| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 16, December 2021, pages 75-82
Antioxidant properties of camelina (Camelina sativa (L.) Crantz) protein hydrolysates
Na Thi Ty Ngo, Fereidoon Shahidi*
Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1C 5S7
*Corresponding author: Fereidoon Shahidi, Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1C 5S7. E-mail: fshahidi@mun.ca
DOI: 10.31665/JFB.2021.16295
Received: December 22, 2021
Revised received & accepted: December 31, 2021
| Abstract | ▴Top |
Camelina seed meal was used to produce protein hydrolysates using Alcalase and Flavourzyme. The hydrolysates were then fractionated by employing ultrafiltration membranes (3, 10 kDa). The antioxidant activities of camelina protein hydrolysates and peptide fractions were investigated. The essential amino acid content of camelina protein isolates and hydrolysates was comparable and adequate. All camelina hydrolysates exhibited the highest radical scavenging activity in both DPPH and ABTS assay compared to camelina protein isolates. When comparing the overall DPPH and ABTS radical scavenging activity of peptide fractions, smaller-size peptides (<3 kDa) displayed considerably higher values and hence more potency than larger-sized peptides (>3 kDa). Peptide fractions with 3–10 kDa had better metal chelation and reducing power than those <3 kDa and >10 kDa. These findings suggest that camelina protein hydrolysates could be employed as bioactive ingredients in the formulation of functional foods and against oxidative stress.
Keywords: Camelina meal; Protein hydrolysates; Peptide fractions; Antioxidant activity
| 1. Introduction | ▴Top |
Proteins are macronutrients in food that provide energy and amino acids required for proper human growth and maintenance. Food proteins have a wide range of physicochemical and sensory properties. Protein hydrolysates, containing bioactive peptides, have recently been demonstrated to have a variety of biological functions in addition to their known nutritional benefits. Interests in studying protein hydrolysates and bioactive peptides has intensified in the recent past, due to their multitude of beneficial effects as functional food ingredients, nutraceuticals, or as medicinals (Ambigaipalan et al., 2015; Senadheera et al., 2021).
Several food from animals, plants, and marine products, have been used as sources of bioactive peptides. Production of such products is generally carried out in pursuit of value-added use of underutilized proteins or protein-rich food industry’s by-products, and utilization of proteins containing specific peptide sequences or amino acid residues of pharmacological interest (Udenigwe and Aluko, 2012). Seed meals and cakes produced from important oilseed crops are attractive co-products due to their high content of protein and presence of bioactive compounds. Therefore, these could be considered as valuable plant-derived feedstocks for food and non-food applications. In this respect, camelina (Camelina sativa (L.) Crantz) meal is gaining attention as an ingredient in functional foods and cosmetic additives in the health sector (Tavarini et al., 2021).
Protein hydrolysates are produced by the cleavage of peptide bonds that can be achieved by enzymatic or chemical processes. Using enzymes to produce protein hydrolysates has gained much attention as a potential method of making functional food ingredients and nutraceuticals for disease prevention and health promotion (Alashi et al., 2014; Ambigaipalan et al., 2015; Senadheera et al., 2021). Generally, an acceptable food protein source must be chosen based on the physiological properties of products and then treated with a single or multiple specific or nonspecific protease to release peptides of interest. The type of enzyme determines the cleavage pattern of peptide bonds. The principal enzymes employed for peptide production are endo- and exo-proteinases such as trypsin, chymotrypsin, and pepsin, as well as commercial proteases such as Alcalase and Flavourzyme. Because of their favorable operating conditions, microbial proteases such as Alcalase and Flavourzyme are used in industrial manufacturing (Kristinsson and Rasco, 2000; Udenigwe and Aluko, 2012).
Several studies on the production of hydrolysates from various protein sources including plants and their by-products have been performed such as using hemp seed (Girgih et al., 2011), rapeseed (He et al., 2013), canola meal (Alashi et al., 2014), kidney bean (Mundi and Aluko, 2014), date seed (Ambigaipalan et al., 2015), and mung bean (Carlos et al., 2017), among others. This research line has shown that hydrolysates and/or peptides reduce oxidative stress and improve health as well as being able to render certain functional properties to food. More importantly, because of their nutritional and biological qualities, active protein hydrolysates and/or peptides can be used as functional food ingredients (Girgih et al., 2011).
Camelina, an ancient oilseed, is also known as Gold-of-pleasure or false flax. It belongs to the Brassicaceae family and is cultivated for its oil, mainly in Europe and in North America. Camelina is a new potential oilseed and a novel source of plant protein (Ngo and Shahidi, 2021; Tavarini et al., 2021). Although camelina meal is rich in protein (40–45%), its current use is mainly limited to animal and aquaculture feeds (Boyle et al., 2018; Hixson et al., 2016). Furthermore, the nutritional quality of camelina protein is similar to that of canola protein and competes with soy protein for some applications targeting the use of plant proteins (Li et al, 2015).
