| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 17, March 2022, pages 11-17
An insight into the glycosidically bound aroma compounds and biological activities in fruits and vegetables
Baojiang Hea, Jufang Haob, Zilong Mac, Fengmei Zhuc, Bin Dud, *
aZhengzhou Tobacco Research Institute of China National Tobacco Corporation, Zhengzhou, 450001, China
bStaff Development Institute of China National Tobacco Corporation, Zhengzhou, 450008, China
cCollege of Food Science and Technology, Hebei Normal University of Science and Technology, Qinhuangdao, 066004, China
dHebei Key Laboratory of Natural Products Activity Components and Function, Hebei Normal University of Science and Technology, Qinhuangdao, 066004, China
*Corresponding author: Bin Du, Hebei Key Laboratory of Natural Products Activity Components and Function, Hebei Normal University of Science and Technology, Qinhuangdao, 066004, China. Tel: +86 335 8387040; Fax: +86 335 8387040; E-mail: bindufood@aliyun.com
DOI: 10.31665/JFB.2022.17299
Received: March 8, 2022
Revised received & accepted: March 30, 2022
| Abstract | ▴Top |
Glycosides are a major source of untapped flavor in fruits and vegetables. This review aims to present an overview of the isolation and characterized of glycosidically bound aroma compounds in fruits and vegetables. The analytical techniques of glycosidically bound aroma compounds is discussed. The characterization of glycosidically bound aroma compounds is comprehensively included. In addition, the acidic and enzymic hydrolysis of glycosidically bound aroma compounds are reviewed as well as the liberation mechanisms. At last, the different biological activities of glycosidically bound aroma compounds were also summarized.
Keywords: The glycosidically bound aroma compounds; Fruits; Vegetables; Enzymatic hydrolysis; Biological activities
| 1. Introduction | ▴Top |
The aroma belongs to one of the sensory traits. The sensory quality is a crucial issue for consumers’ satisfaction (Bianchi et al., 2019). Many aroma compounds occur as glycosidically bound precursors that do not contribute to fruit and vegetable aroma until aglycone release during processing or storage (Hampel et al., 2014). Glycosylated compounds are often considered as transportable storage compounds or detoxification products (Sarry and Günata, 2004). They are more than 10 times abundant of free aroma compounds (Liu et al., 2017). Among the aroma compounds present within fruits and vegetables, a significant part is assumed to come from specific odorless precursors found mainly as glycosidic compounds which are known as bound fraction (Pedroza et al., 2010). Glycosidically bound aroma compounds are typically analyzed following isolation of the glycosides on columns (including solid-phase extraction (SPE) or SPE cartridges) filled with a C18-bonded reversed-phase stationary phase (Hampel et al., 2014).
Not only new analytical data of the glycosides but also the role of the glycosides was paid attention by many researchers (Stahl-Biskup et al., 1993). The first review article on glycosidically bound volatiles was published in 1987 (Stahl-Biskup, 1987). At present, research activities in this field have increased dramatically. Other results show the glycosides as a transport form of the free monoterpenes, on one hand from the site of biosynthesis to the site of accumulation, on the other hand in the service of monoterpene turnover and catabolism. On account of their hydrophilic properties, their role as a transport form seems plausible and opens a wide field for future research work.
Together with the glycosidically bound aroma compounds in case of grapes and wines (Zhu et al., 2016), there is a growing interest for the study of glycosidically bound aroma compounds in fruits, vegetables and aromatic plants. At the same time, aroma compounds, especially glycosidically bound aroma compounds in fruits and vegitables currently represent a hot research field. There are many papers about this research field. However, this field lacks a systematic and comprehensive review paper for glycosidically bound aroma compounds. In this review we will summarize the isolation, identification, the analytical techniques, the acidic and enzymic hydrolysis, the characterization and the biological properties of glycosidically bound aroma compounds in fruits and vegetables (Figure 1). This review helps to provide insights into the release of glycosidically bound aroma compounds.
