| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 16, December 2021, pages 69-74
Formulating a novel fermented soy milk functional meal replacement among overweight individuals: a preliminary weight loss clinical trial
Bin Shen*, Jianbin Liu, Wanyi Cao, Xiaodan Wang
Tianjin Zhongkelanding Natural Medicine Institute, Tianjin 300304, China
*Corresponding author: Bin Shen, Tianjin Zhongkelanding Natural Medicine Institute, Tianjin 300304, China. E-mail: zkld20211115@163.com
DOI: 10.31665/JFB.2021.16294
Received: November 19, 2021
Revised received & accepted: December 31, 2021
| Abstract | ▴Top |
Meal replacement is an optimising strategy in designing structured diets for weight management. In this study, a novel fermented soy-whey based beverage was fortified with dietary bioactive ingredients containing probiotic strains (Lactobacillus bulgaricus and Streptococcus thermophilus). The aim of this study was to evaluate its efficacy on the body weight loss of participants pre-and post-meal replacement. A total of 20 participants underwent the weight loss program. Over the 14-day trial, women volunteers had a body weight loss of 6.70% of their initial body weight, and men had a comparable body weight reduction of 6.18%. In comparison to the blood glucose baseline level of 3.5420 mmol/L, the meal replacement decreased the level of blood glucose by nearly 50%, reaching 1.7785 mmol/L. Total cholesterol level was reduced by the beverage with a 15.7% reduction. The functional drink also decreased the triglycerides and low-density lipoprotein significantly (p < 0.001). A significantly higher level of high-density lipoprotein was obtained at Day 14 (1.73 mmol/L) compared with Day 0 (1.23 mmol/L). The meal replacement was able to provide satiety within the average of 180.7 minutes post-meal. This study supports the soy-based functional milk is of benefit for weight management, glucose homeostasis and blood cholesterol-lowering effect.
Keywords: Meal replacement; Fermented soy milk; Weight loss; Glucose homeostasis; Cholesterol-lowering effect
| 1. Introduction | ▴Top |
Obesity is rising around the world as a result of many factors, including an imbalance in food intake, increased consumption of high-calorie diets, and a lack of physical exercise. Overweight and obesity are highly prevalent among Chinese adults. In a very recent study by Zhang et al. (2020), 441,306 participants were analysed. The prevalence of overweight and obesity were 28.1 and 5.2%, respectively (Zhang et al., 2020). Increasing evidence indicates that obesity has developed into a worldwide public health concern and a considerable cost to the healthcare system. Therefore, the global non-communicable diseases target has been proposed, that controlling obesity and raised glucose are two targets by 2025 with the aim of halt raise (Beaglehole et al., 2014). Obesity is the cause of various chronic diseases such as cardiovascular diseases, type 2 diabetes, cancer, and strokes, which negatively affects longevity and quality of life. Anti-obesogenic approaches which are safe, widely available, and inexpensive are required to combat metabolic diseases.
Current methods of weight loss and control include exercise, surgery, dieting and meal replacement. The failure rate of different weight-loss therapies and the likelihood of not reaching goal weight in obese adults are considered to be high (Jakše et al., 2017). Weight loss therapy must address long-term compliance, diet plan adherence, and weight reduction maintenance. Meal replacement is nutritionally equivalent to a low-calorie normal meal. Due to its simplicity, low cost, and convenience, meal replacement is an important strategy in designing structured diets for weight management and enhancing the long-term effectiveness of weight-loss regimens. Previous studies have shown that substituting a meal replacement results in more weight loss and diet compliance than isocaloric conventional diets (62% vs. 30% at week 40) in a 40-week randomised controlled clinical trial (Davis et al., 2010). In a study of the outpatient weight-loss program by Li et al. (2010), meal replacement supplements were proven to be safe for the liver, kidney, and bone mineral density indicators, even when a greater protein group (2.2 g protein/kg lean body mass) was employed in the 12-month isocaloric meal plan.
Meal replacements may also be utilised to aid with weight reduction and maintenance. Vázquez et al. (2009) reported that meal replacement was effective compared with diet alone in a randomised paralleled clinical trial. Sixty-two obese people with a weight loss of at least 5% of their baseline body weight were randomised to an additional 6 months of calorie restriction (400–500 kcal) or calorie restriction plus meal replacement for dinner. The meal replacement plus diet group lost an extra 3.2 % weight, whereas the diet alone group lost 1.3 percent (Vázquez et al., 2009).
