| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 17, March 2022, pages 18-26
Effectiveness of micellization with polysorbate 80 on the in vitro bioaccessibility of various bioactives
Johanita Kruger*, Quirin Fink, Nadine Sus
Institute of Nutritional Sciences, University of Hohenheim, Garbenstraße 28, 70599 Stuttgart, Germany
*Corresponding author: Johanita Kruger, Institute of Nutritional Sciences, University of Hohenheim, Garbenstraße 28, 70599 Stuttgart, Germany. Tel: +49-711-459 23376; Fax: +49-711-459 23386; E-mail: johanita.kruger@nutres.de
DOI: 10.31665/JFB.2022.17300
Received: February 4, 2022
Revised received & accepted: March 16, 2022
| Abstract | ▴Top |
The improvement in bioavailability of different compounds from PS80 micelles differs widely. This research, for the first time, investigated the effects bioactives, with different physicochemical properties (CoQ10, curcumin, catechin, naringenin, quercetin, β-carotene and retinyl palmitate), have on the micellar characteristics and the in vitro bioaccessibility of the loaded bioactives. There was no link between the physicochemical properties of the bioactives and the loading capacity of the PS80 micelles, which varied between 0.04 and 14.0%, or the average bioaccessibility of the bioactives, which varied between 14 and 86%. Not the ratio of loaded bioactive to PS80, but rather an increased concentration of PS80, increased the bioaccessibility of the loaded bioactive. It is not clear if differences were due to modifications of the PS80 micelles during the digestion process, where e.g. bile salts and/or phospholipids were incorporated into the micelles, or if the micelles totally dissociated and physiological mixed micelles were formed, including the PS80.
Keywords: Surface charge; Size distribution; Digestive stability; Loading capacity
| 1. Introduction | ▴Top |
A substantial number of health-promoting bioactive compounds, including: lipid-soluble vitamins, phytosterols, carotenoids, phenolic compounds and prescription drugs, have very low oral bioavailability (Raikos and Ranawana, 2017). This can be due to low stability during the digestive track, low aqueous solubility and resulting bioaccessibility, poor absorption or high efflux from the enterocytes and/or pre-systemic metabolism in the gut wall. Polysorbate 80 (PS80; polyoxyethylene (20) sorbitan monooleate) is a non-ionic surfactant and emulsifier used in food (Hasenhuettl, 2008) supplements and oral (Cannon and Long, 2008) as well as intravenous (Bian et al., 2016) drug formulations to improve the bioavailability of lipophilic compounds. Addition of amphiphilic compounds (surfactants) such as PS80, facilitates the incorporation of lipid-soluble compounds into emulsions, micelles or liposomes. The exact mechanism by which PS80 micellization improves the bioavailability of various lipophilic compounds is unknown and interestingly, the improvement in bioavailability of various compounds loaded in PS80 micelles differs widely.The effect of PS80 micellization of bioactives on their oral bioavailability has been found to range from negligible for coenzyme Q10 (CoQ10) (1.1-fold) (Weis et al., 1994) to a very high increase of curcumin (185-fold) (Schiborr et al., 2014), with various bioactives in between: digoxin (2.6-fold) (Zhang et al., 2003), resveratrol (5-fold) (Calvo-Castro et al., 2018), astaxanthin (2- to 4-fold) (Odeberg et al., 2003) and progesterone (4- to 6-fold) (Potluri and Betageri, 2006). The differences in the improvement in bioavailability of the different bioactives incorporated into PS80 micelles, could be due to the physicochemical properties of the different compounds resulting in micelles with different characteristics, bioaccessibility and bioavailability.
The composition of PS80 micelles can alter the micellar characteristics, including: loading capacity, size, surface charge, digestive stability and/or bioaccessibility, which all play a role in the bioavailability of the loaded compounds. The physical micellar characteristics (e.g. size and surface charge) affect the interactions between the micelles and the intestinal mucus layer and membrane receptors and subsequently, alter absorption into enterocytes (Borel et al., 2013; Goncalves et al., 2015). Physiological mixed micelles are believed to dissociate during interaction/movement through the intestinal mucus layer, after which the contents are absorbed through their respective passive/facilitated absorption mechanisms (Shiau et al., 1990). Nano-sized particles (including PS80 micelles (<20 nm)), however, are formulated to improve digestive stability, protecting the loaded compound during gastrointestinal digestion (Gaucher et al., 2010; Verkempinck et al., 2018). Nevertheless, vast majority of in vitro research evaluating the absorption mechanisms of nano-sized particles and micelles (including cellular uptake studies), are done without an initial simulated digestion step, and as such, fail to evaluate the effect of digestive conditions on the micellar characteristics and subsequent absorption. While PS80 micelles are formulated to have increased digestive stability, digestive conditions such as; changes in pH, the presence of bile salts and enzymes might change the characteristics of PS80 micelles in as yet, unknown ways. We hypothesise that the characteristics of the PS80 micelles, after in vitro digestion, are different from that of the originally ingested micelle.