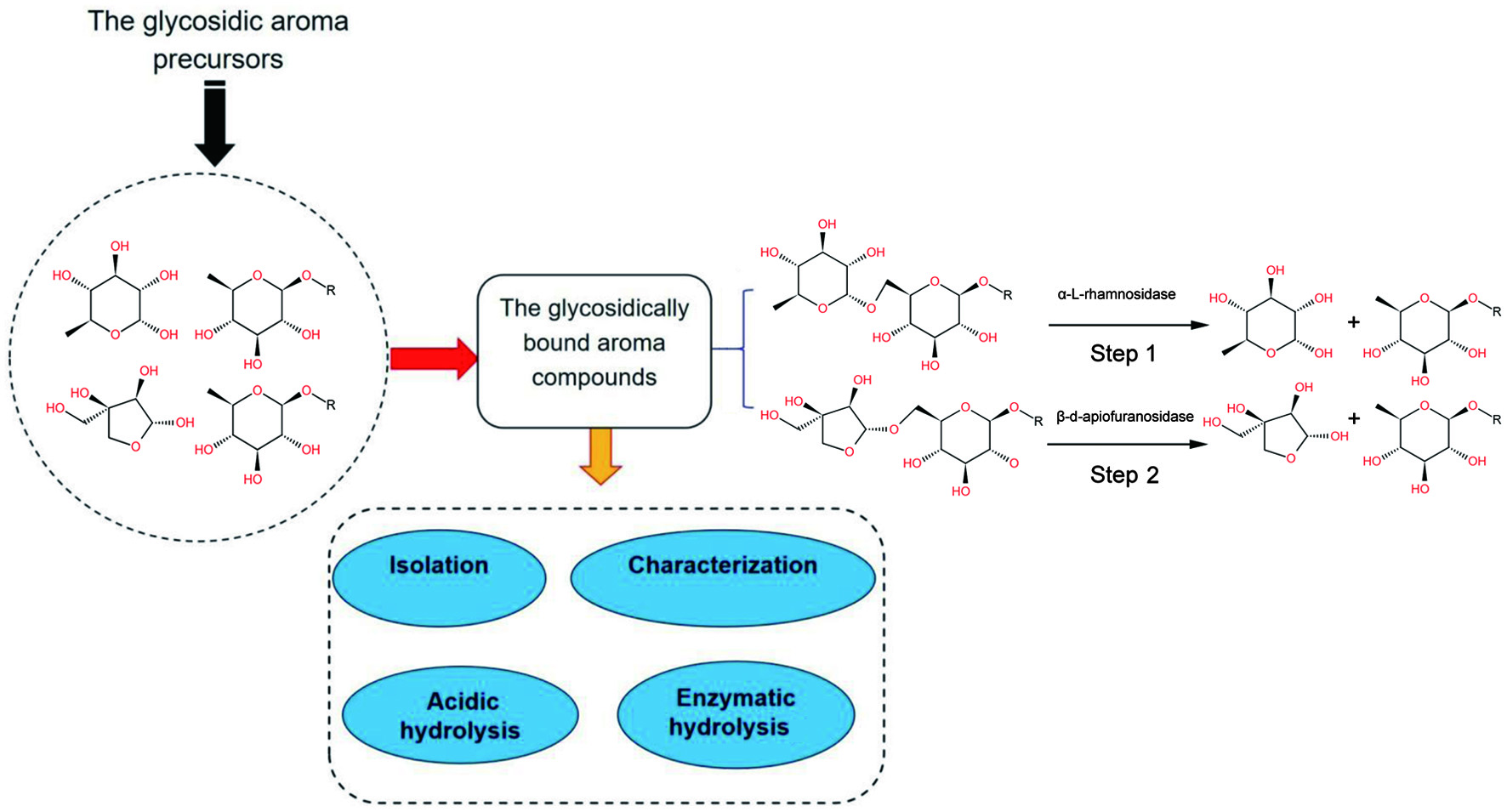 Click for large image | Figure 1. Analysis of glycosidically bound aroma compounds in vegetables and fruits. |
| 2. Occurrence of glycosidically bound aroma compounds in fruits and vegetables | ▴Top |
In fruits and vegetables, many flavor compounds are glycosilated and accumulated as non-volatile and flavorless glycoconjugates. Aroma glycosides have been found and studied in substantial fruits and vegetables, such as celery, pepper, ash gourd, orange, nectarines, mangoes, cherry, mulberry, stawberry, lychees, menthaaquatica, basil and so on. The occurrence of glycosidically bound aroma compounds is typically two to eight times greater than that of their free counterparts (Maicas and Mateo, 2005). Additionally, Aroma glycosides structurally involve an aroma compound (aglycone) and a sugar moiety (glycone) (Liang et al., 2022).
| 3. Isolation of glycosidically bound aroma compounds in fruits and vegetables | ▴Top |
Free and glycosidically bound aroma compounds from Murraya koenigii (L.) Spreng, were isolated and separated by Amberlite XAD-2 column. The fraction containing the free aroma compounds was eluted with pentane: diethyl ether (1:1). Aroma compounds from the bound fraction were released by β-glucosidase hydrolysis. Samples were analyzed by GC and GC-MS. Sixty-seven constituents were found to be present in the bound fraction where linalool was found to be the main constituent. In the free aroma fraction, seventy-eight constituents were present with octyl acetate being found as the main constituent. In the hydrodistilled oil, fifty-six compounds were identified and β-caryophyllene was the prominent compound (Padmakumari, 2008).