Protein beverages have grown in popularity in recent years as consumers aim to enhance their daily protein intakes(Parker et al., 2018). Many studies have reported soy protein for its effect of reducing cholesterol and triglyceride. In a randomised controlled clinical trial by Allison et al. (2003), the soy-based meal replacement was effective in lowering body weight, fat mass and reducing LDL cholesterol (Allison et al., 2003). A recent study of meta-analysis of randomised controlled trials reported that the incorporation of soy milk into the diet exerted beneficial effects in several cardiometabolic risk factors in both healthy and unhealthy individuals (Sohouli et al., 2021).
In this study, we aim to formulate a novel soy-based fermented milk, fortified with wolfberry and other natural diet ingredients. Considering the fact that the majority of the existing meal replacement products in the market are in a solid form that needs to be brewed prior to consumption, this product was formulated in liquid. The efficacy of the liquid meal replacement for weight loss was evaluated over a 14-day period in overweight and obese adults. Other metabolic indicators, including blood glucose and cholesterol level, were also involved and compared pre-and post-meal, providing evidence of whether the beverage could be used as part of a plan for diets that are designed for weight management and health.
| 2. Materials and methods | ▴Top |
2.1. Materials
The ingredients of the meal replacement are shown in Table 1. Materials include the following components: whole milk powder (Fonterra, New Zealand), skim milk powder (Fonterra, New Zealand), isolate soy protein (DuPont, USA), quinoa flour (Puyang, Guangzhou, China), enzymatically digested oat flour (Biograin, Xiamen, China), resistant dextrin (Roquette, France), Inulin (Cosucra, Belgium), isomaltulose (Haiyi, Hubei, China), isolated whey protein powder (Hilmor, CA, USA), pine nut oil powder (Hongsongbao, Liaoning, China), Luo Han Guo concentrate (Huacheng, Hunan, China), wolfberry fruit powder (Nestle, Switzerland), cactus fruit powder (Nexira, France), donkey collagen peptide powder (Donge, China), okra powder (Nexira, France), green coffee powder (Huike, Shaanxi, China), thickener (Siyou, Hefei, China), Streptococcus thermophilus (DSM), Lactobacillus bulgaricus (DSM), food fortification (Jinkangpu, Beijing, China), flavouring (Essen, Tianjin, China).
 Click to view | Table 1. The composition of meal replacement |
2.2. Preparation of fermented soy milk
The fermented soy milk was prepared as follows. Soy protein isolate, whole milk powder, whey protein isolate, skim milk powder, cactus fruit powder and okra powder were added to the preheated drinking water (65 °C) and sheared for 15 min. After hydration for 30 min, the mixture was homogenised at 60 to 65 °C with a pressure of 20 Mpa. The sterilisation was started by heating to 95 °C in a water bath, holding for 10 min. Streptococcus thermophilus and Lactobacillus bulgaricus were added at 42 °C and fermented for 6.5–7 h at 42 °C. The fermentation was terminated when the pH was below 4.3. The fermented milk was stirred while cooling down to below 20 °C, then homogenised at 22 Mpa and filtered through a 120-mesh sieve.
2.3. Preparation of meal replacement
The stabiliser stock was prepared as follows. The thickener with isomaltulose was mixed and dissolved in water until there were no visible particles. Enzymatic oat powder, quinoa powder, resistant dextrin, pine nut oil powder, wolfberry fruit powder, donkey collagen peptide powder, green coffee powder and inulin were added to the mixture.
The stabiliser stock solution was then mixed with the fermented milk. The Luo Han Guo concentrate was then added, followed by the fortification agents and the edible flavour. The fortification compound includes vitamin A, vitamin D, vitamin E, vitamin B1, vitamin B2, vitamin B6, vitamin B12, biotin, niacinamide, folic acid, pantothenic acid, zinc sulphate, magnesium sulphate, manganese sulphate and sodium selenite.
2.4. Diet and supplementation
Twenty overweight and obese adults (14 women; 6 men), with no heart, liver, kidney and other diseases and BMI ≥ 25kg/m2 were invited to participate in this clinical analysis programme. Participants provided their informed consent before the program started. The trial is approved by the medical ethic committees of Tianjin Zhongkelanding Natural Medicine Institute (Ethic Approval Protocol Number: E20200008).