In this study, dietary bioactives, with diverse physicochemical properties were loaded into PS80 micelles. To obtain a better understanding of the mechanism underlying successful PS80 micellar formulation, the following characteristics were investigated: loading capacity, stability upon aqueous solubilisation, micellar size distribution and surface charge as well as in vitro bioaccessibility of the bioactives. The results obtained provide novel insights into the bioaccessibility of different bioactives from PS80 micelles and a better understanding of the factors, which affect the bioaccessibility, that will aid in the development of micellar formulations with optimally improved bioavailability.
| 2. Materials and methods | ▴Top |
2.1. Reagents
For the preparation of the micelles, curcumin (95%) CoQ10 (95%), quercetin (95%), retinyl palmitate (1,7 × 106 IE/g) from Aquanova AG (Darmstadt), catechin (≥98% (+)-catechin) and naringenin (≥98%) from Carl Roth (Karlsruhe) and β-carotene from Sigma-Aldrich (Steinheim) were used. The following digestive compounds used in the in vitro digestion; bile extract (Sigma B8631), pepsin (Sigma P7000; ≥250 U/mg, labeled activity), pancreatin (Sigma P7545; 8 × USP, labeled activity), and pancreatic lipase (Sigma L3126) were of porcine origin and obtained from Sigma-Aldrich.
2.2. Micelle production
Using a solvent evaporation process, polysorbate 80 (Tween®80, Sigma-Aldrich) was used to formulate CoQ10, curcumin, catechin, naringenin, quercetin, β-carotene and retinyl palmitate, micelles (n = 4). For curcumin, catechin, naringenin and quercetin, ethanol (ROTISOLV® HPLC Gradient Grade, Carl Roth) was used as solvent, for CoQ10 and retinyl palmitate, hexane (ROTISOLV® HPLC, Carl Roth) and for β-carotene dichloromethane (ROTISOLV® HPLC, Carl Roth) was used. As control, PS80 empty micelles were produced using ethanol (Figure 1).
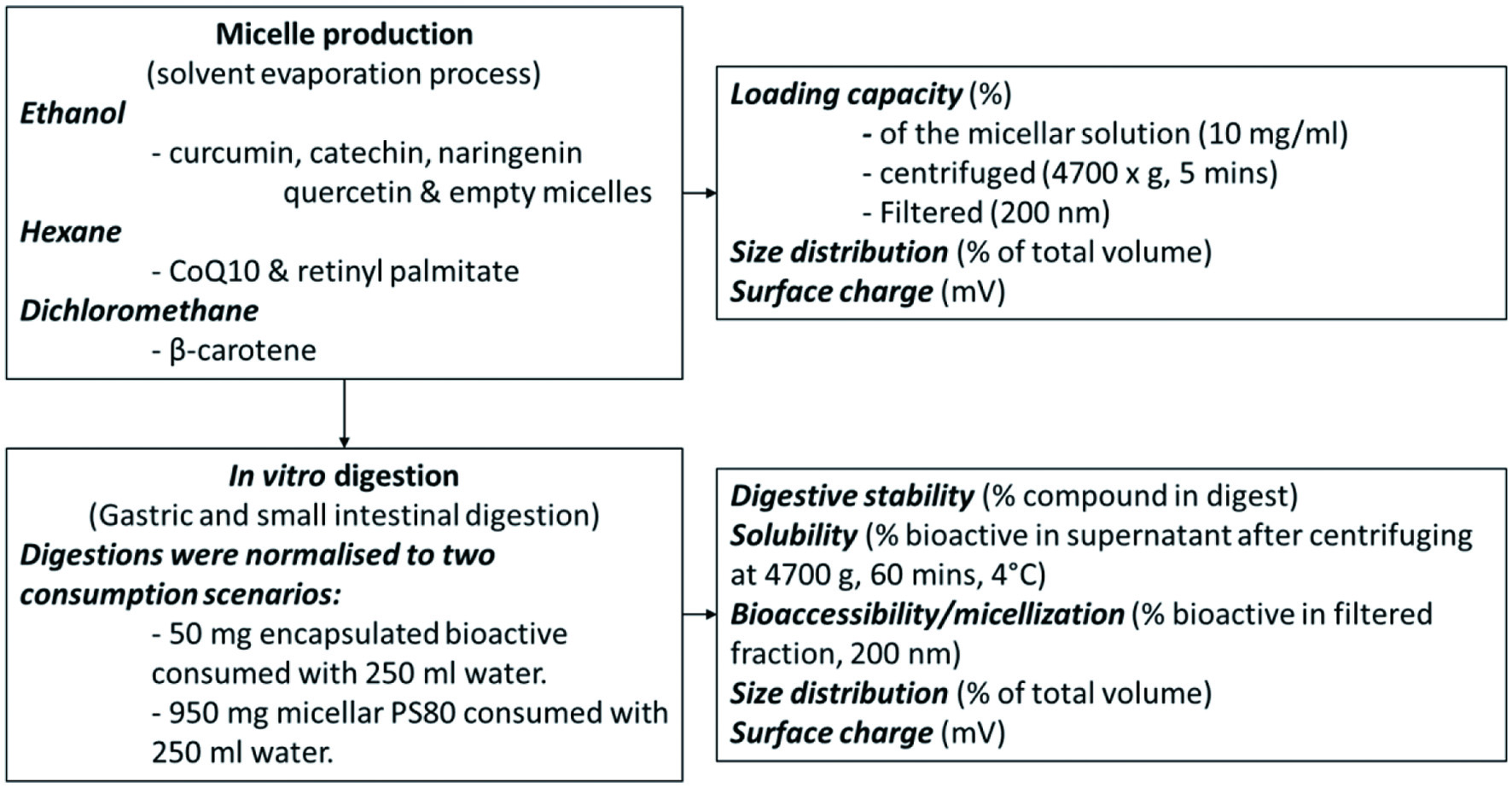 Click for large image | Figure 1. Experimental design. |
PS80 (900 mg) alone (empty micelles) or with each of the above-mentioned bioactives (100 mg) were added to 20 ml of the respective solvents and dissolved in an ultrasonic bath at 25 °C for 15 min. The solvent was evaporated overnight, using a rotary vacuum concentrator, after which 20 ml of H2Odd (distilled and deionized) was added and incubated (3 h, shaking at 150 rpm at 4 °C). Preliminary test showed higher stability of micelles when formed at lower temperatures. After incubation, the mixture was centrifuged (4,700 ×g, 10 min, 4 °C) and filtered (Filtropur S, 200 nm, Sarstedt, Nümbrecht, Germany) to isolate the micelles and remove any precipitated bioactive and, or larger emulsions. The H2Odd was then evaporated overnight, using a rotary vacuum concentrator, and the resulting micellar paste stored at 4 °C until further use.