Apricot glycosidically bound components separated from the heterosidic pool by silica gel chromatography, gel filtration, and preparative overpressured layer chromatography (OPLC) were suggested by negative ion chemical ionization (NICI) and negative ion desorption chemical ionization (NI-DCI) mass spectrometry (MS) and tandem mass spectrometry (MS-MS). The low-energy collisionally activated (CAD) fragmentation patterns and the use of chromatographic retention data have allowed the identification of linalyl, alpha-terpinyl, neryl, geranyl, and benzyl glucosides. The presence of linalyl arabinoglucoside was established by identification of the glucoside derivative obtained by partial enzymatic hydrolysis. The MS and MS-MS spectra agree with the presence of hexyl glucoside and 2-phenylethyl arabinoglucoside. In the presence of ND3 as reagent in mass spectrometry shifts of 3 mass units were indicative of the presence of linalool oxide glucosides (four isomers detected) and shifts of 4 mass units were characteristic of the four dienediol glucosides isolated. One dienediol arabinoglucoside was also tentatively identified using the same method. These results provide some indications that glucosides are the major glycosidically bound compounds present in apricot (Salles et al., 1991).
In another study, free and glycosidically bound volatiles obtained from the guava leaves (Psidium guajava L.) by liquid-liquid extraction and by chromatography, followed by enzymatic hydrolysis with non-selective pectinase respectively, were analyzed by capillary GC and GC-MS analyses. A total of 39 free volatiles were detected, with the major components being α-copaene, β-caryophyllene, and cadina-1,4-diene. Among the 47 detected glycosidically bound compounds , hexanol, benzyl alcohol, and (E)-3-hexenoic acid were found to be the major constituents. In aroma evaluation, the free volatile fraction showed a guava leaf-like aroma whereas the bound fraction was odorless. However, the characteristic herbaceous-green-fatty aroma was noted in the bound fraction after enzymatic hydrolysis. Upon the combination of the free and hydrolyzed bound fractions, a strongly fresh, guava leaf-like aroma was perceived (García et al., 2009).
Wen et al. (2014) isolation the bound aroma compounds by adsorption on Cleanert PEP-SPE resins. Milos and Radonic (2000) analyzed the essential oils in fresh needles and green and mature berries of Juniperus oxycedrus L. (Cupressaceae) through GC-MS. The glycosidically bound aroma compounds amounted to 21 mg kg−1 in needles and 4 mg kg−1 from green berries. Only traces of aglycones were identified in mature berries. There was no similarity between the glycosidically bound aglycones and the corresponding free compounds found in the essential oil.
Perez et al. (1997) presented the glycosidically bound aroma compounds from two banana cultivars. Glycosides were isolated from aqueous extracts of banana pulp by means of an Amberlite XAD-2 column. Aroma compounds were released by enzymatic hydrolysis using almond β-glucosidase and further analyzed by HRGC/MS. The results showed that 25 main aglycons were reported for the first time in banana fruit pulp. These aglycons could be grouped in two biogenetically different groups: 65 fatty acids and shikimic acid-derived compounds. Alcohols, such as decan-1-ol and 2-phenylethanol, and acids, such as 3-oxo-pentanoic acid, 3-methylbutanoic acid, and benzoic acid, were quantitatively the most important aglycons in glycosides isolated from both banana cultivars.
Menon et al. (2001) demonstrated the free and glycosidically bound aroma compounds from fresh green pepper berries (Piper nigrum L.) by an Amberlite XAD-2 adsorption method followed by hydrolysis with β-glucosidase and GC and GC-MS. The results indicated that the major compounds elucidated in the free fraction were the furanoid form of trans-linalool oxide (18.6%), and α-terpineol (25.6%), while major volatile identified in the bound fraction was 3-buten-2-ol (25.8 %).
Sharma et al. (2010) used steam distillation and high vacuum distillation methods to isolate the free and glycosidically bound aroma compounds in ash gourd. Acetoin was identified as the major constituent existing as glycosidic conjugate.