Each bottle of functional soy milk consisted of 162 g of water and equal proportions of other components. Subjects were required to consume one bottle of product at a time, no more than 5 bottles a day, in a 7-day cycle. From day 1 to day 3, the staple foods of breakfast, lunch, and dinner were replaced; day 4 and day 5, the staple foods of lunch and dinner were replaced; day 6 to day 7, staple foods in dinner were replaced by the functional soy milk. During the trial, 2 L and more drinking water daily was advised. High-protein or low-calorie foods such as fish, prawns, beef, egg, lightly cooked vegetables and fruits were advised to intake. During the trial period, no special restrictions were required on participants’ livings, and a moderate appropriate amount of exercise was advised to maintain as usual.
Over the program, the body weight of participants was collected daily. At the beginning and end of the trial period (day 15), blood was collected from eight of the participants. The satiety was recorded when participants started to feel hungry after taking the meal replacement (Solah et al., 2015).
2.5. Data analysis
Data were expressed as mean ± SD. One-way analysis of variance (ANOVA), with the multiple range significant difference (Tukey’s) test (p < 0.05) was carried out using Minitab Express 1.5.0 (Minitab Pty, Sydney, Australia). Figures were created in GraphPad Prism 8.0 (GraphPad, CA, USA).
| 3. Results | ▴Top |
3.1. Body weight
Table 1 summarises the baseline characteristics of participants. Fourteen participants were women, and six participants were men, with a mean BMI of 27.4 ± 2.4 kg/m2 and a mean body weight of 94.09 ± 23.36 kg. None of the participants has been on a diet in the last three months. After two weeks of the experiment, the subject’s mental status, urinary and defecation status, and sleep status were normal.
The body weights of participants were recorded and shown in Figure 1. After the continuous follow-up of 20 participants, the effect of body weight management was obvious, that every participant had a loss of body weight to a different degree. The average weight loss was 5.20 kg, with a range from 1.70 kg to 6.90 kg in different participants. For women volunteers, they had a body weight loss of 6.70% of their initial body weight, and men had a comparable body weight reduction of 6.18%.