2.3. Loading capacity
In order to determine the loading capacity (LC) of the different micellar formulations, the individual micelles (thick paste) were dissolved in H2Odd (10 mg/ml) and the content of each bioactive measured by HPLC (see section 2.4). To evaluate the stability of the micelles, the LC was also calculated after the micellar solution was centrifuged (4,700 ×g, 5 min) and filtered (Filtropur S, 200 nm).
LC was calculated as follows:
2.4. HPLC analysis
2.4.1. CoQ10
CoQ10 was analysed on a SHIMADZU HPLC system (autosampler SIL-20AC HT, degasser DGU-14A, column oven CTO-10AS VP, system controller SCL-10A VP, UV/VIS detector SPD 20A, liquid chromatograph LC-20AT) equipped with a Reprosil-Pur column (C18-AQ, 5 µm, 250 × 4.6 mm, Dr. Maisch GmbH, Ammerbuch, Germany) maintained at 35 °C. After a 10 µl injection, chromatographic separation was achieved with a mobile phase consisting of 85% methanol and 15% 1,4-dioxane at a flowrate of 1 ml/min. The UV/VIS detector analysed CoQ10 at an excitation wavelength of 272 nm.
2.4.2. Curcumin
The different curcuminoids were analysed on a SHIMADZU HPLC system (see CoQ10), with the column maintained at 40 °C. After a 20 µl injection chromatographic separation was achieved using a mobile phase of 55% H2Odd (pH = 3 with perchloric acid) and 45% acetonitrile at a flowrate of 1.4 ml/min. The analyses were carried out with the fluorescence detector, which quantified at an excitation wavelength of 426 nm and an emission wavelength of 536 nm. Peak integration was done with the LabSolution data management software and quantified against external standard curves of curcumin (purity >97%), demethoxycurcumin (purity >98%) and bis-demethoxycurcumin (purity >98%) standards (Chromadex, Irvine, USA).
2.4.3. Polyphenols
Catechin, naringenin and quercetin were analysed on a JASCO HPLC system (LC-Net II ADC, AS-2059-SF Plus, PU-2080 Plus, CO-2060 Plus, DG-2080-53, LG-2080-02 and a photodiode array detector PDA MD-2018; JASCO, Groβ-Umstadt, Germany) equipped with a Kinetex PFP column (100 A, 250 × 4.6 mm, 5 µm, Phenomenex, Aschaffenburg, Germany) maintained at 35 °C. After a 20 µL sample injection, chromatographic separation was achieved using a multistep gradient method (0 min 0% B, 1 min 20% B, 4 min 20% B, 12 min 75% B, 14 min 75% B, 17 min 30% B) with mobile phase A (deionized water with 5% formic acid) and mobile phase B (acetonitrile with 10% deionized water and 5% formic acid) at a flow rate of 1.0 ml/min. Photodiode array detection was monitored at 320 nm for quercetin and 280 nm for naringenin and catechin. Peak integration was done with the JASCO ChromNAV (version 1.19.1) data management software and quantified against external standard curves of (+)-Catechin (purity ≥ 98%, Carl Roth, Karlsruhe, Germany), naringenin (purity ≥ 98%, Carl Roth) and quercitin (purity >95%, Aquanova, Darmstadt, Germany).
2.4.4. β-carotene, retinyl palmitate and retinol
β-carotene, retinyl palmitate and retinol were analysed on a SHIMADZU HPLC system (see CoQ10), with the column maintained at 40 °C. After a 20 µl injection, chromatographic separation was achieved with a mobile phase consisting of 82% acetonitrile, 15% 1,4-dioxane and 3% methanol with 100 mM ammonium acetate and 0.1% triethylamine at a flow rate of 1.5 ml/min. The β-carotene was measured by the UV/VIS detector with an excitation wavelength of 400 nm. Retinyl palmitate and retinol were measured with the fluorescence detector at an excitation wavelength of 325 nm and an emission wavelength of 470 nm. Peak integration was done with the LabSolution (SHIMADZU Corporation, Nakagyoku, Japan) data management software and quantified against external standard curves of β-carotene (≥97.0% purity, Sigma-Aldrich), retinyl palmitate (purity >97%, Sigmal Adrich) and retinol (purity >95%, Sigma-Aldrich (Merck Group KGaA), Darmstadt, Germany).
2.5. In vitro digestion
A simplified version of the IFOGEST method (Rodrigues et al., 2017), consisting of an in vitro gastric and intestinal digestion, was carried out (n = 6). Due to the different LC of the micelles, the digestions were normalised to simulate two concentrations (consumption scenarios). First, the concentrations were normalised to the quantity of micellar PS80 added to the digestion, where oral intake of the micellar paste, at such a quantity that 950 mg of micellar PS80 consumed with 250 ml of water, was simulated. The digestions were then repeated and the concentration normalised to the quantity of the loaded bioactive, where oral intake of the micellar paste, at such a quantity that 50 mg of loaded bioactive consumed with 250 ml of water, was simulated.