Tinjan and Jirapakkul (2007) carried out solvent extraction and solid phase extraction methods (with Amberlite XAD-2 resin,) to extract and isolate the free and glycosidically bound aroma compounds in Kaffir lime leaves. Better result was obtained with the solvent extraction method. Fifty-four free and thirty-nine glycosidically bound aroma compounds were obtained by solvent extraction method and their odor descriptions related to kaffir lime leaf odors.
In another study, Özkaya et al. (2018) determined the glycosidically bound aroma compounds from two major tomato cultivars in Turkey. They used solid phase extraction method to extract bound aroma compounds. Among the glycosidically bound compounds, 2-phenylethanol, guaiacol and eugenol were found to be potential contributors to overall tomato flavor upon hydrolysis.
Wang et al. (2017) optimized the headspace solid phase microextraction (HS-SPME) methods for extracting glycosidically bound aroma compounds in raisins. There were 91 compounds identified in the raisins. Moreover, Politeo et al. (2007) isolated the glycosidically bound aroma compounds from basil (Ocimum basilicum L.) using liquid–solid chromatography containing Amberlite XAD-2 as adsorbent.
In addition, Chen et al. (2015) investigated the glycosidically bound aroma compounds in four mulberry cultivars using solid phase extraction (SPE) and headspace solid phase microextraction with gas chromatography-mass spectrometry (HS-SPME-GC-MS). A total of 57 glycosidically bound aroma compounds were identified and quantified. And glycosidically bound aroma compounds were responsible for the sweet and spicy qualities of mulberry. Ghaste et al. (2015) evaluated the glycosidically bound aroma compounds via LC-HRMS in ten grape cultivars. The results showed that 15 glycosylated precursors of volatiles were identified. Sánchez-Palomo et al. (2015) isolated the glycosidically bound aroma compounds using solid phase extraction (SPE) to later be analyzed using gas chromatography-mass spectrometry (GC-MS). Thirty-six bound aroma compounds were identified and quantified in La Mancha Verdejo wines oven this five-year period.
Wang et al. (2015) analyzed the glycosidically bound aroma compounds in air-dried raisins from three seedless grape varieties using headspace solid phase microextraction (HS-SPME) with GC-MS. The main glycosidically bound aroma compounds were benzyl alcohol and acetoin, with β-damascenone contributing most to the glycosidically bound aroma compounds, enhancing the floral, fruity and fatty aroma.
Twenty-two glycosidically bound aroma compounds were identified via GC-MS in the final wines (Yilmaztekin et al., 2015). Solís-Solís et al. (2007) reported the glycosidically bound aroma compounds from eight apricot varieties via simultaneous distillation extraction (SDE), solid phase extraction (SPE) with reverse phase (C18), liquid–liquid extraction (LLE) and HS-SPME.
Kilic et al. (2005) obtained the glycosidically bound aroma compounds in different parts (leaves and buds) of Laurus nobilis L. by Amberlite XAD-2 adsorption and methanol elution. In the leaves most of the glycosidically bound volatiles occur as a-D-glucopyranosides. Among the disaccharides, primeverosides are predominant; smaller amounts of R-L-arabinofuranosyl-a-D-glucopyranosides, rutinosides, and vicianocides could also be identified.
In addition, aroma compounds were extracted by solid-phase extraction and analyzed by gas chromatography-mass spectrometry. The results showed that geranyl acetate, neryl acetate, citronellyl acetate, and menthyl acetate were formed from the corresponding terpene alcohols (Slaghenaufi et al., 2020).
Generally speaking, the conventional Amberlite XAD-2 adsorption resin method has some shortcomings such as time-consuming and labor-intensive steps and extended concentration processes. Nevertheless, HS-SPME-GC-MS is relatively new modern technique, which with solvent-free sample preparation. The isolation methods of glycosidically bound aroma compounds are indicated in Table 1.
 Click to view | Table 1. The isolation methods of glycosidically bound aroma compounds in fruits and vegetables |
| 4. Characterization of glycosidically of bound aroma compounds | ▴Top |
Recently, there has been interest in more rapid and direct way for characterization and analysis of glycosidically bound aroma compounds. The volatiles of celery (Apium graveolens L.) were separated into glycosidically bound and free fractions by Amberlite XAD-2 column chromatography combined with direct solvent extraction. The most abundant volatiles were phthalides and psoralens. These are the major aroma compounds found in both the glycosidically bound and free fractions. A total of 34 compounds was identified, including 6 alcohols, 9 terpene and sesquiterpene hydrocarbons, 2 ketones, 7 phthalides, and 5 psoralens. Ten of these have not been previously reported (Tang et al., 1990).
