| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 17, March 2022, pages 27-33
Transport mechanism of eurycomanone from Eurycoma longifolia Jack across Caco-2 cells model
Wu-Yan Guoa, #, Huan Zhanga, #, Yue-Yang Zhangb, #, Shu-Yan Wanga, Jian Rena, Yong-Qing Jiac, Rui Liud, *, Bo Zhanga, *
aTianjin Key Laboratory of Early Druggability Evaluation of Innovative Drugs, Tianjin International Joint Academy of Biomedicine, Tianjin 300457, China
bAndrology Department, Wangjing Hospital, China Academy of Chinese Medical Science, Beijing 100102, China
cOreentak (Macau) Biotechnologies Limited Oreentak (Macau) Biotecnologia, S.A. Alameda Dr. Carlos DAssumcau, No. 411-417, Dynasty Plaza, 4m, Macau, China
dState Key Laboratory of Component-based Chinese Medicine, College of Pharmaceutical Engineering of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine. Tianjin 301617, China
#These authors contributed equally to this work.
*Corresponding author: Rui Liu, State Key Laboratory of Component-based Chinese Medicine, College of Pharmaceutical Engineering of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, 10 Poyang Lake Road, West Zone of Tuanbo New City, Jing hai District, Tianjin 301617, China., E-mail: liurui@tjutcm.edu.cn; Bo Zhang, TianJin International Joint Academy of Biomedicine, No. 220 Dongting Road, the Tianjin Economic-Technological Development Area (TEDA), Tianjin 300457, China.+86 022 65378890;, 86-22-65378036; E-mail: zhangbo@tjab.org
DOI: 10.31665/JFB.2022.17301
Received: February 24, 2022
Revised received & accepted: March 26, 2022
| Abstract | ▴Top |
Eurycomanone is the main active ingredient of Eurycoma longifolia Jack with anti-cancer, anti-malaria, improving male sexual dysfunction and other effects. The poor lipid solubility of eurycomanone is a potential reason for its low bioavailability. However, the transmembrane absorption mechanism has not been reported. In this study, the Caco-2 cell monolayer model was used to investigate the influence of different factors on the eurycomanone transmembrane absorption, including time, concentration, temperature, P-glycoprotein inhibitors (verapamil or cyclosporin A) and collateral transport enhancer (sodium desoxycholate or EDTA). The results showed that the apparent permeability coefficient (Papp) of eurycomanone was lower than 10−6 cm/s with poor oral absorption. The Papp was not dependent on concentrations or temperatures. The addition of paracellular transport enhancer promoted the absorption of eurycomanone (P < 0.05), whereas p-glycoprotein inhibitors had no effect. Taken together, this study indicated that absorption of eurycomanone may undergo passive transmembrane transport and paracellular transport.
Keywords: Eurycoma longifolia Jack; Transmembrane transport; Eurycomanone; Caco-2 cells; Membrane absorption
| 1. Introduction | ▴Top |
Eurycoma longifolia Jack has been a well-known herb in Southeast Asia and received considerable attention in recent years due to the nutritional and health protective effects, including aphrodisiac effect, the treatment of malaria and persistent fever and cytotoxic activity towards cancer cells (Hussein et al., 2007). Eurycomanone is the main active ingredient of Eurycoma longifolia Jack with anti-cancer, anti-malaria, improving male sexual dysfunction and other effects (Kuo et al., 2004).
The oral administration is the most common and accessible manner during nutrients absorption and drug delivery, because it provides many advantages, such as being painless, easily self-administered and so on (Dou et al., 2019). A validated HPLC analysis of eurycomanone, in rat plasma following oral and intravenous administration of Eurycoma longifolia Jack extract was developed for pharmacokinetic and bioavailability studies (Low et al., 2005). Studies from a rat model suggested that less lipid solubility of eurycomanone leads to poor absorption, fast metabolic rate, and low oral bioavailability (Low et al., 2011). In addition, intestinal instability, P-gp-mediated substrate efflux and presystemic metabolism were involved in low oral bioavailability of eurycomanone (Ma et al., 2017). Exploring transport characteristics and mechanisms of eurycomanone through intestinal epithelial cells may help us gain a deeper understanding of the eurycomanone and improve its oral bioavailability.