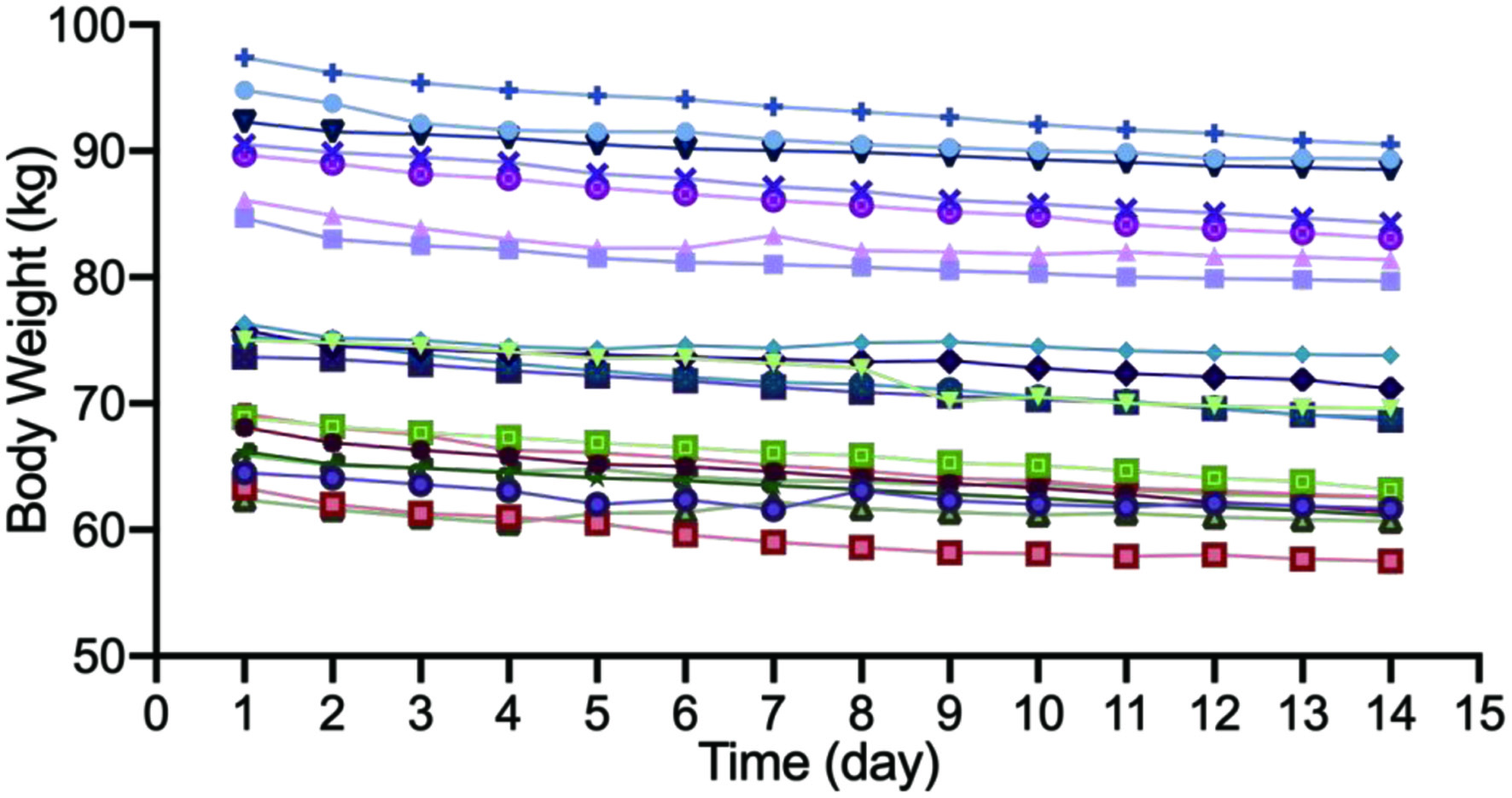 Click for large image | Figure 1. The change of body weight of 20 participants. |
3.2. Blood glucose
Table 2 shows the blood glucose of eight participants. After taking the beverage meal replacement for 2 h, participants were subjected to a hypoglycaemic production index experiment to obtain the area under the curve (AUC) glycaemic response, compared to the AUC of glucose. The results obtained are shown in Table 3.
 Click to view | Table 2. The glucose level of participants after taking meal replacement and ratios of products to glucose (mmol/L). |
 Click to view | Table 3. Baseline participants characteristics and changes of body weight. |
The soy-whey based milk beverage had a blood glucose-lowering effect in individuals. The average blood glucose of participants who had had the beverage was 1.7785 mmol/L, ranging from 0.6090 mmol/L to 2.5548 mmol/L. If compared to the original glucose baseline level of 3.5420 mmol/L, the meal replacement decreased the level of blood glucose by nearly 50%. Although ratios of the meal replacement to glucose ranged greatly from 0.2960 to 0.8388 among participants, this might be explained by the difference in individuals’ metabolic status.
3.3. Cholesterol
The cardiometabolic parameters of participants pre-and post-treatment of the soy-whey based beverage are shown in Figure 2. Compared to baseline, the participants who received the treatment had a significantly greater reduction in total cholesterol (TC). Before the meal replacement, the TC level of participants was 5.89 ± 0.44 mmol/L, and the beverage had a 15.7% reduction effect on TC, maintaining the average level of 4.96 ± 0.49 mmol/L. Similar reductions were observed in other indicators of blood, including triglycerides (TG) and low-density lipoprotein (LDL-C). With regard to TG, the functional drink decreased the TG level from 1.97 mmol/L to a level of 1.53 mmol/L significantly (p < 0.001). And participants had a lower level of LDL-C ranging from 2.50 to 2.98 mmol/L than the baseline level of 3.31 mmol/L. The HDL values observed on day 14 were significantly higher than that of day 0. The baseline values of high-density lipoprotein (HDL-C) were ranged from 0.80 to 1.43 mmol/L, whereas these values were improved significantly by the drink after two weeks trial with an average level of 1.73 mmol/L (p < 0.01).