The glycosidically bound aroma compounds (mainly 12 aglycones) from fresh leaves of Cupressus arizonica Greene var. glauca Woodal, Cupressaceae, were determined by GC/MS (Sánchez-Palomo et al., 2015). The main aglycone was 3-phenylprop-2-en-1-ol. Sharma et al. (2010) used GC-olfactometry (GC-O) and odour activity value (OAV) methods to characterize the active aroma compounds in ash gourd. The results indicated that s-acetoin (9,522.5), octanal (8,571) and nonanal (24,000) were identified.
In another study, Liu et al. (2018b) characterized 111 bound aroma compounds of six currant cultivars grown in China via SPME-GC-MS. They compared with reference standards and matching mass spectrum in the NST11 library. An approach to characterize glycosidically bound precursors of monoterpenoids, norisoprenoids, volatile phenols, aliphatic alcohols, and sesquiterpenoids was investigated in grapes. Monoterpene-triol, monoterpene-tetraol, and sesquiterpenol glycosides were tentatively identified for the first time in grapes, and a C6-alcohol trisaccharide was tentatively identified for the first time in any plant. These findings showed that comparison of glycosylated aroma molecules in Riesling and Muscat of Alexandria grapes showed that the two varieties were distinguishable based on relative abundances of shared glycosides and the presence of glycosides unique to a single variety (Caffrey et al., 2020).
Furthermore, Bahena-Garrido et al. (2019) developed the comprehensive profiles of the glycosidically bound aroma compounds and phenolic compounds in koshu berries. The results indicated that koshu berries had 4-vinyl guaiacol and eugenol. koshu berries containe a distinctly different terpenoid composition.
| 5. Hydrolysis of glycosidically bound aroma compounds | ▴Top |
The hydrolysis of glycosidically bound aroma compounds has been associated with the structure of glycone. They can be hydrolyzed to release free aroma compounds through endo- and/or exo-glucosidase, while their biosynthesis refers to glycosylation process using glycosyltransferases (GTs) (Liang et al., 2022). Moreover, the hydrolysis of glycosidically bound aroma compounds can be considered a contribution to aroma enhancement, immobilization, genetic engineering and biosynthesis and other industrial applications.
5.1. Acidic hydrolysis of glycosidically bound aroma compounds
Milos and Mastelić (1998) revealed acidic hydrolysis of the glycosidic extract from the leaves of Cupressus arizonica Greene var. glauca Woodal. The results showed that acidic hydrolysis additionally released an amount of aglycones, about 8 mg/kg of plant material.
In addition, Ubeda et al. (2012) investigated acidic hydrolysis of the glycosidically bound aroma compounds from four strawberry varieties. The greater the duration of acid hydrolysis, the higher was the content of norisoprenoids, volatile phenols, benzenes, lactones, furaneol, and mesifurane.
5.2. Enzymatic hydrolysis of glycosidically bound aroma compounds
In addition to acidic hydrolysis of glycosidically bound aroma compounds, enzymatic hydrolysis is effective and does not lead to modification of the aromatic composition of the bound fraction (Bisotto et al., 2015).
It is well known that pectolytic enzyme has glycosidase side activity. This commercial enzyme also yielded secondary glycosidase-type activities: β-glucosidase, arabinosidase, rhamnosidase and apiosidase, so that it can hydrolyse most glycosides of aroma compounds including O-β-D-glucosides and O-diglycosides (Baek and Cadwallader, 1999). The volatile substances obtained after enzymatic hydrolysis of purified polar extracts from the aerial parts of the two subspecies of Cedronella canariensis were shown to be, among others, benzyl alcohol (29.4%), chavicol (11.6%), cis-3-hexenol (9.3%), 2-phenylethanol (8.6%), cis-pinocarveol (5.1%), myrtenol (2.2%), 1-phenylethanol (2.0%, tentatively), 1-octen-3-ol (1.8%), 1-hexanol (1.1%) for ssp. canariensis and chavicol (85.1%), benzyl alcohol (2.5%), cis-3-hexenol (2.5%), 1-octen-3-ol (2.3%); 1-hexanol (0.8%) for ssp. anisata. Sugars detected in the polar fraction after hydrolysis were glucose, rhamnose and fructose. The main glycosides obtained from the polar fraction before hydrolysis were chavicol glucoside (6 ppm, ssp. anisata) and benzyl alcohol glucoside (2 ppm, ssp. canariensis) (Coen et al., 1995).
