| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 2, June 2018, pages 91-97
5-Demethylnobiletin more potently inhibits colon cancer cell growth than nobiletin in vitro and in vivo
Yi-Siou Chioua, Yu-Nu Zhengb, Mei-Ling Tsaib, Ching-Shu Laib, Chi-Tang Hoc, Min-Hsiung Pana, d, e, *
aInstitute of Food Science and Technology, National Taiwan University, Taipei 10617, Taiwan
bDepartment of Seafood Science, National Kaohsiung Marine University, Kaohsiung 811, Taiwan
cDepartment of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
dDepartment of Medical Research, China Medical University Hospital, China Medical University, Taichung 40402, Taiwan
eDepartment of Health and Nutrition Biotechnology, Asia University, Taichung, Taiwan
*Corresponding author: Min-Hsiung Pan, Institute of Food Science and Technology, National Taiwan University, No. 1, Section 4, Roosevelt Road, Taipei 10617, Taiwan
DOI: 10.31665/JFB.2018.2143
Received: January 15, 2018
Revised received & accepted: February 19, 2018
| Abstract | ▴Top |
Nobiletin (NOB) and 5-demethylnobiletin (DMNB) are unique polymethoxyflavones (PMFs) found in citrus peel that exhibit anti-tumoral action in several cancer cell models. The differences between NOB and DMNB with respect to their anti-proliferative potencies and underlying molecular mechanism were compared in this contribution. The results of the cell viability assay suggested that DMNB resulted in more enhanced growth inhibitory effects than NOB in human colon cancer cell lines (HCT-116, HT-29 and COLO 205). Flow cytometry data found that DMNB inhibited proliferation in COLO 205 cells by predominantly inducing apoptosis. A xenograft mouse model further demonstrated that DMNB exhibited more preferential anti-colon cancer effects than NOB via its ability to induce p53-regulated cell death signaling (apoptosis and autophagy) and inhibit key cellular markers associated with inflammation and angiogenesis. Taken together, our findings provide evidence for the first time that natural bioactive DMNB might serve as a promising polymethoxyflavone for chemoprevention of colorectal cancer.
Keywords: 5-Demethylnobiletin; Nobiletin; Colorectal cancer; p53; Apoptosis; Autophagy
| 1. Introduction | ▴Top |
Colorectal cancer (CRC) is the third most common malignancy and has been ranked the second driving reason for death from cancer among both men and women (Jemal et al., 2010). Although the dissemination of screening tests has led to a decline in CRC incidence and mortality over the last two decades (Simon, 2016), changes in some modifiable factors such as eating habits and lifestyle patterns have been positively or negatively associated with the risk of CRC development (Tuan and Chen, 2016). Furthermore, the current treatment modalities including surgery, chemotherapy and radiotherapy for colon cancer are limited due to resistance, undesirable side effects and complications, finally leading to therapeutic failure (Denlinger and Barsevick, 2009; Hu et al., 2016). Therefore, there is an urgent need for effective approaches and safer medicines to overcome this public health challenge and improve the quality of life of patients.
Recently, epidemiological and experimental evidence has demonstrated that a diet containing high amounts of fat, red meat and refined sugar appear to increase CRC risk appreciably. Conversely, consumption of dietary phytochemicals from vegetables, fruits and spices has the potential to prevent CRC (Li et al., 2015). Therefore, the use of dietary bioactive compounds either alone or in combination to prevent or treat cancer has received much attention due to their safety record and green status . Polymethoxyflavones (PMFs) belong to the family of unique flavonoids that specifically exist in citrus peels such as sweet oranges (Citrus sinensis), mandarin oranges (Citrus reticulate) and miaray mandarin (Citrus miaray) (Li et al., 2006; Uckoo et al., 2015). The compound 5-demethylnobiletin (DMNB) is an autohydrolysis product of nobiletin (NOB) during storage (Zheng et al., 2013), and both are naturally occurring bioactive constituents of PMFs (Wang et al., 2007). Current studies have shown that NOB enhances the effects of cancer cell growth inhibition in various cancer cell lines such as those of gastric, colorectal, lung and breast (Luo et al., 2008; Morley et al., 2007; Moon and Cho, 2016; Moon et al., 2013; Yoshimizu et al., 2004; Chen et al., 2014; Sp et al., 2017). DMNB has been reported to have rather strong pharmacological properties including anti-atherogenic (Yen et al., 2011), neurotrophic (Chiu et al., 2013), anti-obesity (Tung et al., 2016), anti-lung cancer (Chen et al., 2015; Song et al., 2016; Song et al., 2017) efficacies. Thus, it has been implied that both NOB and DMNB may act as effective and diverse cytostatic anticancer agents.
