| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 18, June 2022, pages 85-89
Hesperidin acts as a novel CaMKII-δ inhibitor to ameliorate cardiac ischemia/reperfusion injury
Wei Zhao, Hui Zhao*
Tianjin Key Laboratory of Food and Biotechnology, Tianjin International Joint Center of Food Science and Engineering, State Experimental and Training Centre of Food and Drug, School of Biotechnology and Food Science, Tianjin University of Commerce, No. 409 Guangrong Road, Beichen, Tianjin 300134, China
*Corresponding author: Hui Zhao, Tianjin Key Laboratory of Food and Biotechnology, Tianjin International Joint Center of Food Science and Engineering, State Experimental and Training Centre of Food and Drug, School of Biotechnology and Food Science, Tianjin University of Commerce, No. 409 Guangrong Road, Beichen, Tianjin 300134, China. E-mail: zhaohui@tjcu.edu.cn
DOI: 10.31665/JFB.2022.18311
Received: June 10, 2022
Revised received & accepted: June 29, 2022
| Abstract | ▴Top |
Hesperidin, a flavanone glucoside, is rich in citrus fruits, especially in citrus peels. Experimental and clinical data has demonstrated that hesperidin is a good candidate in cardiovascular diseases due to its lipid-lowing, antioxidative, and anti-inflammatory properties. A recent report has revealed a new mechanism of hesperidin on myocardial ischemic/reperfusion injury through targeting Ca2+/calmodulin-dependent kinase II δ (CaMKII-δ) kinase. Herein, we highlight the finding and summarize the recently published clinical trials of hesperidin with regard to cardiovascular diseases. Akin to hesperidin, polymethoxylated flavones and flavanone – naringin are also very rich and found in some citrus peels. Therefore, clinical data are needed to address the perspectives of citrus peels in preventing cardiovascular disease.
Keywords: Hesperidin; Cardiovascular diseases; Ischemia/reperfusion injury; Citrus peel; Flavonones
| 1. Ischemia/reperfusion injury (I/R) contributes to adverse cardiovascular outcomes | ▴Top |
According to the data from WHO, cardiovascular diseases, mainly referring to two conditions including heart and blood vessel diseases, are the leading cause of death with an estimated 17.9 million lives each year in the global (2022). Causes of cardiovascular diseases include congenital defects, atherosclerosis, decreased heart blood flow, infection, hypertension, or insulin resistance. Coronary artery disease induced by atherosclerosis is the most common cause of cardiovascular ailments. In addition to diet modification and exercise, treatment of progressive or serious coronary artery disease may include medication, stenting or ablation, and even surgery.
Effective blood flow is vital to cardiovascular homeostasis. Ischemic diseases, i.e. myocardial infarction and cerebral ischemic stroke, are becoming the leading causes of death worldwide. Primarily, distressed or even lack of blood flow leads to an imbalance between the supply and demand of oxygen, which subsequently initiates and exacerbates damage or dysfunction in the area dominated by vessels (Yellon and Hausenloy, 2007). To prevent further damage, interventions for prompt restoration of blood flow in injury area are usually considered as the first-line solution (Heusch and Gersh, 2017). Currently, thrombolysis and percutaneous transluminal coronary angioplasty have been identified as the most effective strategy for rescuing infarcted myocardium and improving the outcome in patients with acute myocardial infarction (Guan et al., 2021).
Although recovery of blood flow is necessary to reverse injury, studies in both animal models and human patients with acute infarction clearly suggest that reperfusion of ischemic vessels account for up to 50% of the infarcted zone (Fernandez Rico et al., 2022). This pathogenesis was therefore termed ischemia/reperfusion injury (I/R) which contributes to adverse cardio- or cerebrovascular outcomes. Accordingly, the underlying molecular mechanism of myocardial I/R injury is key to finding strategies for reducing the affected infarct area. Indeed, increasing therapeutic strategies are translated to bedside from the bench.
Here, we discuss an interesting finding that a citrus flavanone glucoside, hesperidin, acts as a novel CaMKII-δ inhibitor to ameliorate cardiac ischemia/reperfusion injury (Zhang et al., 2022). Progressively, we summarize clinical trials published in recent years with regard to hesperidin and propose perspectives of citrus peels in preventing/ameliorating cardiovascular diseases.
| 2. Identification of myocardic CaMKII-δ9 as the target of hesperidin | ▴Top |
Ca2+/calmodulin-dependent kinase II (CaMKII) belongs to the serine/threonine protein kinase family. As the most abundant CaMKII-δ splice variant, CaMKII-δ9 is mainly located in the human heart acting as a crucial mediator of DNA damage and death of cardiomyocyte (Zhang et al., 2019). Mechanistically, CaMKII-δ9 directly interacted with IκBα (NF-κB inhibitor α) to prompt IκBα phosphorylation and activation of I/R-induced cardiac NF-κB signaling pathway (Yao et al., 2022).