Compared to its use as an animal feed ingredient, however, camelina meal could be used to manufacture bioactive products with value-added benefits. Some previous studies have focused on using camelina meals to produce protein isolates with the aim to improve food formulation or supplying nutritional benefits (Boyle et al., 2018; Li et al., 2014; Ngo and Shahidi, 2021). Research on the production and the characterization of camelina protein is limited. No study has so far been carried out to produce hydrolysates and bioactive peptides and determine antioxidant properties of camelina protein hydrolysates. To the best of our knowledge, this is the first report on using camelina seeds to manufacture protein hydrolysates using Alcalase and Flavourzyme and investigate their antioxidant potential.
| 2. Materials and methods | ▴Top |
2.1. Materials
Camelina seeds were used in this study. Camelina seeds were obtained from Linnaeus Plant Sciences Inc, Saskatoon, SK, Canada. Alcalase (2.4 AU/g) and Flavourzyme (1,000 LAPU/g) were procured from Novozymes, Bagsvaerd, Denmark. All chemicals used were obtained from Fisher Scientific Ltd. (Ottawa, ON, Canada) or Sigma-Aldrich Canada Ltd (Oakville, ON, Canada).
2.2. Methods
2.2.1. Preparation of camelina protein isolates (CPI).
Camelina protein isolates were prepared from defatted camelina meals according to the method described by Ngo and Shahidi (2021). The hexane-defatted samples were suspended in distilled water (DW) (3.0%, w/v). The slurry was stirred with a magnetic stirrer for 30 min and the pH was then adjusted to12 by the addition of a known amount of 2 M NaOH. Subsequently, the suspended sample was placed in a Vevor Digital Ultrasonic bath (Model JPS-30A, China, 180 W, 40 kHz, 20 min) to extract the proteins. Under similar conditions, the residues were re-extracted two more times with DW. The supernatants were combined, and the pH was adjusted to 4.5 with the addition of 2 M HCl and then centrifuged at 10,000 × g for 30 min at 4 °C to precipitate the protein. The pellets were collected and then washed twice with DW. The precipitated protein was redispersed in DW and the pH was adjusted to 7.0 with 1 M NaOH. The extracted proteins were freeze-dried and stored at −20 °C for further analyses.
2.2.2. Preparation of camelina protein hydrolysates and membrane fractions
Hydrolysis of camelina protein isolates was conducted with Alcalase (0.3 AU/g isolates) and Flavourzyme (50 LAPU/g isolates) under different conditions (Ambigaipalan et al., 2015; Senadheera et al., 2021). The samples were hydrolysed batchwise with Alcalase (AL, pH 8, 50 °C) for 4 h, or Flavourzyme (FL, pH 7, 50 °C) for 4 h. Enzyme combination treatments were carried out by first hydrolysing with Alcalase for 2 h (pH 8, 50 °C), and then adding Flavourzyme for 2 h (pH 7, 50 °C). The pH of the reaction mixture was kept constant throughout the process by adding 4 M NaOH. After digestion, the reactions were terminated by heating the mixture at 90 °C for 10 min to inactivate the enzyme. The hydrolysates were collected by centrifugation at 10,000 g for 15 min. A portion of the collected supernatants was freeze-dried and stored at −20 °C as camelina protein hydrolysate, while the remaining portion was passed through ultrafiltration membranes (Amicon Ultra) with molecular weight cut-off (MWCO) of 3 and 10 kDa. The ultrafiltration was conducted by first passing the hydrolysate through the 3 kDa membrane. The resulting retentate was then passed through the 10 kDa membrane. The permeate from each MWCO membrane was separated into three peptide fractions as <3, 3–10, and >10 kDa. All permeates were freeze–dried and kept at −20 °C in a freezer until further analysis.
2.2.3. ABTS radical cation scavenging assay
The radical scavenging activity of protein hydrolysates was evaluated using the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonate) radical cation (ABTS•+) method reported by John and Shahidi (2010) with minor modifications. The samples and reagents were prepared in 0.1 M phosphate buffer (PBS) containing 0.15 M sodium chloride (pH 7.4). ABTS•+ solution was prepared by mixing 2.5 mM 2,2′-azobis-(2-methylpropionamidine) dihydrochloride (AAPH) with 2.5 mM ABTS stock solution at 1:1 (v/v) ratio. The mixture was heated to 60 °C for 20 min before being stored at room temperature in the dark. The samples (40 μL,1 mg/mL) were combined with the ABTS•+ solution (1.96 mL) and allowed to react for 6 min before reading the absorbance at 734 nm. Similarly, a blank was prepared by replacing the sample with distilled water. A standard curve was built by using different concentrations of Trolox (0–1,000 μM). The decrease in the absorbance at 734 nm after 6 min of the addition of a test compound was used in calculating the results. ABTS radical scavenging activity, represented as µmol of Trolox equivalents (TE) per milligram (mg) of protein hydrolysates, was calculated using the following equation:
2.2.4. DPPH radical scavenging assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of camelina protein hydrolysates and its peptide fractions was determined using a previously described method (Aluko and Monu, 2003; Chandrasekara and Shahidi, 2011) with slight modifications. Protein hydrolysates solutions (0.5 mg/mL) were prepared in 0.1 M sodium phosphate buffer, pH 7.0, while DPPH (0.3 mM) was prepared in methanol. The samples (50 µL) were mixed with DPPH (250 µL) and placed into a 96-well microplate. The mixture was allowed to stand in the dark at 25 °C for 30 min. Phosphate buffer (pH 7.0) was used as the control. The control (Ac) and samples (As) absorbance values were measured at 517 nm. Trolox was used to preparing the standard curve (50–500 μM). DPPH radical scavenging capacity, expressed as µmol of Trolox equivalents (TE) per milligram (mg) of a protein hydrolysate, was calculated using the following equation:
2.2.5. Metal chelation activity
Ferrous ion chelating activity was determined according to a previously reported method (Ambigaipalan et al., 2015). Camelina protein hydrolysates (0.5 mg/ml) were prepared in distilled water. The samples (200 μL) were mixed with 1.74 mL of distilled water, 20 μL of FeCl2 (2 mM), and 40 μL of ferrozine (5 mM). The mixture was incubated at room temperature for 10 min, and the absorbance of the solution was read at 562 nm. Distilled water was used as the control. A standard curve was constructed using trisodium salt of ethylenediaminetetraacetic acid (Na3EDTA). Metal ion chelating ability (%) was calculated using the following equation:
2.2.6. Reducing power
The reducing power of camelina protein hydrolysates was evaluated according to the method described by Cumby et al. (2008) and Ambigaipalan et al. (2015). Camelina protein hydrolysates (0.5 mg/mL) were dissolved in phosphate buffer (0.2 M, pH 6.6). The samples (1 mL) were added 2.5 mL of 1% potassium ferricyanide solution, and the mixture was incubated at 50 °C for 20 min. After that, 10% trichloroacetic acid (2.5 mL) was added to the mixture, which was centrifuged for 10 min at 1,000 g. After centrifugation, 2.5 mL of supernatant was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1% ferric chloride solution. The solution’s absorbance was read at 700 nm after being allowed to react for 10 min. The control had no hydrolysates added, whereas the blank had only protein hydrolysates. A standard curve was constructed using different concentrations (0–1,000 μM) of Trolox, and the reducing power was expressed as µmol of Trolox equivalents (TE) per milligram (mg) of protein.
2.2.7. Hydroxyl radical scavenging activity
The hydroxyl radical scavenging capacity was determined using an EPR spectrometric (Bruker E-scan, Bruker Biospin Co., Billericia, MA, USA) method as described by Chandrasekara and Shahidi (2011), with slight modifications. The camelina protein hydrolysates were dissolved in deionized water. The sample (200 μL) was mixed with 10 mM H2O2 (200 μL), 17.6 mM 5,5-dimethyl-1-pyrroline N-oxide (DMPO; 400 μL) and 10 mM FeSO4 (200 μL). After 3 min at room temperature, the mixture was injected into the sample cavity of the EPR spectrometer, and their spectra recorded. Deionized water was used as the control. The EPR spectra were recorded, and Trolox (0–50 μM) was used to prepare the standard curve. The hydroxyl radical scavenging capacity, expressed as µmol of histidine or carnosine equivalents per milligram (mg) of a protein hydrolysate, was calculated using the following equation:
2.2.8. Amino acid composition
The amino acid composition of each protein hydrolysates was analysed at the Analytics, Robotics and Chemical Biology Centre (SPARC BioCentre), the Hospital for Sick Children, Toronto, ON, Canada, as previously reported (Mohan and Udenigwe, 2015). Except for tryptophan, cysteine, and methionine, all other amino acids were analyzed using vapor-phase hydrolysis with 6 M HCl and 1% phenol at 110 °C for 24 hours. Samples were hydrolyzed with 4.2 M NaOH for 24 hours at 110 °C for tryptophan analysis. Before hydrolysis, cysteine was quantified using performic acid oxidation. Norleucine (25 μmol/mL) was used as an internal standard. Following hydrolysis, samples were dried and resuspended in a solution of methanol/water/triethylamine (2:2:1, v/v/v) and vacuum dried for 15 min. This was followed by pre-column derivatization with a derivatizing solution containing methanol/water/triethylamine/phenyl isothiocyanate (PITC) (7:1:1:1, v/v/v). Vacuum-dried derivatized samples were dissolved in sample diluent. Waters ACQUITY UPLC (Milford, MA, USA) was used to analyze diluent aliquots using an UPLC BEH C18 column (0.21 × 10 cm) operated on a modified PICO-TAG gradient at 48 °C. The amino acids were measured using a UV detector at 254 nm.
2.3. Statistical analysis
All experiments were conducted in triplicates and data were reported as mean ± standard deviation. One-way ANOVA was performed, and means were compared by using Tukey’s HSD test (p < 0.05), SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA).
| 3. Results and discussion | ▴Top |
3.1. ABTS radical cation scavenging assay
The Trolox equivalent antioxidant capacity (TEAC) is used to assess the electron donating potential of antioxidants using the ABTS•+ scavenging activity. The blue/green ABTS•+ chromophore is produced by the chemical reaction between ABTS and the strong oxidizing agent potassium persulfate, with maximum absorption at 734 nm (Shahidi and Yeo, 2020). This assay is commonly used to evaluate the antioxidant capacity of bioactive compounds (Shahidi and Zhong, 2015).
The ABTS radical scavenging activity of protein hydrolysates and their peptide fractions obtained from camelina protein isolate (CPI) showed significantly higher values (p < 0.05) when compared to that of camelina protein isolates (Figure 1).
