other vegetable oils such as soybean oil (1.99–2.09), the blends of chia oil and sesame oil (1.96–2.12) and olive oil (2.04–2.48) (Farhoosh, 2007; Farhoosh & Hoseini-Yazdi, 2014; Rodríguez et al., 2020). All these Q10 values around 2.00 indicate that the reaction rate doubles for every 10 °C of temperature increase. Our results also showed that the high-oleic sunflower oil including the optimum composite antioxidant (480 mg/kg TPP and 40 mg/kg AP) had 1.96-fold lengthier shelf life than that of control high-oleic sunflower oil. Though such predictions might lead to some uncertainties, Presa-Owens et al. (1995) reported that the shelf life prediction of an infant formula based on such method provided reasonably results.
| 5. Conclusion | ▴Top |
In conclusion, TPP showed the best antioxidant efficiency amongst all the seven antioxidants used in inhibiting the oxidation of high-oleic sunflower oil. Then, the optimum antioxidant combination of TPP + AP exerted the strongest effect in increasing the stability of high-oleic sunflower oil amongst the composite antioxidants including TPP and one or two of the other six antioxidants (VE, PA, AOB, RE, TP and AP). By optimizing the mixture proportions, the combination of 480 mg/kg TPP and 40 mg/kg AP revealed the strongest stability in high-oleic sunflower oil, which could prolong the shelf life by 1.96-fold compared with the blank sample.
| Supplementary material | ▴Top |
Table S1. Adding methods of antioxidants for different groups (mg/kg).
Table S2. Fatty acid compositions (%) of the high-oleic sunflower oil.
Table S3. Rancimat induction times (ITs) at 130 °C and 140 °C of high-oleic sunflower oil samples containing different antioxidants and antioxidant composites.
Figure S1. ESR spectra (12 h post high temperature induction). Group 1 was the control high-oleic sunflower oil without adding any antioxidants. Groups 2–8 were the high-oleic sunflower oil samples containing vitamin E, phytic acid, antioxidant of bamboo leaves, rosemary extract, tea polyphenols, ascorbyl palmitate and tea polyphenol palmitate, respectively.
Acknowledgments
This work was financially supported by “The National Natural Science Foundation of China (32001654)”, “National Key R&D Program of China (2018YFC1406806; 2018YFC1406805)”, “Dalian Science and Technology Innovation Fund Project (2019J11CY005)” and “Central Funds Guiding the Local Science and Technology Development (2020JH6/10500002)”.
Conflict of interest
The authors declare that they have no conflict of interest.
| References | ▴Top |