To find the therapeutic target for CaMKII-δ9 in the heart, a small-molecule kinase inhibitor library combined with a high-throughput screening system was employed to search for CaMKII-δ kinase inhibitors. Interestingly and unexpectedly, hesperidin, a flavonoid mainly found in citrus peel, was discovered to be a potential CaMKII-δ inhibitor. Furthermore, by using in vitro cultured cardiomyocytes and in vivo rodent models, the protection of hesperadin against I/R injury was discovered. Mechanistic study revealed that hesperidin directly binds to CaMKII-δ and specifically blunts its activation by competition with adenosine triphosphate. Furthermore, both in vivo and in vitro experiments suggest that CaMKII-δ9 is not required for hesperidin inhibition of tumor cells (Figure 1), despite that hesperidin was previously reported to have cellular toxicity to tumor cells as an inhibitor of Aurora B kinase (Hauf et al., 2003, Pollard and Mortimore, 2009).
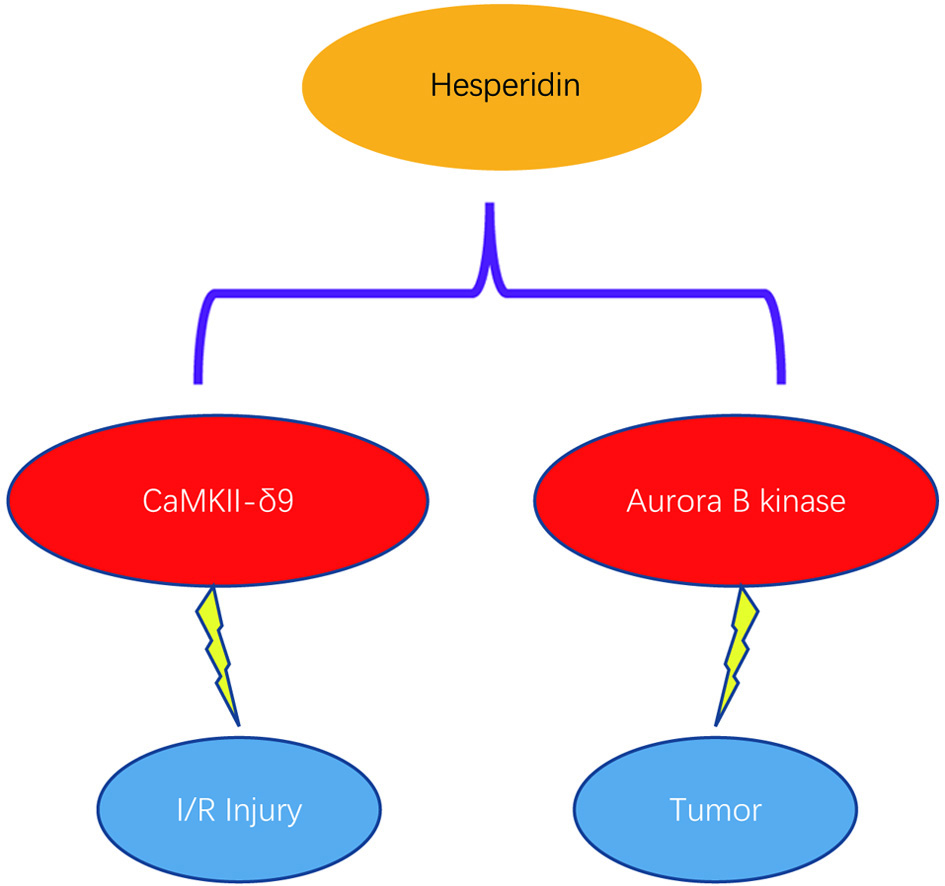 Click for large image | Figure 1. Independent effects of hesperidin in targeting I/R injury via CaMKII-δ9 and tumors via Aurora kinase. |
| 3. Source of hesperidin | ▴Top |
Citrus peel (CP) accounts for around 40–50% of the fresh fruit mass (Singh et al., 2020). It is considered a waste in the citrus juice industry except that tangerine peels have been used as a traditional medicine in China for thousands of years. However, compared with other edible parts of the citrus fruits, research has demonstrated that CP is a substantial source of naturally occurring phenolic compounds and carotenoids with health promoting effects (Wang et al., 2014, Wang et al., 2018). Particularly, flavonoids such as polymethoxylated flavones (notably nobiletin and tangeretin) and flavanones (generally hesperidin and naringin) are richly and almost exclusively found in CP. It has been reported that the more aged CP had more polymethoxylated flavones (Guo et al., 2017). Accordingly, biological activities of the more aged CP including anti-oxidative stress and protection against the risk of many chronic diseases is also higher than other edible fruit parts owing to the more abundance of phenolic compounds in CP (Wang et al., 2018, Li et al., 2014).