The NOB and its colonic metabolites have excellent chemopreventive activity in a colitis-associated colon carcinogenesis mouse model as well as in human colon cancer cell models (Morley et al., 2007; Kawabata et al., 2005; Wu et al., 2017; Wu et al., 2015). However, no information is available on the effects of DMNB on colon cancer cell growth inhibition. More importantly, we have previously found that dietary administration of hydroxylated PMFs produces a stronger chemopreventive effect in azoxymethane (AOM)-induced colonic tumorigenesis (Lai et al., 2011). However, the effects of bioactive hydroxylated PMFs on colon cancer cell growth inhibition have not been systematically elucidated. Therefore, the present study aimed to compare both in vitro and in vivo anti-proliferative effects of NOB and DMNB, and elucidate the underlying molecular mechanisms.
To the best of our knowledge, this work is the first study to demonstrate that DMNB exerts higher anti-colon cancer efficacies than NOB, which is related to its proapoptotic, pro-death autophagy, anti-inflammatory, and anti-angiogenetic activities. These findings support the use of DMNB in colorectal cancer chemoprevention and chemotherapy.
| 2. Materials and methods | ▴Top |
2.1. Chemicals and reagents
The compound NOB was purchased from Quality Phytochemicals LLC (Edison, NJ, USA), while DMNB was synthesized and obtained as a pale yellow crystal solid through acid hydrolysis from NOB as described previously (Zheng et al., 2013). Antibodies against poly-ADP-ribose polymerases (PARP), p53, vascular endothelial growth factor (VEGF) and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti- cyclooxygenase-2 (COX-2) was purchased from BD Transduction Laboratories (Lexington, KY, USA). Anti- light chain 3 (LC3) was purchased from MBL International (San Diego, CA, USA). All other chemicals used in this study were of the highest purity and were commercially available.
2.2. Cell culture
Human colon cancer cells were purchased from the American Type Culture Collection (Rockville, MD, USA) and maintained in 90% RPMI-1640 medium and 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA), supplemented with 2 mM glutamine (Gibco BRL), 1% penicillin/streptomycin (100 U of penicillin/mL and 100 μg streptomycin/mL) in a humidified atmosphere containing 5% CO2 at 37 °C.
2.3. Cell viability assay
Cell viability was determined for indicated compounds (NOB and DMNB) based on the classical MTT assay. Briefly, human colon cancer HCT-116, HT-29 and COLO 205 cells were plated at a density of 1 × 105 cells/mL into 96-well plates. After overnight growth, cells were treated with a series of concentrations of NOB and DMNB for 24 h. The final concentrations of dimethyl sulfoxide (DMSO) in the culture medium were <0.05%. The viability percentage was calculated based on the percentage of unstained cells, as described previously (Pan et al., 2007).
2.4. Apoptosis detection
COLO 205 cells 1 × 105 cells/mL were cultured in 24-well plates and incubated overnight. The cells were treated with various concentrations (5, 10, and 20 μM) of NOB and DMNB for 24 h. The cells were then harvested, washed with PBS, resuspended in 200 μL of phosphate-buffered saline (PBS), and fixed in 800 μL of iced 100% ethanol at −20 °C. After being left to stand overnight, the cell pellets were collected by centrifugation, resuspended in 500 μL of hypotonic buffer (0.5% Triton X-100 in PBS) and 0.5 μg/mL RNase), and incubated at 37 °C for 15 min. Subsequently, propidium iodide (PI) solution (10 μg/mL) was added, and the mixture was allowed to stand at 37 °C for 15 min. Fluorescence emitted from the PI-DNA complex was quantified after excitation of the fluorescent dye by FACScan cytometry (Becton Dickinson, San Jose, CA, USA).
2.5. Xenograft model establishment
Five-week-old male BALB/cAn.Cg-Foxnlnu/Crlnarl mice were purchased from the BioLASCO Experimental Animal Center (Taiwan Co., Ltd, Taipei, Taiwan). All animal experimental protocols used in this study were approved by Institutional Animal Care and Use Committee of the National Kaohsiung Marine University (IAC UC, NKMU) and maintained in pathogen-free sterile isolators according to institutional guidelines and all food, water, caging and bedding were sterilized before use. To assess the efficacy of NOB and DMNB against tumor growth, COLO 205 cells (5 × 106 cell/100 μL) were subcutaneously injected into the ventral flank of each nude mouse. When the diameter of all xenograft tumors reached 100 mm3, the model was considered successfully established and treatment was initiated.
2.6. Animal grouping and treatment
Tumor-bearing mice were randomized into four groups (n = 6) and injected intraperitoneally (ip) daily with DMSO solvent, NOB 100 mg/kg and DMNB 50 and 100 mg/kg, respectively, for 21 days. At the beginning of treatment (day 0), the body weight and tumor volume were measured once every three days. The maximal diameter of the tumor (a) and the short diameter (b) were calculated with a digital caliper. The tumor volume was estimated by using the formula: volume = ½ × a × b2. On day 21, all nude mice were sacrificed and the tumors were excised and then weighed using an electronic balance. During the administration, the activity and diet were also observed.