The amounts of micelles to be added to the in vitro digestions were calculated using the loading capacity:
- 50 mg loaded bioactive consumed with 250 ml water:
- [(50 mg bioactive) × 10 ml]/(250 ml water) (gastric digestion vol-ume) = 2 mg of bioactive to be added to the digestion
- (2 mg bioactive)/[LC (%)] = micelles (mg) added to 10 ml digestion;
- 950 mg micellar PS80 consumed with 250 ml water:
- [(950 mg PS80) × 10 ml]/(250 ml water) (gastric digestion vol-ume) = 38 mg of PS80 to be added to the digestion
- (38 mg PS80)/[100 − LC (%)] = micelles (mg) added to 10 ml digestion.
For the gastric phase, the micelles (quantities according to above calculations) were diluted to a final volume of 8.5 ml, to which pepsin was added (1.5 ml 34 mg pepsin/ml 0.1 N HCl–final conc. 5 mg/ml), the pH adjusted to 2 and the digests incubated (in the dark at 37 °C for 1 h, shaking at 120 rpm). For the small intestinal phase (final volume 15 ml), porcine lipase (9 mg/ml), porcine pancreatin (18 mg/ml) and bile extract (36 mg/ml) were prepared in 100 mM NaHCO3 and added to final concentrations of 0.6 mg/ml lipase, 1.2 mg/ml pancreatin and 7.2 mg/ml bile extract. The pH was adjusted to 6.5 using 1 N NaOH, the digest overlaid with nitrogen gas and incubated, as described above, for 2 h. The final digests were centrifuged (4,700 ×g, 60 min, 4 °C) and filtered (Filtropur S, 0.2 µm) to separate the soluble and bioaccessible fraction, respectively. All collected samples were overlaid with nitrogen gas and stored at −80 °C for a maximum of two weeks before HPLC analysis.
The digestive stability (%) in this study is defined as the amount of bioactive not degraded during digestion (amount in the whole digest), as a percentage of the total amount of bioactive digested. The aqueous solubility (%) in this study is defined as the amount of bioactive solubilised after digestion (amount in supernatant after centrifuging), as a percentage of the total amount of bioactive digested. Bioaccessibility (%) in this study is defined as the amount of bioactive incorporated into soluble micelles, smaller than 200 nm (amount in supernatant after centrifuging and filtration) (Figure 2).
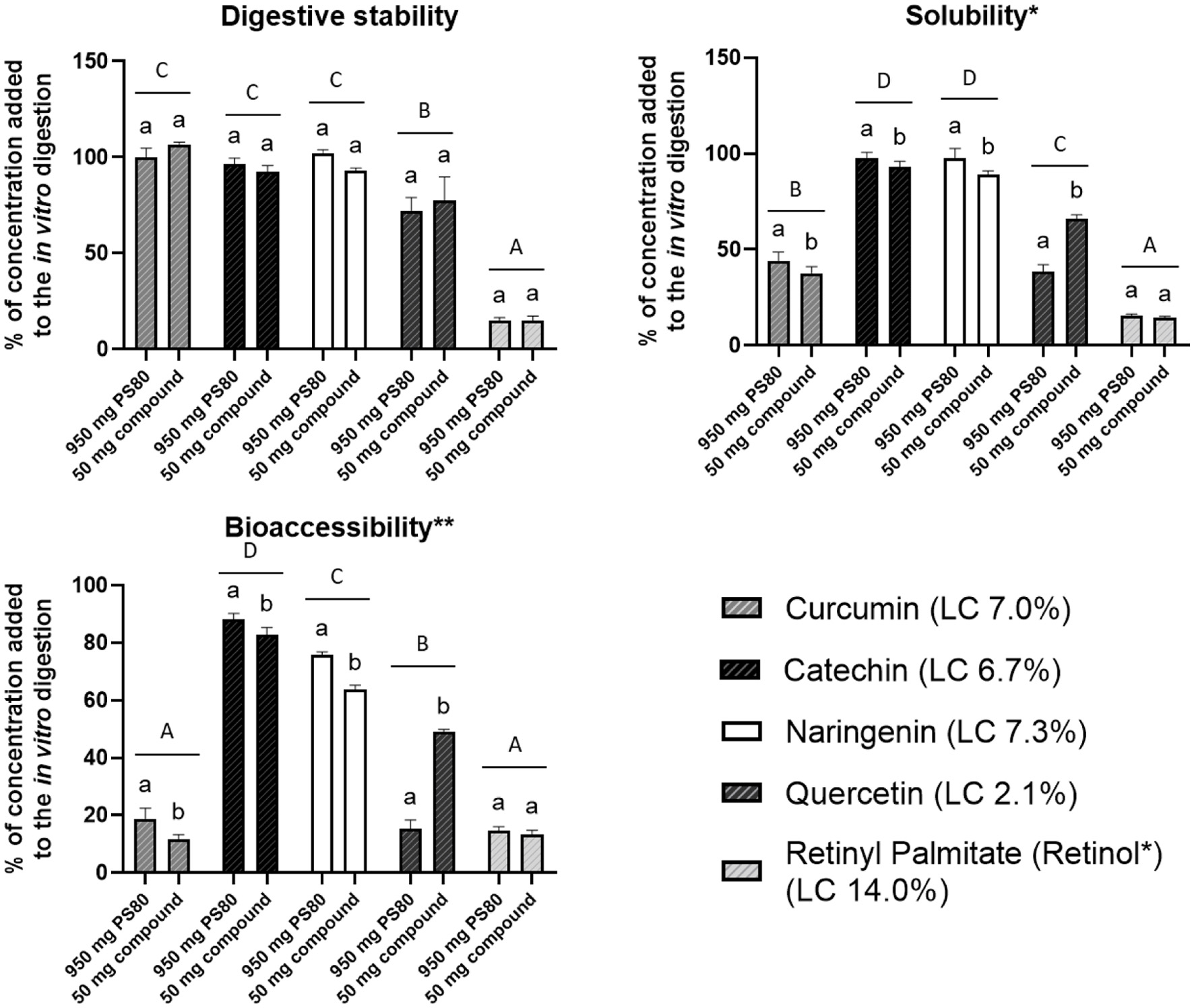 Click for large image | Figure 2. The in vitro digestive stability (%), solubility (%) and bioaccessibility (%) of bioactives loaded in PS80 micelles. Micelles were digested at two consumption scenarios; either 950 mg micellar PS80 or 50 mg loaded bioactive consumed with 250 ml water. Data presented as mean, and error bars indicate one standard deviation (n = 6). Bars with different lowercase letters of the same bioactive and different capital letters between different bioactives, differ significantly (p < 0.05). *Supernatant taken after the in vitro digests was centrifuged (4,700 ×g, 60 min, 4 °C). **Supernatant taken after the in vitro digests was centrifuged and filtered (200 nm). |
2.6. Size distribution and surface charge determination
The size distribution and surface charge of micelles from digestion fractions (50 mg of loaded bioactive/250 ml water) were analysed with a photon correlation spectrometer (Zetasizer Nano, Malvern Instruments, Malvern, USA).