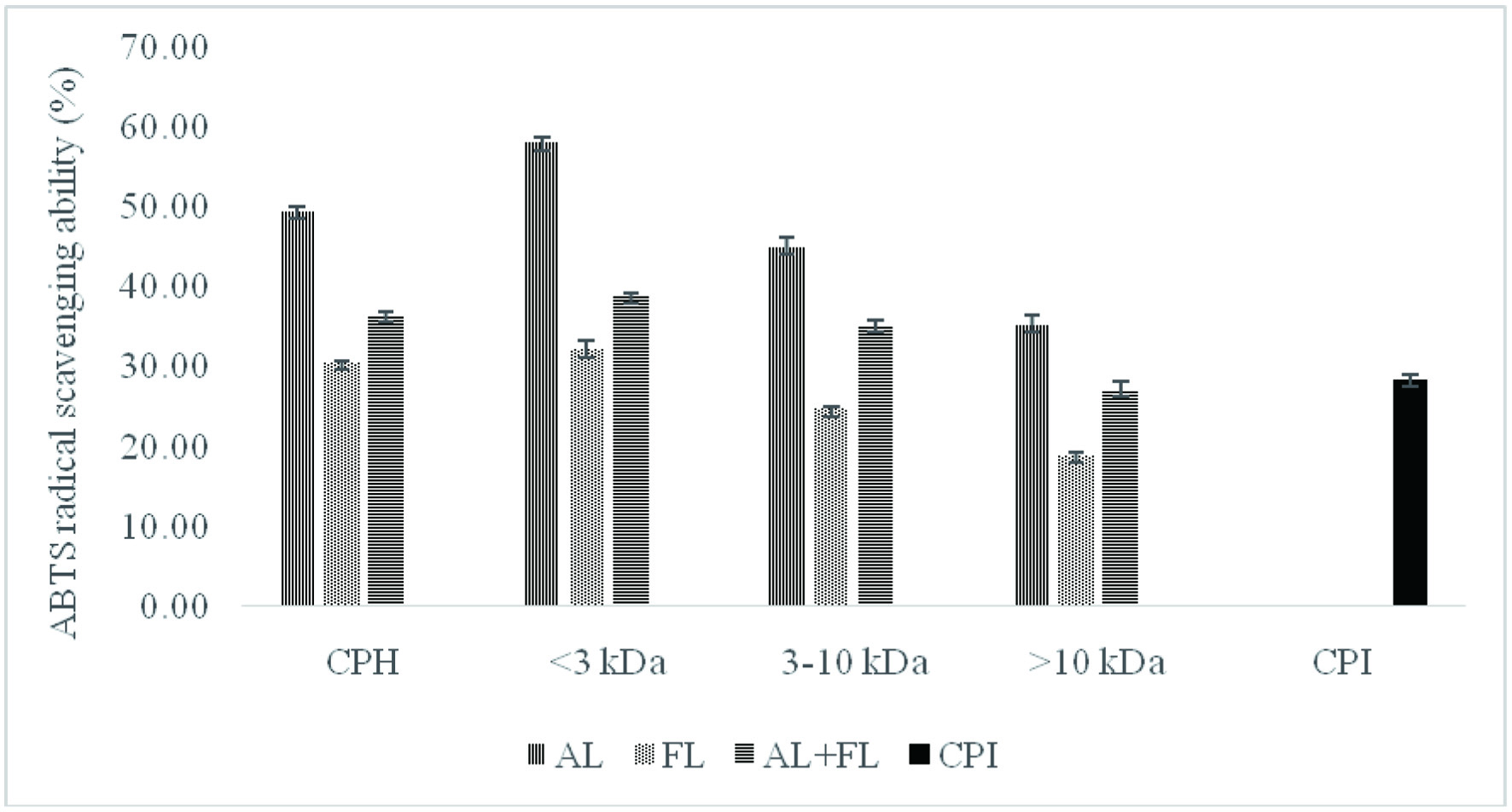 Click for large image | Figure 1. ABTS radical scavenging activity of camelina protein isolates (CPI), hydrolysates (CPH) and their peptide fractions prepared by using enzymatic treatment (AL, Alcalase; FL, Flavourzyme; and AL + FL, Mixture of Alcalase and Flavourzyme). |
The considerable difference between the unhydrolyzed and hydrolyzed samples suggests that antioxidant peptides were released from CPI during enzyme digestion, and that these peptides can donate hydrogen atoms to reduce ABTS•+ (Alashi et al., 2014). Hydrolysates prepared using Flavourzyme had the lowest ABTS radical scavenging activity (30.6%), while Alcalase hydrolysates showed the highest values (49.49%). Furthermore, Figure 1 shows that the Alcalase <3 kDa permeate peptide fraction exhibited the highest ABTS radical scavenging activity (50.02%) while the lowest activity (18.94%) was observed for Flavourzyme >10 kDa. When comparing the overall ABTS radical scavenging activity of peptide fractions, smaller-size peptides (<3 kDa) displayed considerably higher values and hence more potency as ABTS•+ scavengers than larger-sized peptides (>3 kDa). The ABTS radical cation scavenging activity of camelina peptide fractions were also similar to those of canola peptide fractions (Alashi et al., 2014) and corn meal (Hu et al., 2020).
These findings indicate that the antioxidant activity of peptides could be influenced by their amino acids sequences, the amount of free amino acids present, the degree of hydrolysis and molecular weight of peptides (Ambigaipalan et al., 2015). Alcalase and Flavourzyme have different hydrolysis processes because Alcalase is an endopeptidase, whereas Flavourzyme has both exo- and endopeptidase activity. As a result, the varying antioxidant activity of different enzyme treatments could be related to differences in their composition (Senadheera et al., 2021). Overall, smaller peptides were found to be more effective antioxidants against ABTS radicals.