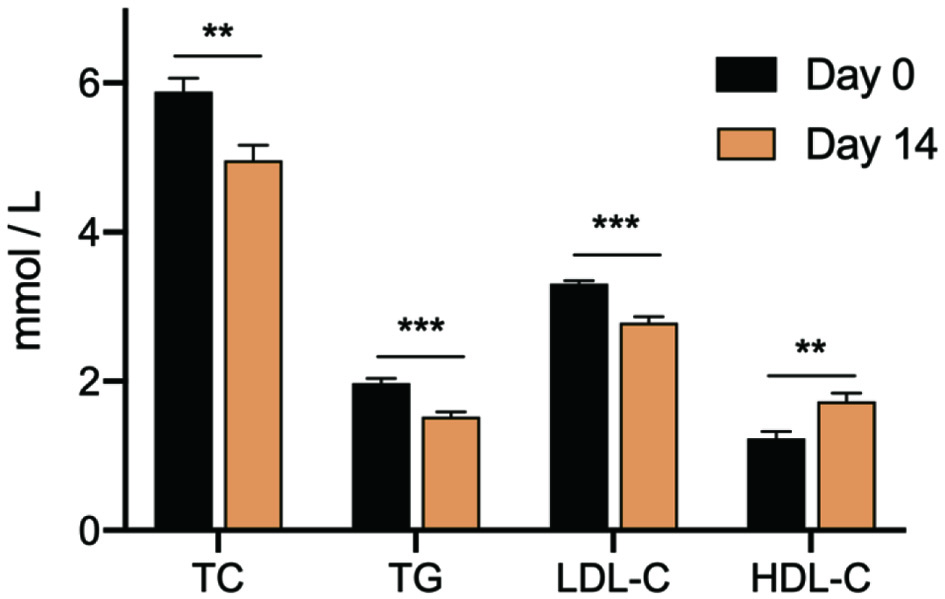 Click for large image | Figure 2. The blood cholesterol level of participants before and after taking the meal replacement. TC: cholesterol; TG: triglycerides; LDL-C: low-density lipoprotein; HDL-C: high-density lipoprotein. **represents p < 0.01; ***represents p < 0.001. |
3.4. Satiety evaluation
The results of the satiety experiment are shown in Table 4. The satiety was recorded when they started to feel hungry after taking the meal replacement. As shown in Table 4, one bottle of this product can basically maintain satiety within 200 minutes with an average of 187.5 minutes.
 Click to view | Table 4. The satiety level of participants. |
The most often used experimental method for determining satiety is to have individuals consume the test item or meal and rate their feelings of fullness or hunger. On a line scale with descriptive anchors, fullness and hunger are recorded post-meal. The awareness of satiety could be affected by multiple factors. Personal perception, emotions, time of the day, and even the level of hunger and proximity to food would affect the reliability and consistency of the results (Flint et al., 2000). Solah et al. (2015) also reported that satiety measurement should be performed by trained panellists since they found a significant difference in satiety evaluation between “after the training” and “before training” (Solah et al., 2015).
Further studies could also be designed to measure several satiety hormones levels, for instance, glucagon-like peptide-1 (GLP-1). GLP-1 is a specific gut neuropeptide released from gut endocrine L-cells, has the physiological effects of satiety induction and suppression of gastric emptying (Hira et al., 2019). Compared to the sensory test, GLP-1 measurement in the blood may provide reliable and trusted evidence in improving the precision of satiety evaluation.
| 4. Discussion | ▴Top |
In this clinical program, it has been demonstrated that the soy-whey fermented milk meal replacement is of benefit in promoting weight loss over a two-week period. Participants lost a significant amount of weight with a concomitant reduction in blood glucose and cholesterol. On average, those who consumed the meal replacement lost 6.4% of their initial body weight. The majority of the participants (n = 14) in the clinical trial were women and had a significant controlling effect of body weight from baseline, with an average body weight loss of 4.7 kg. Six male participants lost a substantial amount of weight of an average of 5.7 kg during the 2-week trial. A Higher loss of body weight was reported by Allison et al. (2003), in which the soy-based beverage group had a 7.00 kg weight loss. This could be due to the longer period of the trial conducted over 12 weeks.