Generally, the Caco-2 cell monolayer (Caco-2 Cell Monolayer, CCM) was used as an in vitro absorption model (Kansy et al., 2000). Because the Caco-2 cell monolayer permeability is associated with absorption in vivo, over the past few years, CCM has been used as models of drug permeability screening trials to predict drug intestinal permeability and human oral doses. Previous studies demonstrated that CCM is in widespread use for investigating the absorption mechanisms of the drug, including passive transport and paracellular transport, vector-mediated resorption, and efflux mechanisms (Kansy et al., 2000; Yamashita et al., 2000). These experimental theories and literature will provide good direction for us to explore the transport mechanism. The permeability of eurycomanone was investigated by using the parallel artificial membrane permeability assay system and Caco-2 cultured cells. The result suggests that eurycomanone is not readily absorbed across biological membranes. The stability of eurycomanone in Caco-2 cell model was good. The report also confirmed that eurycomanone was a very polar compound with high stability at different pHs, in the plasma and in liver microsome. No major degradation of the compound in the gastrointestinal tract and blood plasma was noted and eurycomanone also exhibited low plasma protein binding ability (Ahmad et al., 2018).
In our previous studies, we successfully prepared the quassinoids-phospholipid complex and in vivo study had shown that the quassinoids-phospholipid significantly improved the oral absorption and bioavailability of eurycomanone and overcame the limited absorption of eurycomanone (Guo et al., 2021). However, there is still a lack of a better understanding of eurycomanone absorption. In this study, we used the Caco-2 cell monolayer model to investigate the transmembrane transport mechanism of eurycomanone by examining the effects of different factors on cells uptake and transport.
| 2. Materials and methods | ▴Top |
2.1. Materials
Eurycomanone (mass fraction >95%; Chengdu alpha Biological Technology Co. Ltd. Chengdu, China); DMEM medium (Thermo Scientific .Carlsbad, CA, USA); fetal bovine serum (Excell Bio, Beijing, China); green-streptomycin (100×, Beijing Solarbio Science & Technology Co., Ltd. Beijing, China); nonessential amino acids (Thermo Scientific .Carlsbad, CA, USA) 0.25% trypsin–0.02% EDTA digestion solution (laboratory homemade); Hank’s Buffer (Hank’s balanced salt solution), (Thermo Scientific .Carlsbad, CA, USA); MTT Reagent (Sigma Aldrich, Merck KGaA, Darmstadt, Germany); Verapamil hydrochloride (Tianjin heowns Biological Technology Co. Ltd. Tianjin, China); Cyclosporin A (Tianjin heowns Biological Technology Co. Ltd. Tianjin, China); Sodium deoxycholate (J&K Scientific Co., Ltd. Beijing, China); EDTA (Tianjin heowns Biological Technology Co. Ltd. Tianjin, China); Transwell cell culture plate (Corning Incorporated, New York, USA); Caco-2 cell line (Jiangsu Keygen Biotech Co., Ltd. Nanjing, China).
2.2. Cell culture
Caco-2 cells were cultured in complete medium (DMEM medium supplemented with 10% (v/v) fetal bovine serum (FBS), 1% nonessential amino acids, 1% penicillin-streptomycin solution (100×)) and incubated in a humidified 5% CO2 incubator at 37 °C. The cells were maintained routinely on 100 mm cell culture dishes and the culture medium was replaced every other day. The cells were split with 0.25% trypsin-EDTA when reaching 80–90% confluence and then transferred on the 96-well plate for cell viability determination as well as for uptake and transport experiments.
For cell uptake experiments, Caco-2 cells, in logarithmic growth phase, were suspended in cell culture medium at 6∼8 × 104 cell/mL. The cell suspension was added to a 6-well plate (2 mL per well) with a gentle shake. The medium was replaced every two days thereafter. Cell monolayers were used for experimentation at 10–14 days after seeding.
For transport experiments, Caco-2 cells suspension was seeded into 12-well polycarbonate Transwell plates at a density of 6∼8 × 104 cells/mL (0.5 mL) in the upper chamber (apical side, AP), and 1.5 mL of fresh complete medium was added into the lower chamber (basolateral side, BL). Medium was changed every 2 days in the initial one week, and subsequently changed once a day. About 3 weeks after culture, monolayer morphology existing on the polycarbonate membrane could be used for transport experiments.