The size distribution of particles in solution was measured by dynamic light scattering and the distribution curve (% of total volume) in addition to the polydispersity index (PDI) of the individual micelles were obtained. Size distributions with a PDI value ≥0.7 were excluded in this work, as larger values indicate an overly extensive size distribution and are therefore not appropriate for the technique of dynamic light scattering (Danaei et al., 2018). Measurements were repeated at least 4 times with 15 runs each at 25 °C.
The surface charge (mV) was measured with laser Doppler microelectrophoresis. Measurements were made at least 4 times with 20 runs each, at 25 °C.
2.7. Statistical analysis
Normality of data and equality of variance were assessed using the Shapiro-Wilk and Levene’s tests, respectively (GraphPad Prism 8.4.1, GraphPad Software Inc., La Jolla, USA). For the particle diameter data (not normally distributed), a Brown-Forsythe and Welch ANOVA (Analysis of Variance), followed by Dunnett’s T3 multiple comparison test were done. In the case of the other data (all normally distributed), a one-way ANOVA followed by Tukey’s posthoc test were performed. The main effects (of the two digestion scenarios) for each bioactive (results not shown) were analysed with a two-way ANOVA. With all analyses p < 0.05 was considered statistically significant.
| 3. Results and discussion | ▴Top |
3.1. Micellar characteristics and aqueous stability
The loading capacity (LC) of the various bioactives varied greatly, from very low for β-carotene (0.04%) and CoQ10 (0.48%), to high for retinyl palmitate (RP) (14.0%) (Figure 3). Various solvents (hexane, ethyl acetate, ethanol) were tested to improve the LC of the β-carotene and CoQ10 micelles, but the LC was similarly low with all solvents. The RP powder used in the micellar formulation contained small amounts of retinol (<1%), of which the LC is displayed. The LC of the various phenolic bioactives ranged from 2.1% for quercetin, 6.7% for catechin to 7.3% for naringenin. The curcumin powder used, also contained demetoxycurcumin and bisdemetoxycurcumin (≈20%), and the LC of curcumin and the total curcuminoids were 7.0% and 8.6%, respectively. There was no clear link between the loading capacity of the micelles and the physicochemical characteristics of the loaded bioactives (Table 1). For example, despite the large difference between the LC of RP and β-carotene, these bioactives have similar physicochemical properties, including molecular weights and hydrophobicity (logPoct/wat). The polar surface area (PSA) of RP (26.3 Å2) is higher than that of β-carotene (0 Å2). However, CoQ10 also has a higher PSA (52.6 Å2), but the LC was equally low. The same was found in a study where the solubility of 30 compounds with diverse physicochemical properties in lipid-based formulation excipients were evaluated (Persson et al., 2013). The solubility of the compounds in PS80 ranged from ≈1 to 340 mg/g, and while a partial least squares model, using calculated molecular descriptors, resulted in excellent predictions of solubility in soybean oil (R2 0.81) and Captex355 (R2 0.84), for PS80 the predictive value was low (R2 < 0.62).