3.2. DPPH radical scavenging activity
DPPH radical scavenging is a determination of antioxidative properties of compounds as free radical scavengers or hydrogen atom donors and is widely used in the evaluation of peptide, phenolic and food antioxidant capacity (He et al., 2013; Intarasirisawat et al., 2012). There are many parameters that influence the radical scavenging ability of food proteins and their hydrolysates, including their size and the amino acid composition of the peptides, specificity of the protease and DPPH assay conditions (Girgih et al., 2011). Additionally, hydrophobic groups in the hydrolysates increase the peptides’ solubility in a non-polar environment, thus providing a greater access to DPPH. This promotes peptides to interact with radicals and trap them so that the chain reaction can be terminated (Senadheera et al., 2021).
Figure 2 reveals that the untreated counterparts, CPI showed significantly lower DPPH radical scavenging activities compared to the samples hydrolysed with Alcalase, and Flavourzyme. The study found that camelina protein hydrolysate contains peptides that act as electron donors and free radical scavengers to terminate the chain reaction. These findings lend support to the previously reported study on the radical scavenging activities hydrolysates from various plant proteins (He et al., 2013; Hu et al., 2020; Senadheera et al., 2021). According to the authors, hydrolysates produced by alkaline proteases such Alcalase and Flavourzyme have stronger antioxidant activity than those manufactured with other enzymes as Neutrase, papain, bromelain, and pepsin.
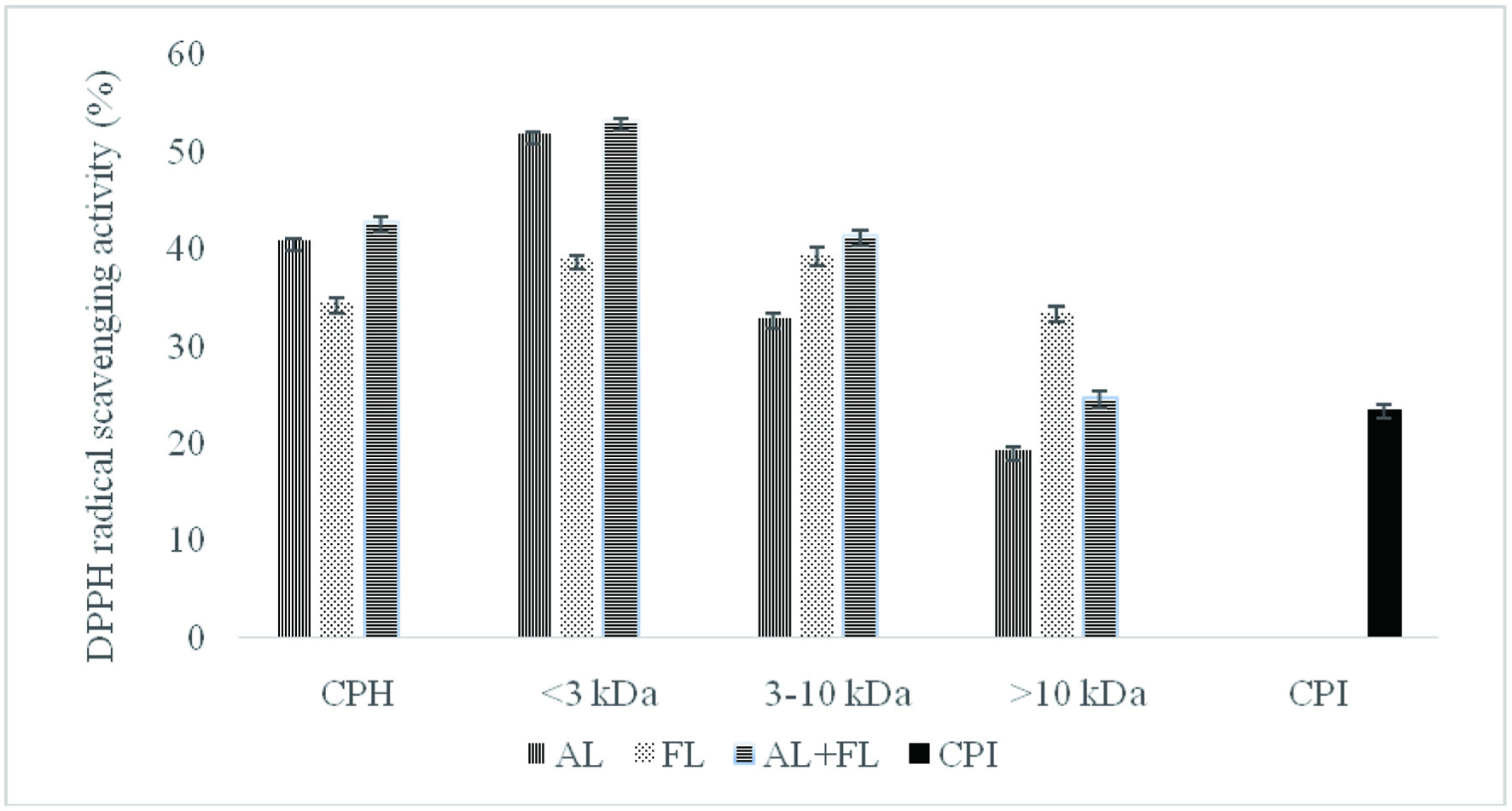 Click for large image | Figure 2. DPPH radical scavenging activity of camelina protein isolates (CPI), hydrolysates (CPH) and their peptide fractions prepared by using enzymatic treatment (AL, Alcalase; FL, Flavourzyme; and AL + FL, Mixture of Alcalase and Flavourzyme). |
The DPPH radical scavenging activity of camelina protein hydrolysates and their peptide fractions (Figure 2) show that hydrolysates prepared with Alcalase, and the combination of Alcalase and Flavourzyme, respectively, were the most active against DPPH, while Flavourzyme CPH was the least active. The DPPH inhibitory activity of <3 kDa fractions was significantly higher than activities of 3–10 and >10 kDa. Some researchers have also reported that the DPPH inhibitory activity of peptide fractions depended on the molecular size (Aluko and Monu, 2003; Girgih et al., 2011; Hu et al., 2020).