2.3. Cell viability
Caco-2 cells in the 96-well plate were seeded at a density of 5 × 104 cells/well and incubated at 37 °C for 24 h. Before MTT test, eurycomanone with indicated concentrations was added to the cultured cells and incubated for 24 h and then the medium was discarded. After that, 100 μL of MTT solution (1.0 mg/mL) was added and incubated for 4 h in cell culture incubator, then the medium was replaced with 100 μL of DMSO (dimethyl sulfoxide). The absorbance at 562 nm was measured with Multiskan™ FC microplate reader (Thermo Fisher Scientific, USA). The cell viability was calculated as the following Equation 1:
2.4. Verification of Caco-2 cells monolayer model
2.4.1. Cell morphology
The Trans-endothelial electrical resistance (TEER) detection was used to evaluate the monolayer modelas reported previously (Hidalgo, 2001). Briefly, after incubation, the Caco-2 cells were washed with prewarmed PBS twice, then, the electrodes soaked in PBS overnight were inserted into AP side and BL side of transwell chamber respectively, next, the TEER value was detected at days 3, 5, 7, 14, 21. The monolayer was not used until the TEER was above 400 Ω·cm2.
Additionally, mannitol transmittance detection was used to observe the membrane integrity and transport function of the Caco-2 cells monolayer model. Briefly, 100 μg/mL of sodium fluorescein was added to AP side, 100 μL aliquots were taken from the BL side at 0.5, 1.0, 1.5, 2.0, 3.0 h. Then, 20 μL sample of the clear supernatant was injected onto a high-performance liquid chromatography (HPLC) system. Generally, when the transmittance is ≤0.5 %/h/cm2, the integrity of cell monolayer is considered to be good (Bravo et al., 2004).
2.5. Uptake studies
Uptake studies were done based on previously reported literature (Iwazaki et al., 2016). Briefly, Caco-2 cells were seeded at a density of 1 × 105 cells/well on 6-well plates (Costar Corning, NY, USA). Before uptake studies, the culture medium was removed. The Caco-2 cell monolayers were rinsed twice with pre-warmed (37 °C) PBS buffer and then preincubated with HBSS buffer for 30 min at 37 °C. The monolayers were subsequently incubated with HBSS buffer contained eurycomanone at 4 °C or 37 °C, 25, 50, 100, 200 μg/mL of eurycomanone were added to investigate the influence of various concentrations on uptake. Furthermore, verapamil hydrochloride, cyclosporin A, sodium deoxycholate and EDTA were added to investigate the effects of uptake inhibitors or enhancers on eurycomanone uptake. To evaluate the effects of time on eurycomanone uptake, times of incubation were as follows: 10, 30, 60, 120 min. Then the culture medium was discarded and the monolayers were rinsed three times with ice-cold HBSS buffer (4 °C). Then after, 0.15 mL of cell lysis buffer was added to each well. Each 100 μL sample was mixed with 5 μL perchloric acid to precipitate the protein. And the suspension was centrifuged at 9,000 g for 10 min. Then, the supernatant was collected and the absorbed concentration of eurycomanone was evaluated using HPLC analysis. The protein contents of samples were determined by Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). The eurycomanone uptake of the cells was normalized to protein content and expressed as µg/mg protein.
2.6. Transport studies
Transport studies were performed as reported in literature (Iwazaki et al., 2016). The Caco-2 cells monolayer Transwell chamber models were successfully cultured for 21 days, which were washed three times with the preheated HBSS before each experiment and then left at 37 °C in a CO2 incubator for 30 min to reach equilibrium. Immediately after preincubation, HBSS buffer was removed and the HBSS contained test compounds were added to the apical side (0.5 mL) or the basolateral side (1.5 mL) while HBSS buffer was then added to the opposite side. We then investigated the effects of different conditions on the transport characteristics of eurycomanone based on the methods listed in Table 1. Then, 100 μL of the samples were taken from the receiver chamber, and the same volume of fresh HBSS buffer was added. The samples were determined by HPLC as described above. All drugs were freshly prepared prior to the experiment. All trials were performed in triplicate.
 Click to view | Table 1. Effects of some factors on the transport of samples |
The transport characteristics of eurycomanone in different conditions is represented by the apparent permeability coefficient (Papp) and efflux ratio (ER), and the calculation method is as the following Equations 2 and 3:
During the experiments, the integrity of the cell monolayers was assessed by measuring the TEER value and mannitol transmittance. When the TEER value reached 400 Ω·cm2 and the mannitol permeability was ≤0.5%·h−1·cm−2, the cell monolayer membrane was considered to be integrated.