Milos and Mastelić (1998) carried out enzymatic hydrolysis of the glycosidic extract from the leaves of Cupressus arizonica Greene var. glauca Woodal using β-glucosidase. The liberated volatiles amounted to 75 mg/kg of plant material. Liu et al. (2018a) released the bound volatiles compounds by pectolytic enzyme (AR2000 enzyme). Ubeda et al. (2012) evaluated enzymatic hydrolysis of the glycosidically bound aroma compounds from four strawberry varieties. Eight hundred microlitre of a pectinase enzyme solution with 200 mg/mL of AR 2000 was used. A total of 38 aglycones have been described for the first time in strawberry.
Bloem et al. (2008) demonstrated the glycosidically bound aroma compounds from oak wood using Oenococcus oeni. The results indicated that strains selected with high activities toward glycoside substrates could hydrolyze vanillin glycoside precursors from oak wood with the same efficiency as commercial enzymes. It is a promising method to understand their possible role in the metabolic pathway of plant.
Kilic et al. (2005) analyzed the glycosidically bound aroma compounds in different parts (leaves and buds) of Laurus nobilis L. after enzymatic hydrolysis by GC-MS or directly after trifluoroacetyl (TFA) derivatization by GC-MS in EI and NCI mode.
5.3. Others
Recent studies have emerged that a new, simple, environmentally friendly, fast and effective method-ultrasound hydrolysis to liberate the glycosidically bound aroma compounds in orange juice. The results showed that more types of aglycones were released under ultrasound than enzymatic hydrolysis. Alcohols and esters were the principal aglycones under enzymatic hydrolysis, and terpenoids, esters and aldehydes were the main aglycones under ultrasound hydrolysis (Sun et al., 2020).
| 6. Biological activities of the aroma compounds | ▴Top |
According to some reports, aroma compounds exhibited many biological activities, such as antioxidant, antimicrobial, alpha-glucosidase inhibitory, tyrosinase inhibitory and anti-inflammatory activities. The biological activities of aroma compounds are partly due to the presence of phenolic acid and flavonoid. In one study, some glycosidically bound aroma compounds from Bosnian Pine, had remarkable antioxidant properties, such as free radical scavenging activities (Maric et al., 2007). Moreover, the phenolic compounds and PPO activity of aroma properties from sugarcane juice reduced during thermal processing. This research provided a theoretical basis for future studies on the aroma quality control and parameter optimization during the processing of fruit and vegetable juice (Wang et al., 2020).
In another research, Matebie et al. (2019) evaluated the antimicrobial activities of aromas from Phytolacca dodecandra using microdilution method against Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Candida albicans. However, no obvious antimicrobial activity was observed. Rjeibi et al. (2017) investigated Pittosporum tobira seeds essential oils showed significant antioxidant activity in DPPH (IC50 value = 1.5 mg/mL), H2O2 scavenging assay (IC50 value = 159.43 μg/mL) and reducing power test (IC50 value = 0.982 mg/mL). The the biological activities of glycosidically bound aroma compounds are shown in Table 2.
 Click to view | Table 2. The the biological activities of glycosidically bound aroma compounds in fruits and vegetables |
| 7. Conclusions and future prospectives | ▴Top |
Since volatiles can be released from glycosidically bound aroma compounds, these compounds can be considered as a deep possible source of volatile substances and may contribute to the biological activity of plant. Some hidden possible substances from glycosidically bound aroma compounds play a crucial role in fruits and vegetables. It is critical to optimize flavor attributes using glycosidically bound aroma compounds in fruits and vegetables. The challenges and prospects of glycosidically bound aroma compounds will be future discussed. Future research should be focused on glycoside structures to improve understanding of glycosidic hydrolysis mechanisms and enhance ability to quantify volatile aroma glycosides.
Acknowledgments
This research was supported by National Natural Science Foundation of China (31570374) and Key Agricultural Common Technology Project (18227105D) from Hebei Provincial Science and Technology Department.
Conflict of interest
Authors declare that there is no conflict of interest.
Baojiang He wrote this manuscript. Bin Du and Zilong Ma designed this manuscript. Jufang Hao and Fengmei Zhu contributed to literature searching, article selections and edited this manuscript.
| References | ▴Top |