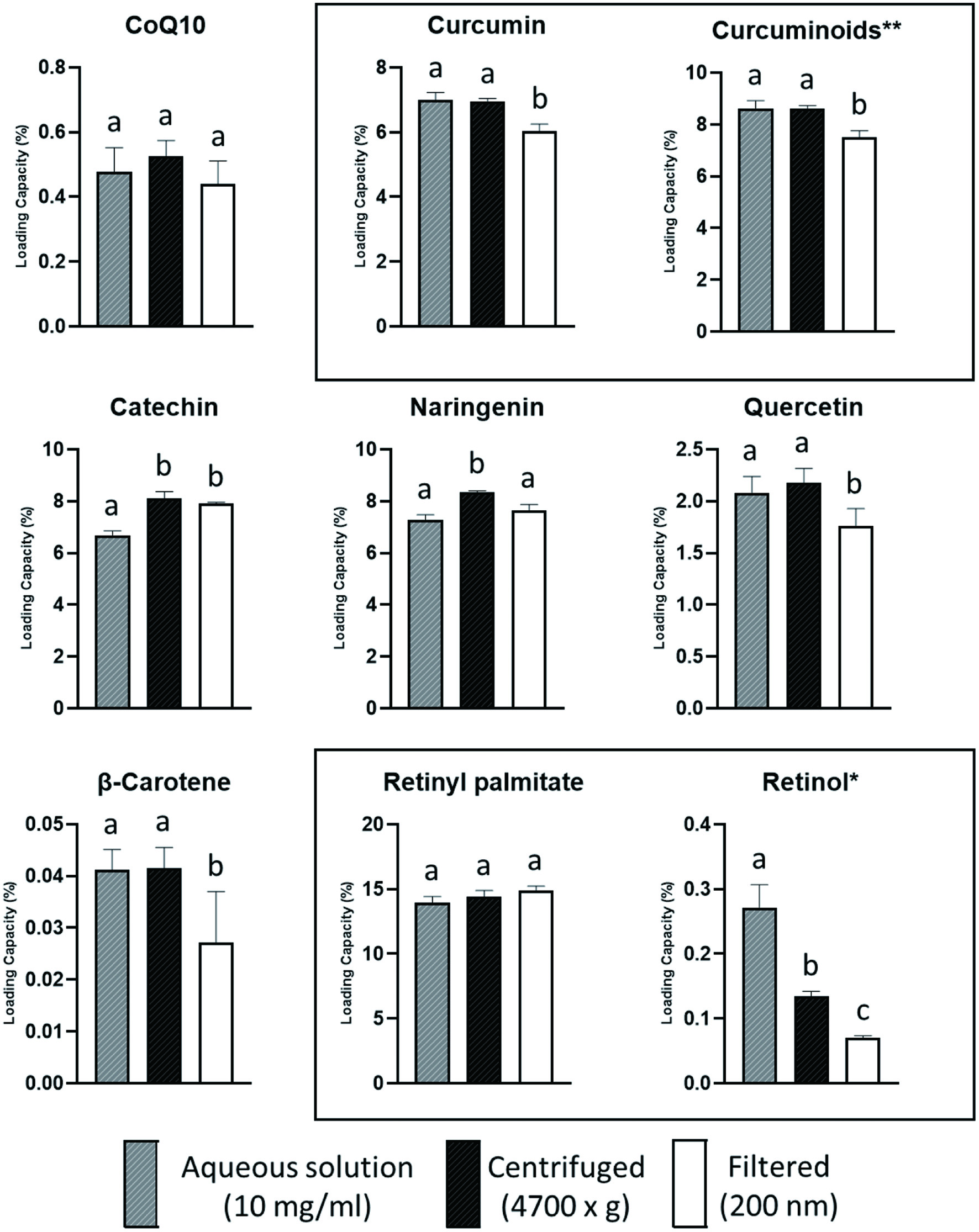 Click for large image | Figure 3. Loading capacity (%) of aqueous solutions of various PS80 micelles (10 mg/ml) before and after centrifuging (4,700 ×g) or filtering (200 nm). Data presented as mean, and error bars, indicate one standard deviation (n = 6). Bars with different lowercase letters of the same bioactive, differ significantly (p < 0.05). *Retinol included in the retinyl palmitate micelles **Sum of curcumin, demethoxycurcumin and bisdemethoxycurcumin in curcumin micelles. |
 Click to view | Table 1. Physicochemical properties of investigated bioactives |
To evaluate the stability of the various micelles, the LC was also measured after the micellar solutions were centrifuged or filtered. Except for the retinol in the RP micelles, centrifuging did not reduce the LC of the micelles, indicating that there was no significant flocculation in the aqueous solution. Filtering resulted in a substantial loss of β-carotene (34%) and retinol (75%) and smaller losses of curcumin (14%), total curcuminoids (13%) and quercetin (14%), indicating that there was aggregation of micelles forming particles larger than 200 nm.
The surface charge is an important indicator of the stability of colloidal dispersions (Mourdikoudis, et al., 2018). There was no substantial difference in the surface charge between the empty PS80 micelles and those loaded with the various bioactives (−3.63 vs. −3.96 to −7.03 mV) (Table 2). The surface charge of all the micelles was close to neutral (> −10 mV), indicating that these micelles could be prone to aggregation, compared to highly positively or negatively charged particles (> ±20–30 mV) which tend to repel each other, forming stable colloidal solutions (Mourdikoudis et al., 2018). The surface charge, however, does not seem to be the only factor affecting aggregation, as the LC of the retinol from the RP micelles decreased upon filtering, but not that of the RP.
 Click to view | Table 2. The surface charge (mV) of PS80 micelles loaded with various bioactives before (micelles) and after in vitro digestion |
There was no substantial difference in the size distribution between the empty PS80 micelles and those loaded with the various bioactives (Figure 4). Loading of the micelles resulted in slight increases in micellar size, but the average diameter of the main peaks (>99% of the volume, Figure 4) were all below 20 nm (results not displayed): empty micelles (9.2 ± 1.3 nm), curcumin (11.3 ± 2.0 nm) catechin (15.3 ± 1.2 nm), naringenin (14.6 ± 0.5 nm), quercetin (12.2 ± 0.4 nm), and RP (19.1 ± 4.7 nm).