2.7. Statistical analysis
All data in the graphs were presented as the mean value ± standard deviation from three independent measurements. Statistical significance was performed using one-way ANOVA followed by Dunnett’s multiple comparisons test with Graph Pad Prism 6.01 software (Graph Pad Software, Los Angeles, CA, USA). The statistical significance was assigned at P < 0.05.
| 3. Results and discussion | ▴Top |
3.1. Caco-2 cell viability
Great Caco-2 cell viability is necessary for uptake and transport studies. The MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, Thiazolyl Blue Tetrazolium Bromide) method was applied for the Caco-2 cell viability. As shown in Figure 1, there was little impact on Caco-2 cell viability when we incubated Caco-2 cells for 24 hours with concentrations of eurycomanone range from 1 to 400 μg/mL. The cell viabilities of eurycomanone at different concentrations were all higher than 80%, and there was no significant difference when compared with the control group. Thus, we performed following uptake and transport studies within this concentration range of eurycomanone.
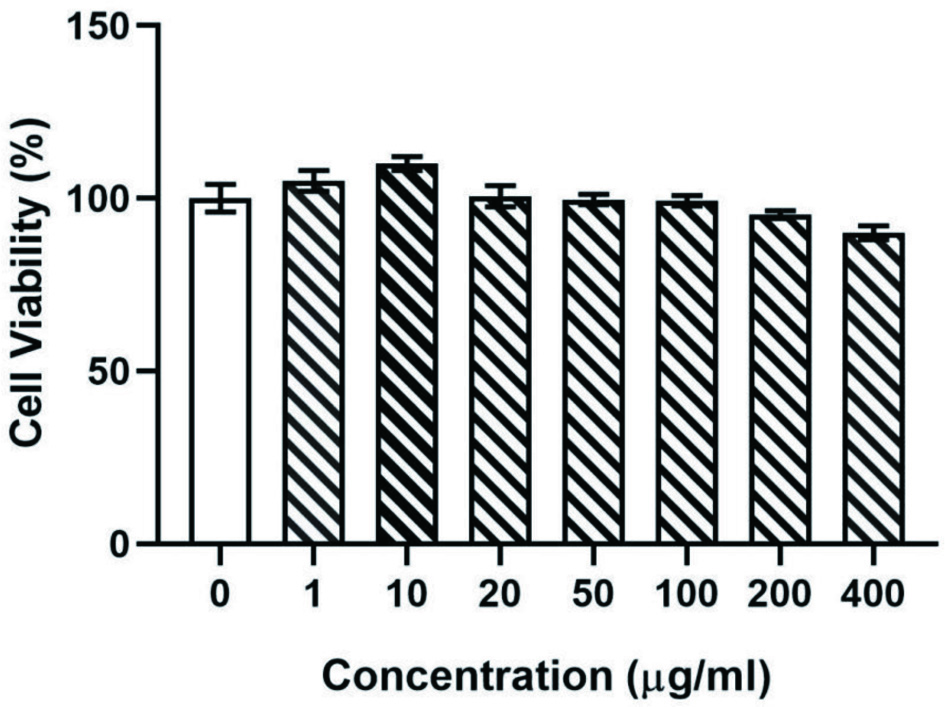 Click for large image | Figure 1. The survival rate of Caco-2 cells after incubation for 24 h at different concentrations of eurycomanone (μg/mL). The results showed that addition of eurycomanone had no significant impact on cell viabilities compared with the control group. |
3.2. Evaluation of the Caco-2 Cell Transport Model (Single Layer Integrity)
3.2.1. TEER detection
The integrity of the Caco-2 cell monolayers was evaluated by measuring the TEER, which was used for the transport study. As shown in Figure 2, the resistance value grew slowly in the first week of the cell growth, because the cell was in the logarithmic growth period in the first week, and the cell had not fully covered the Transwell polycarbonate membrane. In the second week, the resistance values fused rapidly and reduced the gap between cells. On day 14, the transmembrane resistance value reached 500 Ω·cm2, after which the resistance value became flatter, Caco-2 cell could slowly differentiate to form a cell monolayer that possesses many functions of the small intestinal villus epithelium. On day 21 the transmembrane resistance value was >500 Ω·cm2, indicating that Caco-2 cells had formed a complete and dense monolayer.