2.7. Western blotting
Following experimental treatments, all tumors were harvested and lysed with lysis buffer [10% glycerol, 1% Triton X-100, 1 mM Na3VO4, 1 mM ethylene glycol tetraacetic acid (EGTA), 10 mM NaF, 1 mM Na4P2O7, 20 mM Tris buffer (pH7.9), 100 μM β-glycerophosphate, 137 mM NaCl, and 5 mM ethylenediaminetetraacetic acid (EDTA)] containing 1 Protease Inhibitor Cocktail Tablet (Roche, Indianapolis, IN, USA) on ice for 1 h, followed by centrifugation at 17,500 × g for 30 min at 4 °C. Total proteins were extracted as previously described (Chiou et al., 2013). For immunoblot analysis, equal amounts of lysates (50 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrically transferred to a polyvinylidene difluoride (PVDF) membrane (Merck Millipore, Darmstadt, Germany). After blocking, the membrane was incubated overnight with specific primary antibodies at 4 °C, washed three times with PBS-tween 20 (PBST), and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 1h. The membrane was then exposed to X-ray film (FUJIFILM, Super RX, Düsseldorf, Germany) (Eastman Kodak Co., Rochester, NY, USA) and the protein bands were visualized using ECL chemiluminescence reagents (Amersham, Arlington Heights, IL, USA).
2.8. Statistical analysis
All data were repeated in a minimum of three separate experiments, and the data were presented as the mean ± standard deviation (SD) or standard error (SE). SigmaPlot 10.0 software was used for illustration and analysis. P values were calculated using Student’s t-test or one-way ANOVA followed by Duncan’s multiple-range comparison. A P value < 0.05 was considered statistically significant.
| 3. Results and discussion | ▴Top |
3.1. DMNB showed stronger cytostatic effects than NOB on colon cancer cells
Despite significant interest in the health-promoting effects of PMFs such as tangeretin (TAN) and NOB, hydroxylated PMFs have been demonstrated to exhibit more potent biological activities than their permethoxylated counterpart PMFs (Sergeev et al., 2007; Charoensinphon et al., 2013; Ma et al., 2014; Kimura et al., 2013). Our previous study has also shown that hydroxylated PMFs cause much stronger growth inhibitory effects on colon cancer cells (Qiu et al., 2010). Here, we therefore investigated the structure–activity relationships between NOB and DMNB on three human colon cancer cell lines: HCT-116, HT-29 and COLO205. The structures of both PMFs is illustrated in Fig. 1A. The MTT assay was performed to examine the influence of NOB and DMNB on cell viability. Fig. 1B illustrates that DMNB significantly reduced cell survival on three human colon cancer cell lines at high concentrations (5-40 μM) in the following order: COLO205>HCT-116>HT-29, but NOB showed little effect at the same concentrations. However, at 40 μM, NOB had a markedly pronounced cytotoxicity, but this effect was lower than that of DMNB treatment (Fig. 1B). On the other hand, DMNB may be more effective than NOB against colon cancer, indicating the pivotal role of the 5′-hydroxyl group on the A-ring moiety of natural PMFs for enhanced anti-proliferative activity.