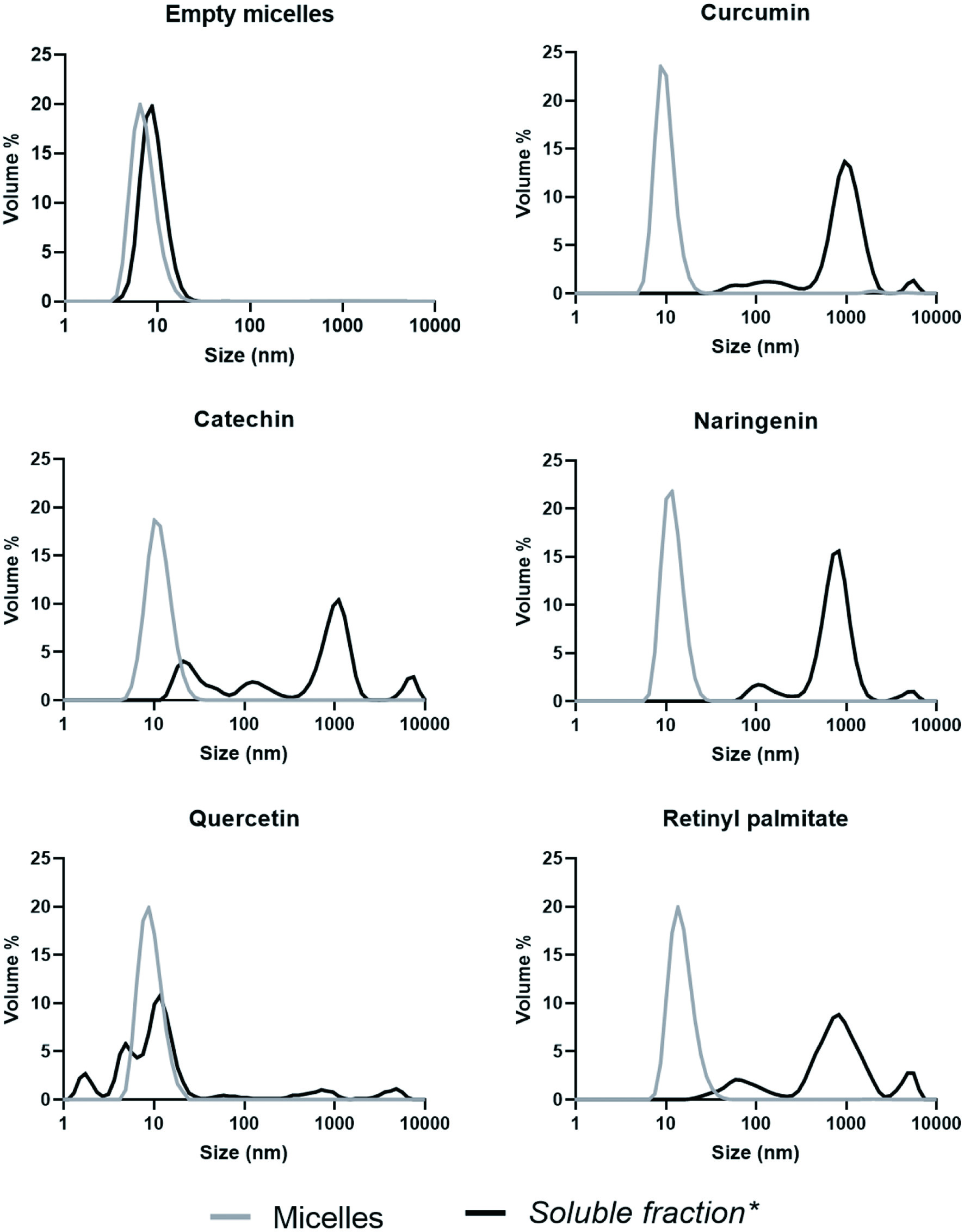 Click for large image | Figure 4. Size distribution (Percentage of total volume) of micellar solutions before and after in vitro digestion. *Supernatant taken after the in vitro digested micelles was centrifuged (4,700 ×g, 60 min, 4 °C). Measurements with a PDI > 0.7 were not included (n ≥ 5). |
3.2. In vitro digestion
As the LC of the micelles differed, digesting the same amount of each micelle, would make comparison difficult, as both the quantity of micellar PS80 and the quantity of the loaded bioactive in the digestions would differ. As such, the amount of micelles added to the digestions was normalised to both the quantity of micellar PS80 and quantity of loaded bioactive. First the digestion was simulated as 950 mg of micellar PS80 consumed with 250 ml of water, then as 50 mg of loaded bioactive consumed with 250 ml of water (see section 2.5 for calculations). The CoQ10 and β-carotene micelles were not included in the in vitro digestion, as their LC were too low.
There were no differences between the digestive stability (%) of the bioactives when the micelles were digested at the two concentrations (Figure 2). The digestive stability (average of two concentrations) of curcumin, catechin and naringenin was high at 103%, 94% and 97% respectively, with that of quercetin lower, at 75%. Technically, the digestive stability of the RP was 0%, as no RP was detected after the digestion. The concentration of the retinol however, increased, indicating that RP was converted to retinol, and as such, the digestive stability, solubility and bioaccessibility data of retinol (15%) are displayed. The digestive stability of the bioactive compounds could be indicative of the stability of the PS80 micelles. A reduction in digestive stability could mean that the bioactive compound leached from the micelle, or that the micelle dissociated, it is however no possible to speculate to which. In both scenarios however, the bioactive would have been exposed to the aqueous digestive environment where it could have been degraded or metabolised (e.g. RP conversion to retinol), or precipitated out of solution.
