| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 2, June 2018, pages 82-90
Absorption of polymethoxyflavones and their derivatives
Ruolin Wanga, Shiming Lia, b, *, Chi-Tang Hoa, *
aDepartment of Food Science, Rutgers University, New Brunswick, NJ, 08901-8520, USA
bHubei Key Laboratory of Economic Forest Germplasm Improvement and Resources, Huanggang Normal University, Huanggang, Hubei 43800, China
*Corresponding author: Chi-Tang Ho, Shiming Li, Department of Food Science, Rutgers University, New Brunswick, NJ, 08901-8520, USA. E-mail:,
DOI: 10.31665/JFB.2018.2142
Received: January 5, 2018
Revised received & accepted: February 8, 2018
| Abstract | ▴Top |
Polymethoxyflavones (PMFs) are a group of flavonoids found exclusively in citrus genus that have been identified with many potent biological activities, including anti-inflammation, anti-cancer, anti-atherosclerosis, and antioxidant effects. However, the bioavailability of PMFs is seldom studied. Evaluating by aqueous solubility and permeability, absorption is the first indicator of bioavailability. In this research, we systemically investigated permeability and solubility of six PMFs and their derivatives, namely, sinensetin (SIN), 5-demethylsinensetin (5-OHSIN), 5-acetylsinensetin (5-AcSIN), 3,5,6,7,8,3′,4′-heptamethoxyflavone (HeptaMF), 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone (5-OHHeptaMF), and 5-acetyl-3,6,7,8,3′,4′-hexamethoxyflavone (5-AcHeptaMF). From octanol/water solubility test, we found that PMFs and their derivatives had very low aqueous solubility. Permeability experiment via Caco-2 cell monolayer transport model indicated high permeability of all the tested compounds. Furthermore, PMFs had the greatest permeability, followed by 5-acetyl PMFs and 5-hydroxylated PMFs. Considering permeability and solubility together, PMFs and their derivatives are expected to have good absorption. This study provides indicative information on the bioavailability of PMFs and their derivatives, which offers clues on the application of PMFs in functional food or nutraceutical products.
Keywords: Polymethoxyflavones; bioavailability; permeability; solubility; Caco-2
| 1. Introduction | ▴Top |
1.1. General
In recent years, food bioactive compounds have gradually become popular due to many potential health benefits. Flavonoids, as a group of polyphenolic compounds originating from plant sources, have proved to have powerful biological functions. Plenty of flavonoids can be found in citrus, polyhydroxylated flavonoids (PHFs) in flesh and polymethoxylated flavonoids (PMFs) and PHFs in peels. According to the newest report on world citrus market from the United States Department of Agriculture (USDA, 2017), the global orange production was 50.2 million metric tons for the year of 2016/17 with 13.9 million metric tons for processing, generating a large volume of peels as by-product. Polymethoxyflavones (PMFs) are found almost exclusively in citrus genus, especially in the peels of sweet orange (Citrus sinensis) and mandarin (Citrus reticulata Blanco). Up to date, more than 20 polymethoxylated flavonoids have been isolated and identified from different tissues of citrus plants (Li et al., 2006). Among them, there are six major PMFs (Figure 1), namely sinensetin (SIN), 3,5,6,7,3′,4′-hexamethoxyflavone (HexaMF), nobiletin (NOB), 5,6,7,4′-tetramethoxyflavone (TetraMF), 3,5,6,7,8,3′,4′-heptamethoxyflavone (HeptaMF), and tangeretin (TAN), which are all present in orange peels. NOB and TAN are the two most abundant naturally occurring PMFs, followed by SIN or HeptaMF. Subsequently, 5-demethylnobiletin (5-OHNOB) and 5-demethyltangeretin (5-OHTAN) are the two most common and abundant hydroxylated PMFs which are rich in the aged citrus peels. Other hydroxylated PMFs, including 5-hydroxylated PMFs other than nobiletin and tangeretin, as well as hydroxylated ones at C3′, C4′ and C7 positions are present in trace amounts. As the chemical structures of PMFs indicate (Figure 1), they all have poor aqueous solubility, which would greatly affect their bioavailability. There are perceptions that some 5-OHPMFs generally have better biological effects than their PMF counterparts. However, in reality, 5-OHPMFs have even lower aqueous solubility than PMFs since the 5-hydroxyl group tends to form an inner hydrogen bond of a six-member ring with its adjacent C4 carbonyl group.