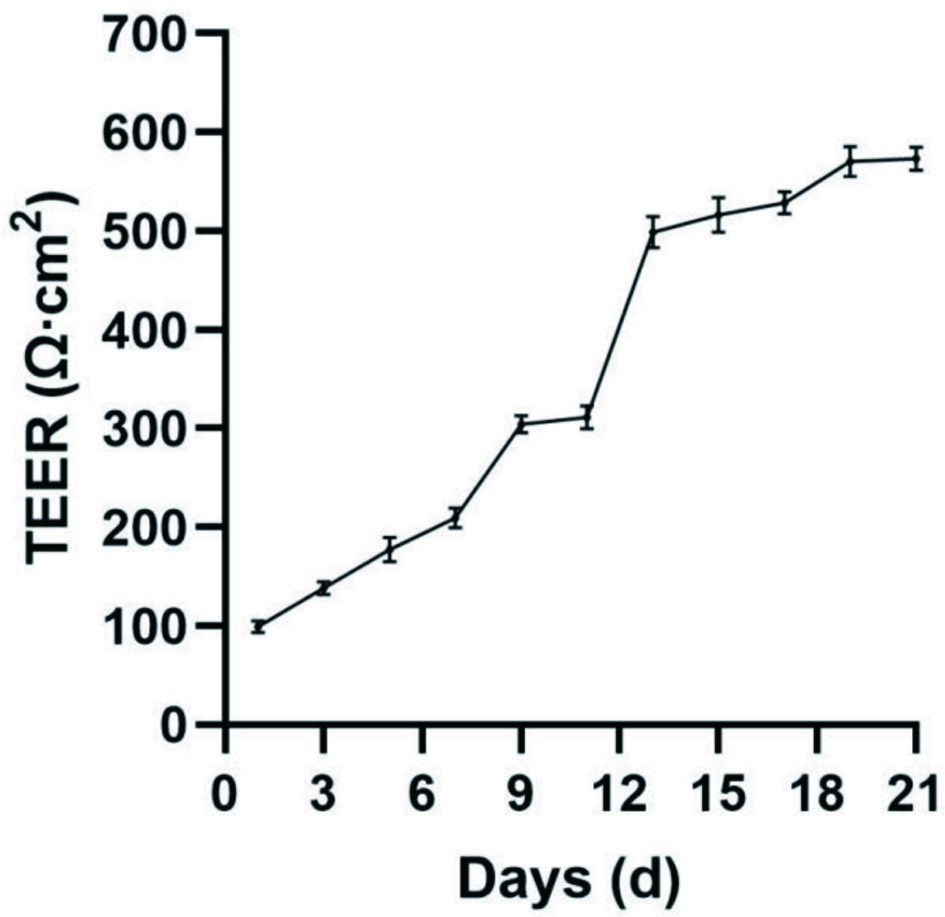 Click for large image | Figure 2. Establishment of a 21-days Caco-2 cells monolayer model by real-time monitoring of change by TEER value. All experiments were performed three times. |
3.2.2. Mannitol transmittance detection
In order to obtain the monolayer cells, we also performed mannitol transmittance detection in Caco-2 cells. We found that the value of mannitol transmittance (0.5% /h/cm2) was not significantly different among sites between any time point (Table 2), consistent with the requirements of monolayer cells.
 Click to view | Table 2. Mannitol transmittance of Caco-2 cells at different time points (x ± s, n = 3) |
In summary, through transmembrane resistance detection and mannitol transmittance detection, the cultured Caco-2 cell single layer met the integrity requirements and could be used for subsequent experiments.
3.3. Effect of differnt factors on the uptake of eurycomanone in Caco-2 cells monolayer
As shown in Figure 3a, the uptake of eurycomanone increased linearly within 60 min (R = 0.9889). As a result, 60 min’ time was chosen as optimal. Thus the time of 60 minute ingestion was taken as the uptake time for subsequent experiments to ensure a maximum absorption. The results demonstrated that the uptake of eurycomanone by the cells increased with increasing concentration of eurycomanone (25∼200 μg/mL), exhibiting a linear dependence at 60 min (R2 = 0.9884). These data indicated that cellular eurycomanon uptake mainly proceeds through passive diffusion. Furthermore, the adding of two P-glycoprotein inhibitors (Verapamil and Cyp-A) (Bohets et al., 2001) did not attenuated the uptake of eurycomanone (P > 0.05) (Figure 3c), and indicated that eurycomanone was not a substrate for P-glycoprotein mediated efflux transport.