Furthermore, differences in amino acid compositions, sequence, and structure could explain the difference in antioxidant activity between all treatments (Ambigaipalan et al., 2015). For instance, Aromatic amino acid residues (Tyr, His, Trp, Phe) can donate hydrogen atoms to electron deficient radicals via resonance stabilization, which improves the radical-scavenging properties of the amino acid residues (Ambigaipalan and Shahidi, 2017). This research indicates that low-molecular-weight peptides have better radical scavenging properties than high-molecular-weight ones, consistent with previous findings for quinoa, hemp seed, and canola protein hydrolysates fractions (Alashi et al., 2014; Aluko and Monu, 2003; Girgih et al., 2011). The trends observed for both ABTS and DPPH radical scavenging activity of the camelina protein hydrolysates and their peptide fractions prepared by using Alcalase followed comparable patterns. The difference between the hydrolysates produced by different enzymes dictates the antioxidant potential of the final products (Cumby et al., 2008). Our research on date camelina protein hydrolysates found that the peptides in the mixture could act as electron donors, inhibiting the radical chain reaction and generating non-radical products.
3.3. Metal chelation activity
Iron, a transition metal, is involved in the Fenton reaction, and stimulates lipid oxidation and hence acting as a prooxidant, which eventually leads to degrading hydroperoxide into volatile compounds (Shahidi and Zhong, 2015). Certain phenolic antioxidants and some peptides can form complexes with transition metal ions, thus retarding the oxidation process. This ability is attributed to the presence of amino acid residues including histidine, cystine, tryptophan, aspartate, and glutamate, which have been shown to bind divalent metal ions when exposed on the surface of proteins and polypeptides (Udenigwe et al., 2016).
Figure 3 showed the Fe2+ chelating effects of the camelina protein hydrolysates and their peptide fractions.
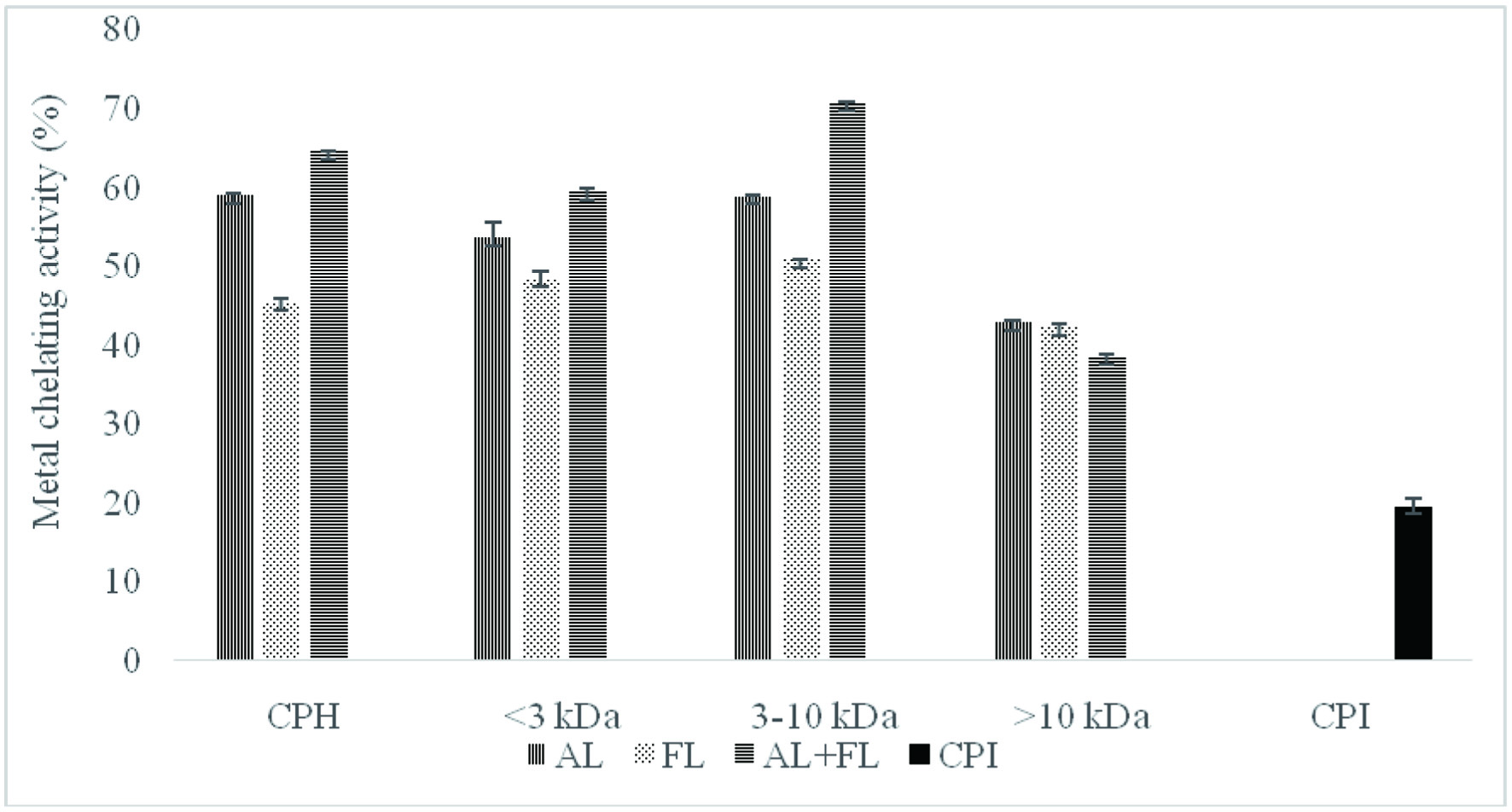 Click for large image | Figure 3. Metal chelating activity of camelina protein isolates (CPI), hydrolysates (CPH) and their peptide fractions prepared by using enzymatic treatment (AL, Alcalase; FL, Flavourzyme; and AL + FL, Mixture of Alcalase and Flavourzyme). |
Clearly, chelation of metal ions by camelina protein hydrolysates treated with the enzyme exhibited significantly higher values than those of their protein isolates. Moreover, ferrous ion chelation of camelina protein hydrolysates varied significantly and followed the order of AL + FL < AL < FL. Interestingly, we observed that the highest metal chelation activity was noted in the 3–10 kDa peptides fraction (70.64%) prepared by the combination of Alcalase and Flavourzyme. The <3 kDa fraction showed low chelating activity compared with the 3–10 kDa peptides fraction. The highest amount of negatively charged amino acids was found in 3–10 kDa fraction in a prior study, which could have contributed to greater electrostatic attraction for positively charged amino acids (Girgih et al., 2011). Moreover, the discrepancies between the samples may be due