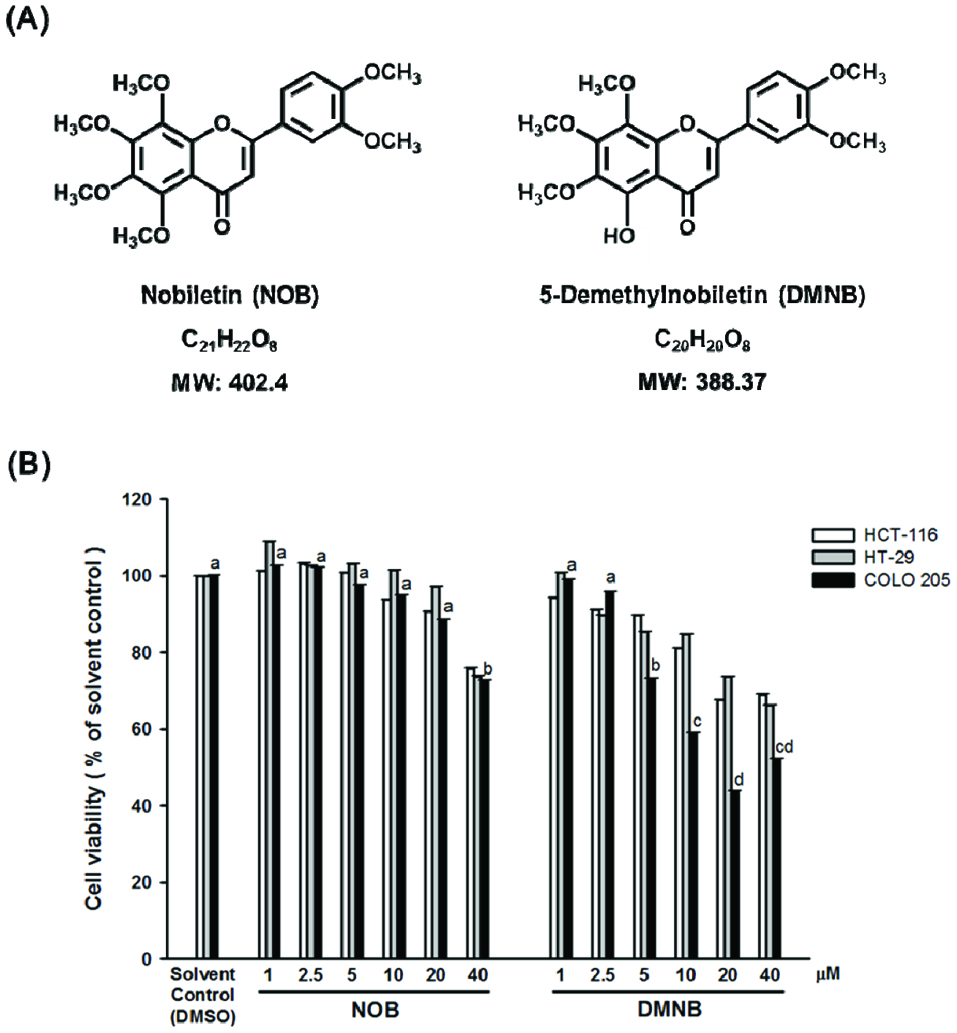 Click for large image | Figure 1. Effect of NOB and DMNB on the growth of HCT-116, HT-29 and COLO 205 cells. |
3.2. DMNB caused cell death through induction of pro-apoptotic signaling
Programmed cell death (PCD), referred to as apoptosis, is a homeostatic mechanism to maintain cellular balance and genome integrity via removing aging or unhealthy cells (Ouyang et al., 2012). It is generally believed that inappropriate apoptosis or uncontrolled cell proliferation may be an important factor in cancer development. Furthermore, evasion of apoptosis is a fundamentally important factor to serve both tumor progression and chemo-resistance in CRC (Abraha and Ketema, 2016). Thus, targeting the apoptotic pathway is considered vital in colorectal cancer therapies. Previously, DMNB has been reported to exert an anti-lung cancer effect with its induction of tumor cell cycle arrest, apoptosis and autophagy (Song et al., 2017; Chen et al., 2015; Charoensinphon et al., 2013). By applying the same rationale, we wanted to determine whether the DMNB-induced growth inhibition was caused by the induction of apoptosis.
As Figure 2A illustrates, when COLO 205 cells were treated with DMNB at a high concentration (20 μM) for 24 h, the characteristics of apoptosis such as shrunken, apoptotic bodies and cell detachment were clearly visible. However, these morphological changes were absent from the control and NOB-treated cells (Figure 2A). Flow cytometry analysis demonstrated a significant apoptosis ratio (∼26%) induced by DMNB, while NOB treatment at any concentration was not observed (Figure 2B). In addition, we also adopted immunoblotting to confirm that DMNB significantly induced apoptosis in a dose-dependent manner (Figure 2C), with application of 10 and 20 μM DMNB resulting in cleavage of PARP (cPARP), which is the hallmark of apoptosis. These data indicate that pro-apoptotic activity is critical for enhancing DMNB-induced cell death up to a level of 50%.