The in vitro solubility (average of two concentrations) of catechin and naringenin was high at 95% and 93%, respectively; with that of quercetin and curcumin lower at 52% and 41%, respectively; and RP (retinol) the lowest at 15% (Figure 2). The bioaccessibility (average of two concentrations) of catechin and naringenin was high at 86% and 70%, respectively; with that of quercetin lower at 32%; and curcumin and RP (retinol) the lowest at 15% and 14%, respectively. One could think that an increased bioactive digestive stability would lead to increased solubility and bioaccessibility. This was however not the case, where a simple regression analysis showed low assosiation between the digestive stability and solubility of the bioactives (R2 = 0.608), and even lower between bioaccessibility (R2 = 0.248). As with the LC, there was no link between the physicochemical properties (Table 1) and the bioaccessibility of the bioactives. In a study investigating the in vitro release of 10 compounds packaged into PS80/caprol 3GO/Captex 200P/capmul MCM/soybean oil self-microemulsifying drug delivery systems, there was also no clear link between the physicochemical properties of the compounds used and the in vitro release from the self-microemulsifying delivery systems (Thi et al., 2009).
Interesting is the comparison between the bioaccessibility data from the digestions at the two different concentrations, which differed for all the bioactives, except RP (retinol). The bioaccessibility of curcumin, catechin and naringenin, from the digestions normalised to 50 mg of the loaded bioactive, was significantly lower (38, 6 and 16%, respectively), compared to the digestions normalised to 950 mg of the micellar PS80 (Figure 2). The bioaccessibility of quercetin, from the digestions normalised to 50 mg of loaded bioactive, was significantly higher (221%), compared to the digestion normalised to 950 mg micellar PS80. The differences between the two consumption scenarios could be ascribed to the different concentrations of PS80 in the digestions. The loading capacity of the micelles was inversely related to differences in the concentrations of the PS80 between the 950 mg PS80 and 50 mg loaded bioactive digestion. With the quercetin micelles (LC 2.1%), the PS80 concentration in the 950 mg PS80, compared to the 50 mg loaded bioactive scenario, was 2.5-fold higher, compared to RP micelles (LC 14.0%) where the PS80 concentration was only 32% higher. For curcumin (LC 7.0%), catechin (LC 6.7%) and naringenin (LC 7.3%), the PS80 concentrations were 67–73% higher. This indicated that the higher the concentration of the PS80 in the digestion, the higher the bioaccessibility of the loaded bioactive. A linear regression, found a high correlation (R2 = 0.844) between the difference in PS80 concentration and difference in bioactive bioaccessibility between the two digestions. This supports the hypothesis that the concentration of PS80 in the digestion plays a substantial role in the bioaccessibility of the loaded bioactive. So, while a high LC is generally considered a desirable aspect, consider the following: when the same amount of bioactive (e.g. 100 µg) is consumed from PS80 micellar formulations with a low (e.g. 5%) and high (e.g. 20%) LC, respectively, the bioactive from the micellar formulation with a lower LC, will have higher bioaccessibility and possibly, subsequent bioavailability.
The size distribution of the empty PS80 micelles, did not change substantially after the in vitro digestion (Figure 4). The sizes of the particles of the digests after the loaded micelles were digested, were however, in general, much larger than that of the original micelles. This again could have been due to aggregation of the PS80 micelles, or formation of larger sized mixed micelles. The size distribution of the naringenin, curcumin and RP micelles were similar with a small peak at ≈100 nm, the main peak at ≈1,000 nm and another small peak between 5,000 and 10,000 nm. The catechin micelles also had a substantial peak at ≈40 nm. The size distribution of quercetin was totally different, with increasing peaks at ≈3 nm, ≈6 nm and ≈15 nm in addition to small peaks at ≈80 nm, ≈900 nm and ≈6,000 nm. On average 37, 91, 75, 62 and 93% of the soluble curcumin, catechin, naringenin, quercetin and RP (retinol), respectively, were assayed as bioaccessible (packed into micelles smaller than 200 nm). This suggests that for curcumin, and to a lesser extent for quercetin and naringenin, the bioactives were loaded into particles larger than 200 nm. Therefore, either PS80 micelles aggregated or larger mixed micelles, containing the bioactives, were formed. There was no link between the size distribution of the micelles and the bioaccessibility of the bioactives, where quercetin had the smallest micelles, but the bioaccessibility was lower than that of naringenin and catechin.
Digestion decreased the surface charge of all the micelles by 7 to 15-fold (soluble fraction) (Table 2). Except for naringenin and quercetin, where the surface charge of the micelles in the bioaccessible fraction was lower (19–53%) than that of the empty micelles. The reduction in surface charge was probably due to the bile salts (Wickham et al., 1998), either adsorbing to the PS80 micelles, or the micelles dissociating and physiological mixed micelles (including PS80 and bile salts) forming. It has been found that above their critical micelle concentration (CMC) (2 to 4 mM), bile salts can displace PS80 and other surfactants from oil in water interfaces (Golding and Wooster, 2010).
| 4. Conclusions | ▴Top |
Of the bioactives investigated, there is no link between their physicochemical properties and the loading capacity or stability of their respective PS80 micelles. Not the ratio of loaded bioactive to PS80, but rather an increased concentration of PS80 in the digestion seems to increase the bioaccessibility of the loaded bioactive. In general, it is not clear if the differences in the size and surface charge of the PS80 micelles after digestion, were due to modifications during the digestion process, where for e.g. bile salts and/or phospholipids were incorporated into the micelles, or if the micelles totally dissociated and physiological mixed micelles were formed, including the PS80. When considering the conversion of RP to retinol during digestion, it is probable that the RP micelles dissociated, as it has previously been found that digestion conditions lead to the conversion of RP to retinol (Courraud et al., 2013).
Acknowledgments
This research and J Kruger were funded by the Deutsche Forschungsgemeinschaft (DFG), who was not involved in the study design or collection, analysis and interpretation of data, in the writing of the report and/or the decision to submit the article for publication.
Conflict of interest
There are no conflicts of interest to declare.
| References | ▴Top |