to differing charged amino acid side chain residues that can remove transition metal ions. Furthermore, due to its imidazole group, the presence of histidine at the N terminal can be linked to a significant metal ion chelation (Ambigaipalan et al., 2015). These findings suggest that peptide cleavages may promote metal ion binding, eliminating prooxidative metal ions from the system due to the increased concentration of carboxylic groups and amino groups in the side chain of acidic and essential amino acids, respectively.
3.4. Reducing power
The ferric reducing antioxidant power assay is widely used to estimate natural antioxidants’ ability to donate an electron or a hydrogen atom, and the compounds exhibiting reducing power can reduce the oxidized intermediates of peroxidation (Ambigaipalan et al., 2015). Some research has indicated a correlation between reducing power and antioxidant activity of protein hydrolysate fractions (Girgih et al., 2011; He et al., 2013).
Table 1 shows the reducing power of camelina protein hydrolysates, and its fractions measured at 700 nm with values ranging from 0.65 ± 0.02 to 1.44 ± 0.01 µmol TE/mg of hydrolysate fraction.
 Click to view | Table 1. Ferric reducing antioxidant power assay (FRAP) of camelina protein hydrolysates and peptide fractions prepared using enzymatic treatment |
The highest reducing power was found in hydrolysates treated with Alcalase. These findings match those of ABTS and DPPH radical scavenging activities. In addtion, a higher reducing power was observed for 3–10 kDa fraction for all the three types of hydrolysates, varying from 1.07 ± 0.02 to 1.44 ± 0.01 μmol TE/mg of proteins. There was an increase in reducing power of the camelina protein fractions with increasing peptide size (3–10 kDa fractions were better than <3 kDa fraction), indicating additive effects of active groups within the peptide molecules. The reducing power of the 3–10 kDa fraction was similar to the trend for the metal chelating effects of the corresponding samples. The camelina protein hydrolysates showed considerably higher values than both the <3 kDa and >10 kDa fractions, implying that the presence of 3–10 kDa peptides contributed to the activity of the camelina protein hydrolysates.
The current results are supported by previous findings that ferric reducing antioxidant power was directly influenced by the type of protease used for hydrolysis (Ambigaipalan et al., 2015; Udenigwe et al., 2016). The presence of hydrophobic amino acids or peptides that can react with free radicals to form more stable products, according to the authors, may be responsible for the differences in the activity. In addition, the presence of amino acids leucine, isoleucine, histidine, methionine, tyrosine, lysine, and tryptophan may have contributed to the reducing power activity observed for protein hydrolysates (Girgih et al., 2011). According to Udenigwe et al. (2016), electron donation by amino acid residues, including the sulfhydryl group of cysteine, also contributes to the reducing capacity of peptides. As a result, the presence of sulfhydryl groups or their oxidized forms directly impacts the reducing ability of protein hydrolysates.