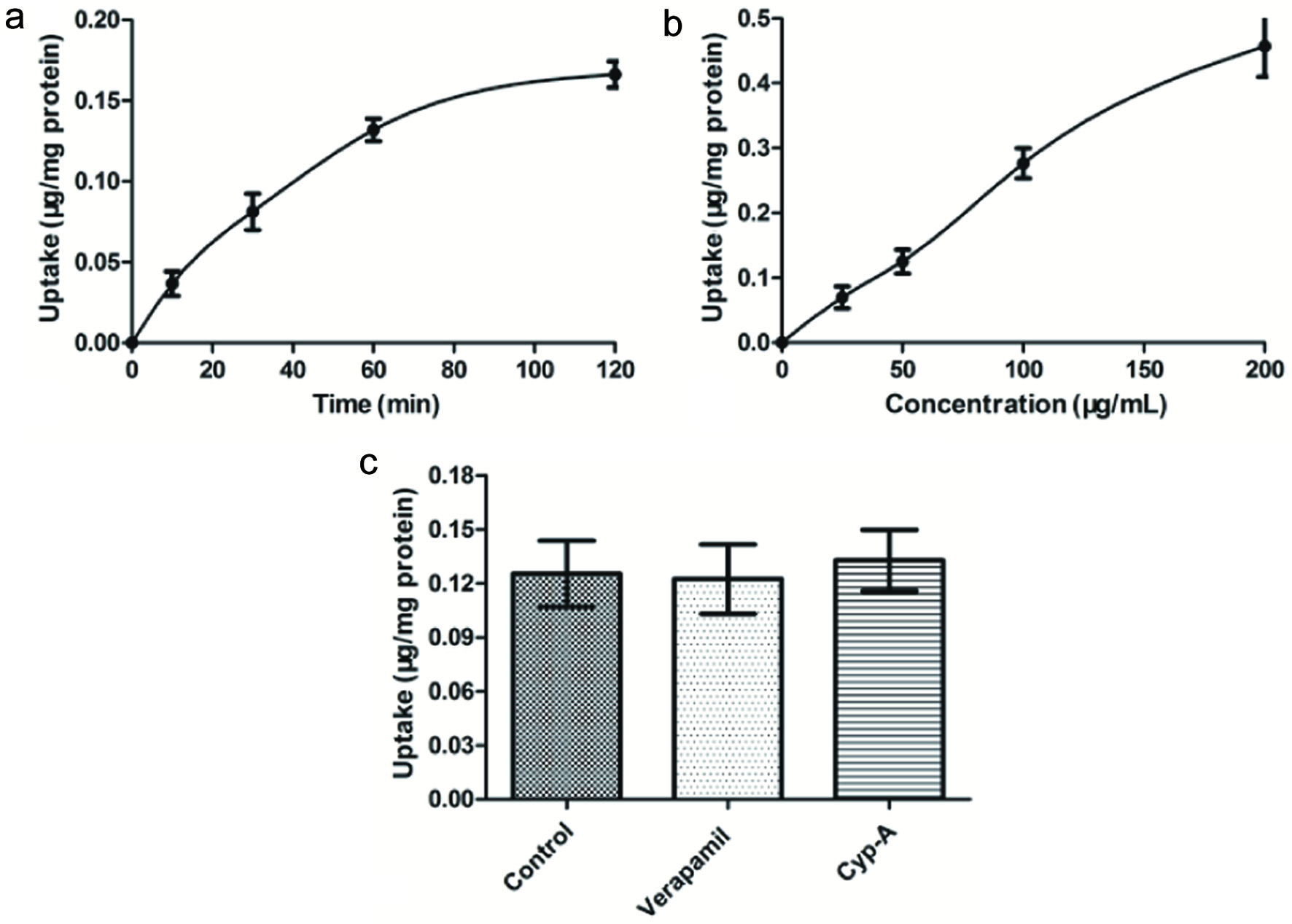 Click for large image | Figure 3. Effect of incubation time (a), concentration of eurycomanone (b) and P-glycoprotein inhibitor (c) on the uptake of eurycomanone in Caco-2 cell. (a) Caco-2 cells were incubated at 37 °C in HBSS containing 50 μg/mL of eurycomanone for 120 min. (b) Caco-2 cells were incubated for 60 min at 37 °C with different concentration (0, 25, 50, 100, 200 μg/mL) of eurycomanone. (c) Caco-2 cells were incubated for 60 min at 37 °C with eurycomanone (50 μg/mL) alone, eurycomanone (50 μg/mL) with Verapamil (100 μg/mL), and eurycomanone (50 μg/mL) with Cyp-A (100 μg/mL), respectively. Each point is presented as mean ± SD, n = 3. |
3.4. Transport characteristics of eurycomanone on both sides of Caco-2 cells monolayer
The absorption of the apical side to the basolateral side (AP→BL) and the basolateral side to the apical side (BL→AP) are shown in Figure 4a and b and Table 3. The drug transport mode can be judged by the efflux rate (ER). At various concentrations, there is no significant difference in the apparent permeability coefficient (Papp). When the ER is between 0.5 and 1.5, it indicates that the drug is mainly passive transport, and further study is needed to investigate whether a carrier participated. When Er is greater than 1.5, the drug may be actively transported with the participation of carrier protein (Gao et al., 2005).