Since it was first isolated in 1828 by Lebreton from the spongy inner portion of orange peel, hesperidin has been broadly identified in various citrus fruits (Manthey and Grohmann, 1998). Peterson et al. (2006) demonstrated that the concentration of hesperidin is high in the fresh fruit of Citrus sinensis (15.25 ± 8.21 mg/100g) and Citrus reticulate (19.26 ± 11.56 mg/100g). According to the analyses of phytochemicals in ‘Gold Lotion’, formulated by an extract of multiple varieties of citrus peels (Lai et al., 2013, Guang et al., 2020) (Table 1), the content of total flavanones is over 3.5 times that of polymethoxylated flavones (358.3 ppm w/w versus. 100.5 ppm, w/w). Of the verified flavanones, structural analogue naringin (253.6 ppm, w/w) is around 2.5 times that of hesperidin content (104.7 ppm, w/w).
 Click to view | Table 1. Flavanones of Gold Lotion (GL) formulated by an extract of multiple varieties of citrus peels (Lai et al., 2013) |
| 4. The health potential of hesperetin in cardiovascular disease | ▴Top |
Notably, hesperidin was frequently used for ischemic cardiovascular conditions such as high blood pressure (Morand et al., 2011, Lu et al., 2022) and atherosclerosis (Salden et al., 2016) through multiple mechanisms. These include upregulation of endothelial NO-synthase activity (Rizza et al., 2011) and Ca2+ sensitization of vascular smooth muscle contraction (Lu et al., 2022). Therefore, it has intrinsic potential to protectively affect ejection fraction (13). Akin to hesperidin, naringin and polymethoxylated flavones are also beneficial to lower the risk of cardiovascular diseases (Mahmoud et al., 2019, Haidari et al., 2015).
Although research suggests that the bioavailibity of hesperidin and its in vivo metabolite hesperitin have low aqueous solubility, poor absorption and rapid elimination, half-life of hesperidin is around 6 hours which represents a reasonable availability due to the prolonged absorption phase by a long Tmax (Li and Schluesener, 2017).
After preclinical studies indicated that hesperidin had evident effects on cardiovascular diseases, accumulating clinical trials involving its oral safety profile, preliminary and further well-designed randomized controlled clinical trials have supported hesperidin as a cardioprotective agent. There are at least 28 clinical trials registered in clinicaltrials.gov (2022). Table 2 summarizes the recently published clinical trials with regard to hesperidin. Of these clinical studies, hesperidin is obviously a good dietary supplement candidate for prevention oft cardiovascular disease, diabetes and hypertension.
 Click to view | Table 2. A summary of published clinical trials with regard to hesperidin |
| 5. Perspectives | ▴Top |
The findings of Zhang et al. (2022) suggest that hesperadin is a promising compound for the joint treatment of cardiovascular diseases and cancer. However, toward better clinical translation, there are at least two questions that need to be urgently investigated in future: (1) Given similarity of structures and abundance in citrus peel, whether the cardiovascular I/R injury protection and mechanism of hesperitin are also available in flavanone glucoside naringin and particularly polymethoxylated flavones which have been considered to be more potent than hesperidin. Furthermore, it is expected to explore clinical trial with citrus peels on the ischemic cardiovascular diseases; and (2) Citrus polymethoxylated flavones as well as hesperidin and naringin may affect blood clotting and platelet activation which also contribute to cardiovascular protection. However, there is no observation regarding coagulation in rodents after long-term consumption of hesperidin (Zhang et al., 2022). Given that anticoagulant/antiplatelet medications usually monitored in clinical study, the safety profile of hesperidin supplements in individuals needs to be established.
In summary, flavanone glucosides including hesperidin are abundant in citrus fruits. It will undoubtedly accelerate the translation utilization of orange peel accompanied by emerging development of nutraceuticals in citrus fruits and peels.
| References | ▴Top |