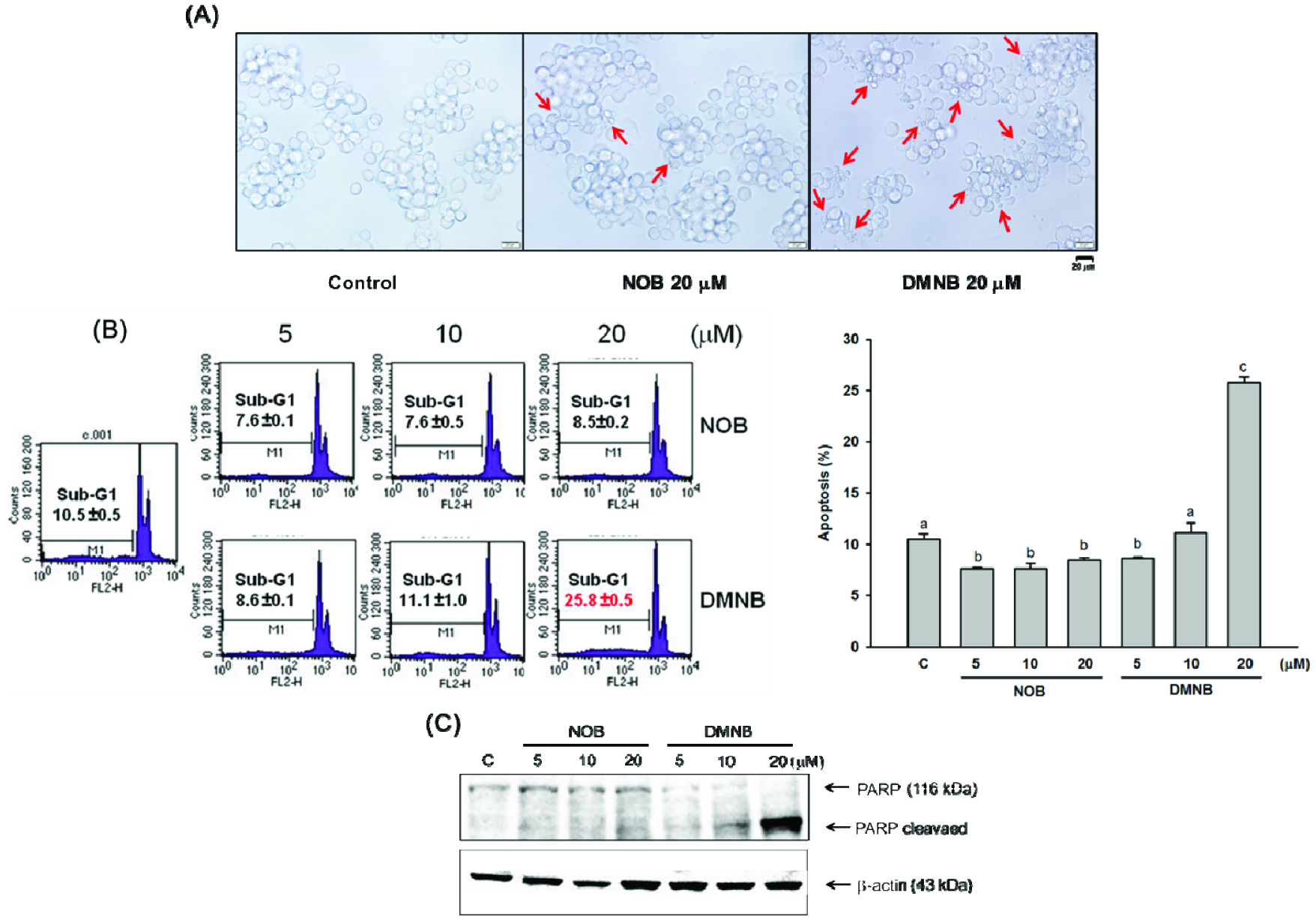 Click for large image | Figure 2. Apoptotic effects of NOB and DMNB on COLO 205 cells. |
3.3. DMNB had a higher potential than NOB as a chemotherapeutic agent against colon cancer
Clinical chemotherapeutic drugs show many toxic side effects, which places significant limitations on their clinical application. In contrast, healthy dietary patterns used for cancer risk reduction have been approved. The naturally occurring constituents of the human diet have been intensively valued in developing new medicines and functional foods for the cancer chemoprevention field. To further confirm that DNMB-induced anticancer activities are better than NOB, we established a colorectal cancer xenograft mouse model and determined its effects on tumor growth and the related molecular mechanisms. Over the treatment period, the body weight of nude mice in each group did not differ and no signs of toxicity were observed, suggesting that long-term treatment of both PMFs did not cause any noticeable side effects (Figure 3A). In contrast, the tumor growth inhibition was most evident in mice treated with 50 and 100 mg/kg of DMNB via i.p injection. As shown in Figure 3B, the tumor volumes in the 50 and 100 mg/kg DNMB groups were stabilized in a steady state starting from Day 12, and were much slower than the positive or NOB-treated groups. In addition, the eventual tumor size and tumor weight of the DMNB-treated group were also much smaller than the control (P < 0.05) at the end of the experiment (Figure 3C and 3D).