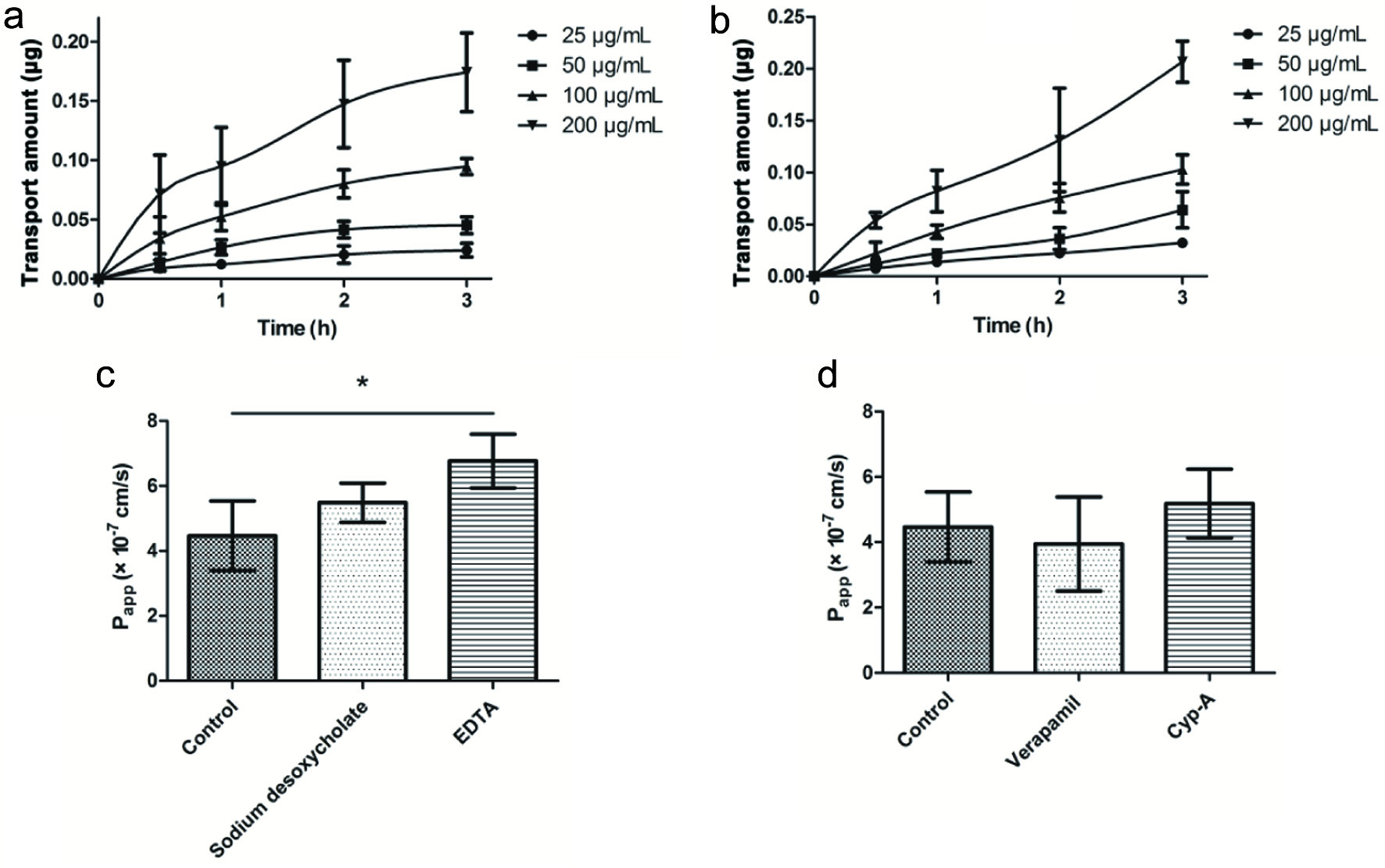 Click for large image | Figure 4. AP→BL (a) and BL→AP (b) are the effect of incubation time on cell transport; the effects of paracellular enhancers (c) and P-gp (d) inhibitors on transmembrane transport of eurycomanone. |
 Click to view | Table 3. Effect of concentration on bidirectional transport (x ± s, n = 3) |
The results showed that ER was between 0.5 to 1.5 and there was no significant difference in bidirectional Papp, and the transport mechanism of eurycomanone in the Caco-2 cell model was mainly passive diffusion. To investigate the effects of paracellular transport enhancers on the absorption of eurycomanone, sodium desoxycholate and EDTA were introduced in experiments. As shown in Figure 4c, although both the two paracellular transport enhancers improved the absorption of eurycomanone (Song et al., 2014), EDTA was prior to sodium desoxycholate in this aspect (P < 0.05). Meanwhile, these findings suggested that there is a paracellular pathway in the passive diffusion absorption of eurycomanone. In theory, water-soluble small molecule compounds are common drugs for the transport of the paracellular pathway (Guan et al., 2012). Interestingly, eurycomanone is well water-soluble with a molecular weight of 408.403, which meets the potential to transport by the paracellular pathway.
Given that P-glycoprotein (P-gp) represents an important intervention in drug absorption, the effect of P-gp inhibitors on eurycomanone absorption was also evaluated (Figure 4d). However, P-glycoprotein inhibitors of Verapamil and Cyp-A had no interference with eurycomanone absorption (P > 0.05), indicating that eurycomanone is not P-gp substrate.
As shown in Table 4, the change of temperature (4 °C and 37 °C) had no significant effect on Papp and ER (P > 0.05), indicating that there was no active transport in the absorption process of eurycomanone. Because of the decrease of temperature, the production of adenosine triphosphate (ATP) is blocked (Guan et al., 2012), the active transport will be inhibited, and the absorption and efflux rate of drugs will change significantly, while the absorption and efflux rate of eurycomanone will not change significantly due to the change of temperature.