3.5. Hydroxyl radical scavenging activity
Hydroxyl radicals are considered the most reactive free radicals in biological systems, reacting with biomolecules such as amino acids, proteins DNA, and membrane lipids to induce severe damages to cells through oxidative stress (Girgih et al., 2011; Shahidi and Yeo, 2020). As a result, removing excess level hydroxyl radicals is recognized to be one of the most effective defense approaches in the prevention of a variety of cellular diseases, including cancer, cardiovascular disease, and diabetes, among others (Girgih et al., 2011). The hydroxyl radical scavenging activity of camelina protein hydrolysates and their fractions is shown in Table 2 and the values varied between 1.57 and 3.8 μmol histidine equivalents /mg of protein.
 Click to view | Table 2. Hydroxyl radical scavenging of camelina protein hydrolysates and peptide fractions prepared using enzymatic treatment |
Hydrolysates prepared using Alcalase had a higher hydroxyl radical scavenging ability compared to all other enzyme treatments in each group. The hydroxyl radical scavenging activity in terms of percentage ranged from 33 to 59 %. Similar observations were made by Ambigaipalan et al (2015) for hydrolysates prepared from date seed. In addition, Alcalase hydrolysed <3 kDa fraction had the highest value amongst all tested samples. This indicates that that the lower molecular weight peptides had a greater the hydroxyl radical scavenging activity. The camelina seed peptides are effective scavengers of hydroxyl radical when compared to those of the hemp seed (Girgih et al., 2011). According to Cumby et al. (2008), the radical scavenging activity of peptides or protein hydrolysates is related to the hydrogen donor activity of the hydroxyl groups of aromatic amino acid residues (tyrosine, histidine, tryptophan and phenylalanine). Radical scavenging activity of these aromatic amino acid residues improves through resonance stabilization. Thus, the presence or lack of certain amino acids in peptides, as well as their position in the peptide sequence, has an impact on antioxidant activity.
3.6. Amino acid composition
The amino acid composition of protein hydrolysates significantly impacts their antioxidant activity. The presence of Tyr, Met, His, and Lys has been demonstrated to contribute to the potency of antioxidant peptides (Alashi et al., 2014; He et al., 2013). Camelina protein isolates and hydrolysates digested with Alcalase were further analysed for their amino acid profiles. Table 3 shows that the hydrolyzation process had no negative impact on the hydrolysates’ amino acid composition. Such finding lends support to the previous finding on canola meal (Alashi et al., 2014).
 Click to view | Table 3. Amino acid composition of camelina protein isolates and hydrolysates (Alcalase) (g/100g) |
In addition, the amino acid composition of this study correspond with the previous study on the camelina seeds (Russo and Reggiani, 2015). In camelina protein samples, leucine was the most abundant essential amino acid (EAA), while tryptophan, histidine and methionine contributed a relatively low amount. During the analysis procedure, sensitive amino acids such as methionine and tryptophan may be impacted (Senadheera et al., 2021). Moreover, camelina protein hydrolysates’ amino acid profiles satisfy the World Health Organization/Dietary and Agriculture Organization (WHO/FAO) 1991 recommendations for the quantity of most essential amino acids in food products. According to the present work, camelina protein hydrolysates can be employed as a good source of dietary protein.
Table 3 shows that the amino acid composition of camelina protein hydrolysates was rich in Glu, Asp, Arg, and Leu. According to reported studies, negatively charged amino acids such as Glu and Asp have potent antioxidant properties due to being a rich source of electrons that can be donated to free radicals. The high quantities of lysine and leucine found in camelina protein hydrolysates could enhance superoxide radical scavenging (Udenigwe and Aluko, 2011; Zou et al., 2016). Furthermore, hydrophobic amino acids including tyrosine, methionine, histidine, cysteine, and tryptophan can positively impact the antioxidant activity. For instance, tyrosine residue could serve as a potent hydrogen donor, and histidine can be credited with strong radical scavenging activity due to the decomposition of its imidazole ring (Senadheera et al., 2021). Therefore, it has been suggested that the amino acid profile can be used to predict the antioxidant activity of protein hydrolysates and peptides (Udenigwe et al., 2016).
| 4. Conclusion | ▴Top |
The camelina protein hydrolysates and their fractions was found to possess potent antioxidant effects in vitro. Furthermore, they had the ability to scavenge free radicals which was mainly dictated by the peptide size. Thus, low molecular weight peptides had a stronger activity. The <3 kDa peptide fractions were generally the most effective scavengers of free radicals (DPPH, ABTS and hydroxyl radicals). The 3–10 kDa fractions had better metal chelation and ferric reducing power than the <3, and >10 kDa fractions. According to our findings, camelina seeds could be a good source of antioxidant hydrolysates and peptides. Hydrolysates may also serve as a natural value-added component in the formulation of functional foods and nutraceutical, and a good source of dietary protein due to their rich profile of essential amino acids. However, more research is needed to determine the amino acid sequence essential for antioxidant and other biological functions.
Acknowledgments
We are grateful to the Natural Science and Engineering Research Council (NSERC) of Canada for financial support. Ngo thanks support from Vietnam International Education Development for a scholarship.
| References | ▴Top |