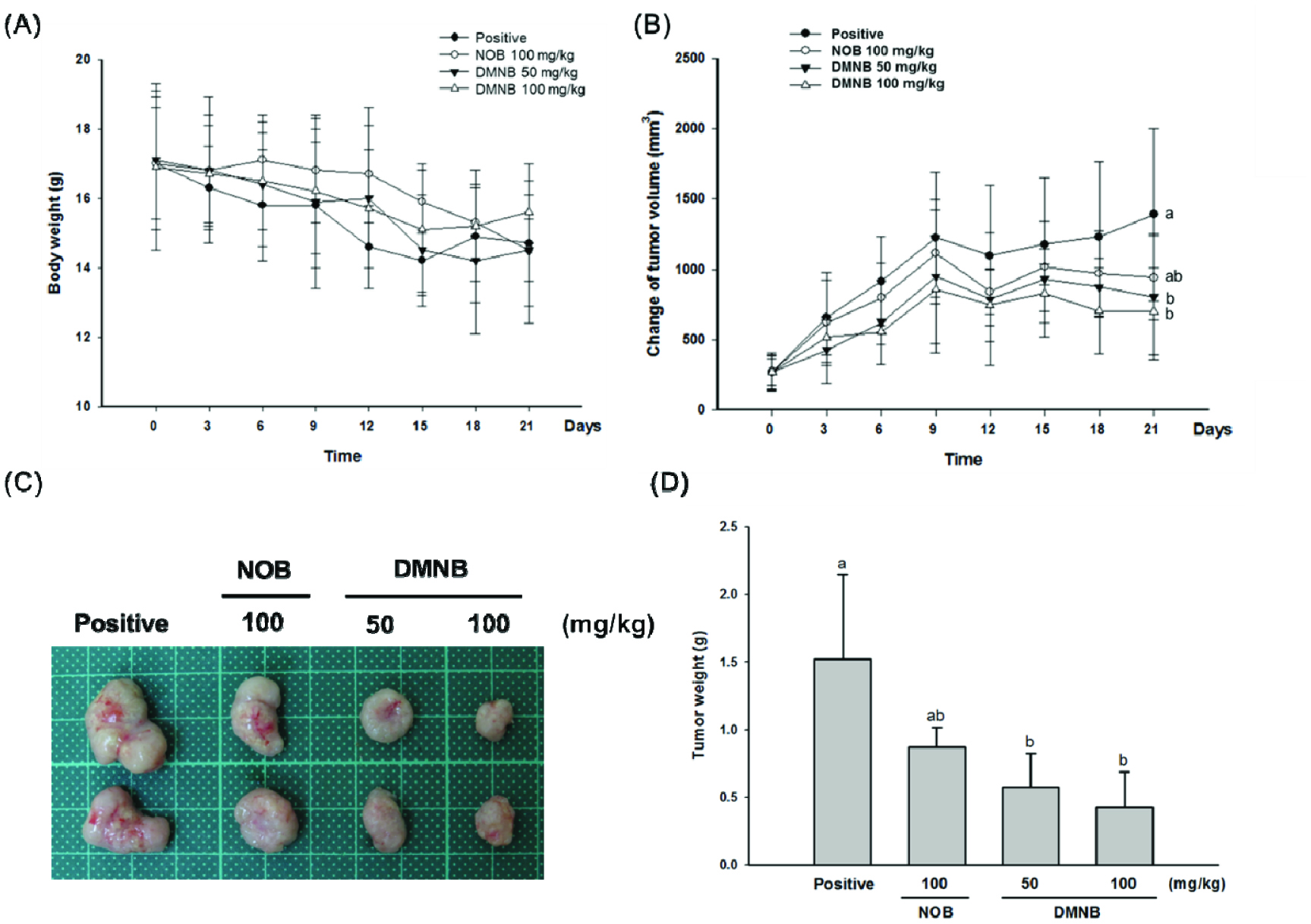 Click for large image | Figure 3. DMNB effectively inhibits tumor growth of COLO 205 tumor xenografts in nude mice. |
To validate our in vitro results, we also detected apoptosis markers. As shown in Figure 4A, after treatment with 50 and 100 mg/kg of DMNB, the ratio of cleaved-PARP was increased in tumor tissues. Previously, DMNB-induced autophagy was found to be associated with mitigated apoptosis (Chen et al., 2015), which exemplifies the importance of autophagy signaling in cell death. Inconsistent with the results of the previous study, the expression of LC3, which is a key regulator involved in the formation of the autophagosome, was significantly increased (Figure 4A), suggesting that DMNB-induced cell death was closely correlated with the pro-death function of autophagy, but predominantly through apoptosis.