 Click to view | Table 4. Effect of temperature on bidirectional transport (x ± s, n = 3) |
There have been many articles (Hussein et al., 2007; Kuo et al., 2004) reporting that eurycomanone shows a lot of pharmacological effects. However, the effects of eurycomanone can be limited due to its low bioavailability. The aim of this study is to provide the preliminary research to uncover transport mechanism of eurycomanone. We have incorporated eurycomanone into phospholipid complexes, with subsequent improvement in bioavailability and increased its efficacy (Guo et al., 2021).
| 4. Conclusion | ▴Top |
This paper investigated the transmembrane transport mechanism of eurycomanone, a representative compound of quassinoids. It was confirmed that the absorption mechanism of eurycomanone is passive transmembrane transport and paracellular pathway transport, and the factors affecting transmembrane absorption and transport were mainly time and drug concentration, independent of temperature. Bi-directional transport experiments demonstrated the transport process of eurycomanone with no apparent directionality.
The experimental results also demonstrated that eurycomanone was not a substrate for P-glycoprotein and was not involved in P-glycoprotein-mediated efflux, and that paracellular transport enhancers could promote the absorption of eurycomanone. Moreover, the poor absorption of eurycomanone was indicated by a low effective permeability coefficient. Thus, these results provided updated information concerning the transport mechanism, which may provide some insights for the research and application of eurycomanoneoral formulations with intestinal absorption. However, oral absorption mechanism of eurycomanone performed by us needs further exploration, such as in vivo experiments and exocytosis process.
Acknowledgments
This research project was supported by Young Talent’s Training Scheme in Binhai New Area, China Postdoctoral Sceience Foundation (2020T130093ZX).
| References | ▴Top |