Fermented milk is known as a dairy product containing more nutrients and has a longer shelf life than milk due to the presence of lactic acid bacteria. The meal replacement in this study was formulated by fermented milk, which was inoculated with two thermophilic strains, Lactobacillus bulgaricus and Streptococcus thermophilus, which have long been used as starter cultures to produce yoghurt. Recent studies have developed fermented milk as a matrix to study the probiotic stability and compounds bioavailability aiming at functional foods. For instance, Gnaphalium affine (Gao et al., 2018), pomegranate peel extract (Chan et al., 2018), Guabiroba pulp (Prestes et al., 2021), have been used as supplementations to develop fortified fermented milk. In a study by Benzie et al. (2006), zeaxanthin bioavailability was enhanced 3-fold by the homogenisation of wolfberry in the milk-based formulations (Benzie et al., 2006). In our soy-whey based beverage, wolfberry was used with a concentration of 0.6%. Further studies towards the bioavailability of bioactive components in the soy-whey based fermented milk as well as their stability should be conducted.
As a combination of soy protein, whey protein was used as another protein source in this meal replacement. Pal and Ellis (2010) reported that, compared with tuna, turkey, and egg albumin, whey protein might be more effective in suppressing appetite and promoting satiety (Pal and Ellis, 2010). Taking together with our results of body weight and satiety measurement, the improvement in weight management may be attributed to whey protein and its peptides’ physiological action of satiety by regulating satiety hormones, shown by the study of Luhovyy et al. (2007) as increased cholecystokinin (CCK) and GLP-1 production and decreased level of ghrelin (Luhovyy et al., 2007).
Humans are most familiar with the disaccharide sucrose, often known as table or granulated sugar. Although it has the same calories as other carbohydrates, namely 4 kcal/g, at current consumer sugar consumption levels, its total calorie contribution to a daily diet totals 500 kcal (Miele et al., 2017). Excess sucrose consumption is hypothesised to be associated with risk for metabolic disease. In the study by Imamura et al. (2015), the metanalysis of 17 cohorts (38,253 cases/ 10,126,754 person years) indicated that consumption of sugar sweetened beverages was associated with a greater incidence of type 2 diabetes.
Replacing drinks and table sugars with natural or artificial sweeteners has been considered effective in helping weight management. In this study, Luo Han Guo was used as the sweetener. Luo Han Guo, referred to as Momordica grosvenorii, is native to southern China, where it has been utilised over the years as a culinary component and herbal medicine (Çiçek et al., 2021). It has been reported that the sweetness of Luo Han Guo is up to 300 times greater than that of sucrose. The compounds in Luo Han Guo providing the sweetness are triterpene glycosides, known as mogrosides (Mora and Dando, 2021) .
The threshold of natural Luo Han Guo should be considered in improving the product sweetness. A study conducted by Parker et al. (2018) reported that when at higher usage levels (above 25% of a sweetener blend), bitter and metallic side tastes were prominent (Parker et al., 2018), despite the reason could be partially due to the off-flavours of stevia in the blend. In this study, the concertation of Luo Han Guo was used as 0.7%, and no off-flavours or bitter taste were reported by participants.
Few human studies have been conducted to evaluate the effects of Luo Han Guo on people with type 2 diabetes (Mejia and Pearlman, 2019). In a study of streptozotocin induced diabetic mice, a dose of 300 mg/kg of mogroside-rich extract increased glucose tolerance, lowered HOMA-IR, and elevated insulin sensitivity (Liu et al., 2019). In contrast, Tey et al. (2017) reported that minimal influences were obtained in postprandial glucose blood glucose and insulin levels in the study of healthy males (30 participants) who consumed Luo Han Guo and other sweeteners (stevia and aspartame). Further designing structured diets related to human trials are necessary to provide deep insights into Luo Han Guo efficacy for weight management, glucose tolerance, insulin sensitivity, and other metabolic syndromes.
| 5. Conclusion | ▴Top |
In conclusion, the soy-whey fermented milk meal replacement is effective in promoting weight loss over a two-week period. Participants lost a significant amount of weight with a concomitant reduction in postprandial blood glucose and cholesterol. Our study offers experimental tests of the efficacy of a novel soy-based meal replacement in weight management and a potential anti-obesity program. Different formulations could be explored; recovery and maintaining of bioactive compounds in food matrices and their optimal bioavailability in the body should be studied, aiming to deliver expected health benefits to humans.
| References | ▴Top |