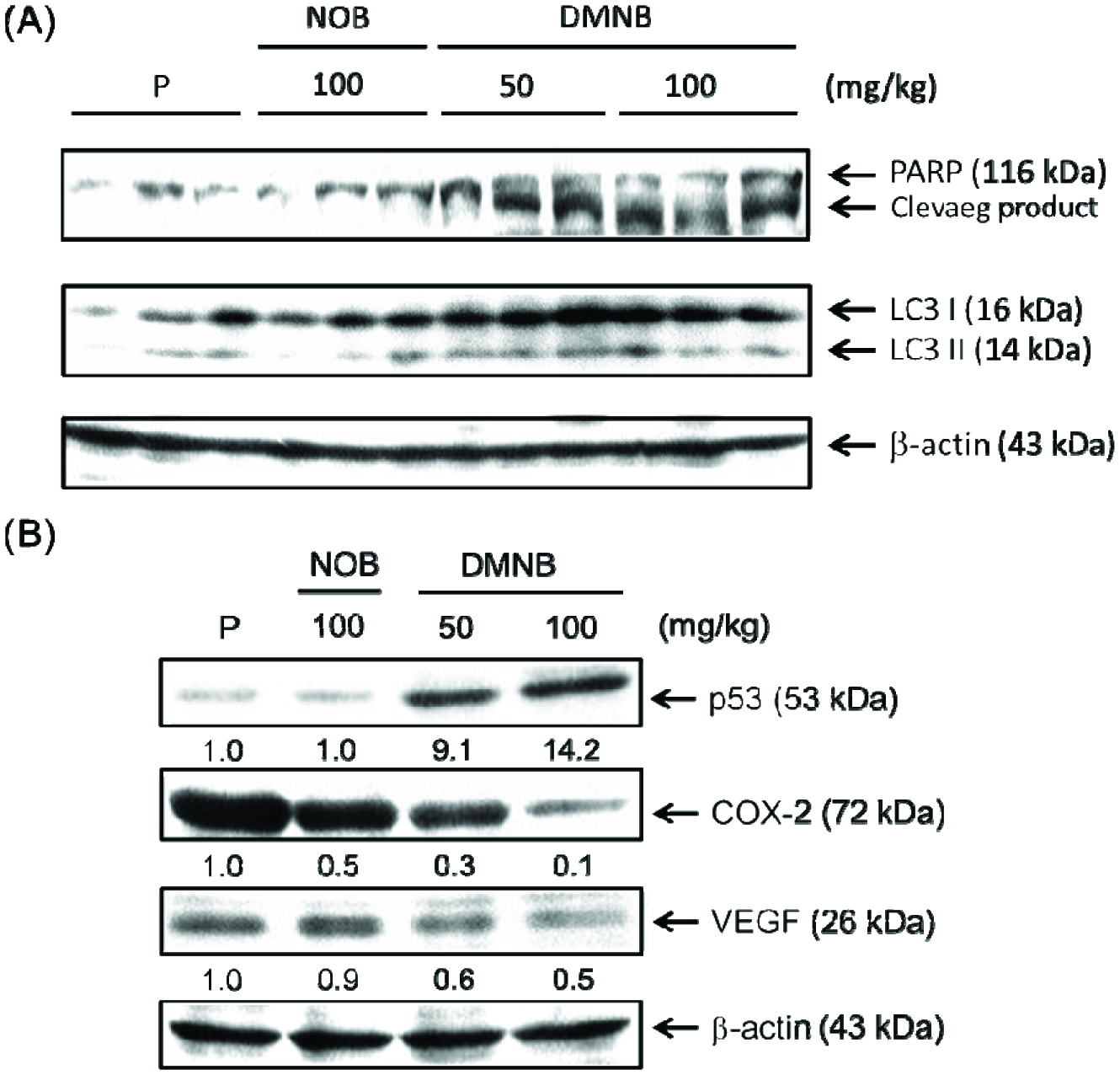 Click for large image | Figure 4. Effects of DMNB on pro-death, inflammation and angiogenesis signaling pathways in tumor tissue. |
The p53 signaling pathways are considered to be an interconnected network for regulation of apoptosis and autophagy (Nikoletopoulou et al., 2013). Owing to the multiplicity of its function, mutation or dysregulation of p53 is tightly associated with tumor progression. In order to characterize the involvement of p53 signaling in DMNB-induced cell death, we detected the p53 protein level. As shown in Figure 4B, promising results were obtained from western blotting and confirmed that p53 up-regulation by DNMB could actually provoke the pro-apoptotic and pro-death autophagy-targeted genes, a novel finding.
To further explore how DNMB exerted its antitumor effects by interfering with pro-inflammatory and pro-angiogenetic pathways, we also detected the expression of COX-2 and VEGF after DMNB treatment. As shown in Fig. 4B, tumor-related increases of COX-2 and VEGF expression were markedly impeded in the presence of DMNB, and these effects were also stronger than that of NOB. The results suggest that the anti-proliferative effect of DMNB was accompanied by inducing pro-death signaling (apoptosis and autophagy), anti-inflammation and anti-angiogenesis (Figure 5). Thus, these findings demonstrate that DMNB had higher potential than NOB as a useful approach for colon cancer therapeutics.
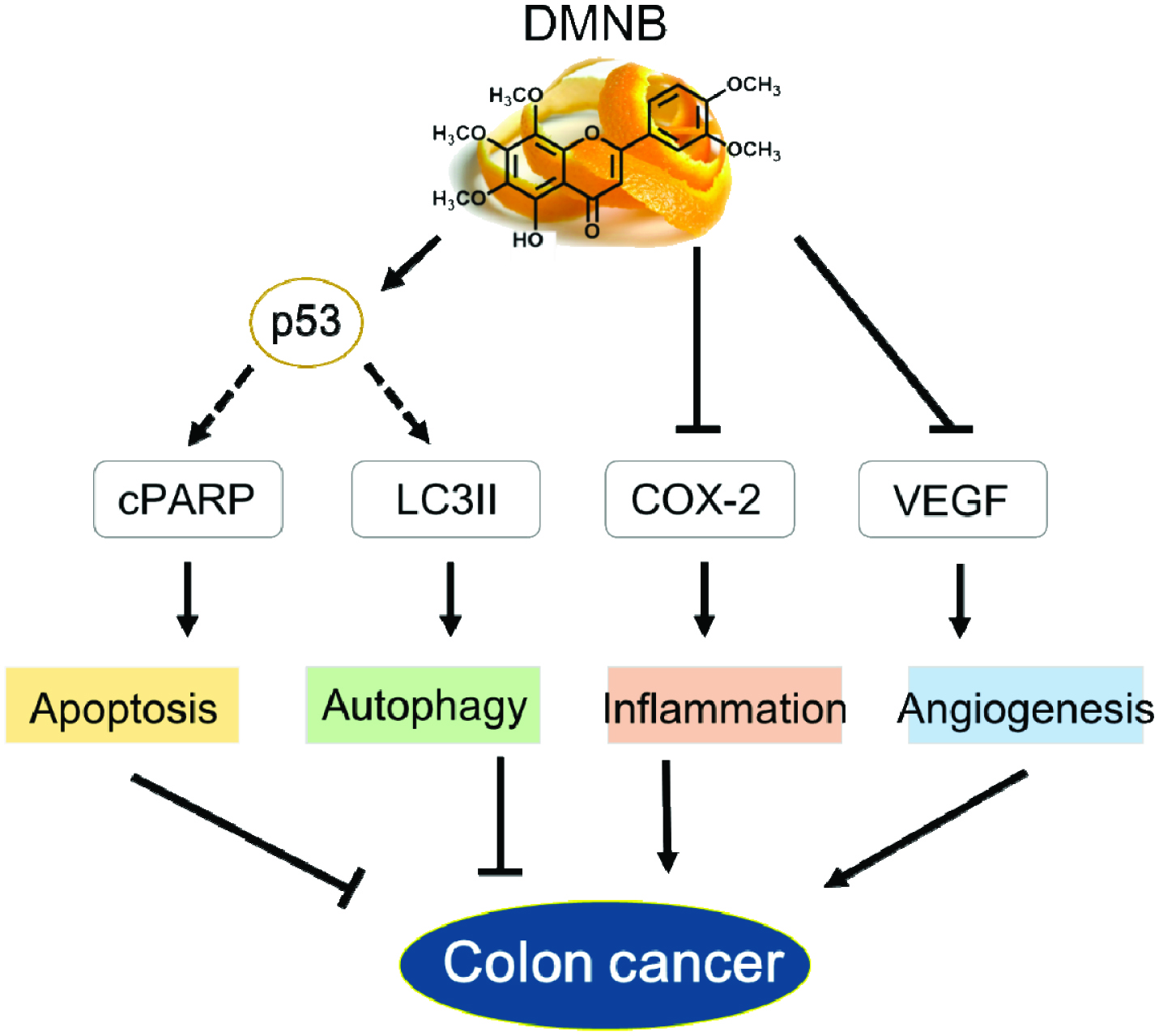 Click for large image | Figure 5. Schematic diagram showing the mechanisms underlying the anti-colon cancer effects of DMNB. |
| 4. Conclusion | ▴Top |
This is the first report showing that the naturally hydroxylated PMF, DMNB, holds much higher potential promise for colon cancer treatment than NOB in both in vitro and in vivo studies, an effect that is significantly mediated by its pro-apoptotic and pro-death autophagy properties. More importantly, we found for the first time that specifically increasing p53 by DMNB might be involved in cell death signaling. This strongly implies that DMNB may have potential as an ideal therapeutic agent, particularly for colorectal cancer patients with wild-type p53 expression. Furthermore, results presented here also reveal that DMNB produced anti-inflammatory and anti-angiogenetic efficacies which play a synergistic role in further enhancing the growth inhibition induced by DMNB. Overall, these findings provide a powerful theoretical and experimental basis for developing DMNB as a more effective and safer agent and functional food for colon cancer prevention and therapy.
Acknowledgments
This study was supported by the Ministry of Science and Technology [105-2320-B-002-031-MY3, 105-2628-B-002-003-MY3].
Conflict of interest
The authors declare no conflict of interest.
| References | ▴Top |