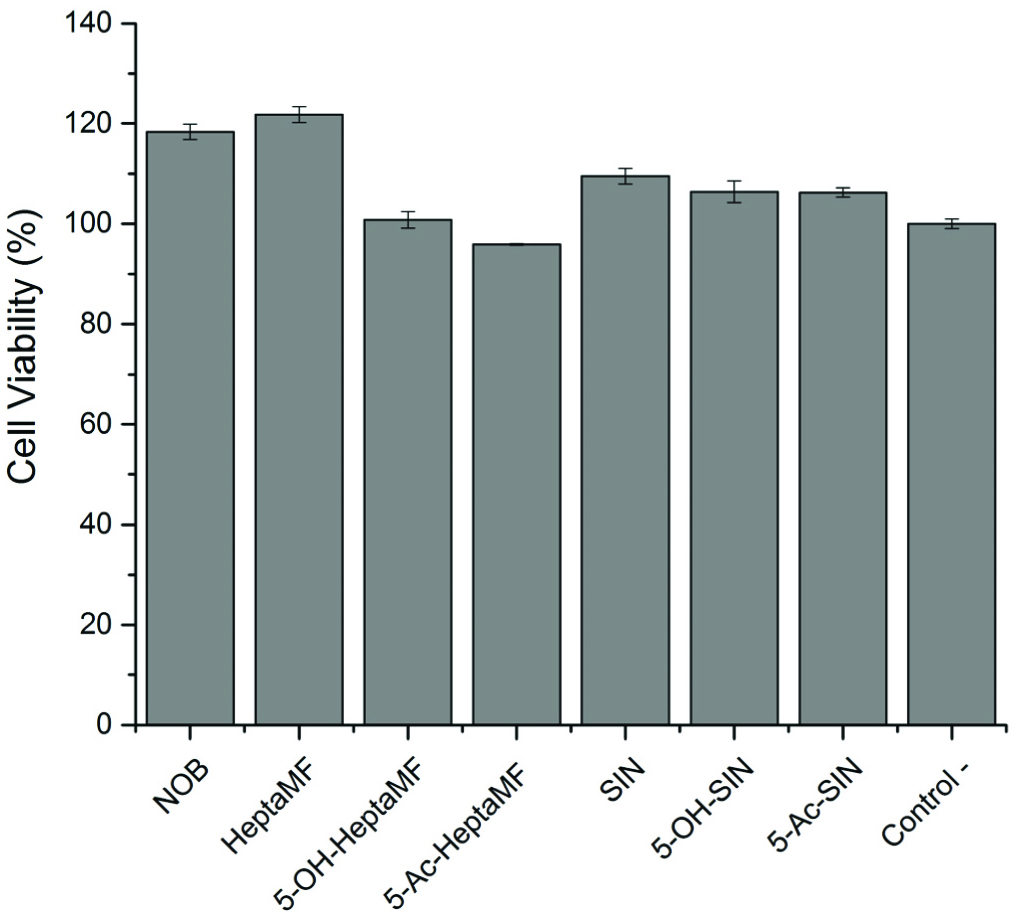 Click for large image | Figure 1. Caco-2 cell viability after treated with PMFs and their derivatives at a concentration of 20 μM. |
Bioavailability of a drug or a bioactive molecule is the overall effect of absorption, distribution, metabolism, and excretion (ADME). Solubility and permeability are the two deciding factors of absorption, which is the first pass affecting the bioavailability of a drug. The bioavailability of PMFs has rarely been reported, which makes the present study stand out for its systemic comparison of PMFs and their derivatives bioavailability. It has previously been reported that nobiletin shows 48.1% permeability from apical to basolateral side in a Caco-2 monolayer transport study during a 4-hour incubation with a preference of accumulating in the monolayer (Murakami et al., 2001). Furthermore, by testing on the SD male rat, nobiletin exhibited a wide distribution and accumulation in organs including stomach, small intestine, large intestine, liver, and kidney after 4 hours of a single dose treatment (Murakami et al., 2002). Another study showed that the overall solubility of PMFs was low, however, it was found that the permeability of 3,5,6,7,8, 3′,4′-heptamethoxyflavone, 3′-hydroxy-5,6,7,4′-tetramethoxyflavone, and 3-hydroxy-5,6,7,8,3′,4′-hexamethoxyflavone through the Caco-2 monolayer was excellent (Li et al., 2008). These previous studies lead to the hypothesis that PMFs could potentially have high bioavailability. One of the important factors of absorption is solubility, which mainly refers to aqueous solubility across a pH range. The chemical structures of PMFs, which have multiple methoxy groups, clearly indicate their poor aqueous solubility. The lyophilisation solubility assay (LYSA) revealed that the overall solubility of PMFs is poor, but the more hydroxyl groups the PMF has, the better soluble it would be (Li et al, 2008). In the concept of bioavailability, solubility is not an independent parameter, which means it works with other parameters like permeability. The Lipinski’s rule marks the criteria of an orally active drug for its pharmacokinetics in the body, including absorption, distribution, metabolism, and excretion (ADME). The rules are as follows: (i) no more than 5 hydrogen bond donors; (ii) no more than 10 hydrogen bond acceptors; (iii) molecular weight of less than 500 daltons; and (iv) the octanol-water partition coefficient (Log P) of not greater than 5 (Lipinski et al., 2012). All the PMFs used in the study have less than 5 hydrogen bond donors, less than 10 hydrogen bond acceptors, and less than 500 Da of molecular weight. There is no previous publication recording the octanol-water partition coefficient, thus, we performed related experiments on selected PMFs.
This study focused primarily on evaluating the solubility and permeability of two PMFs, their corresponding 5-OHPMFs and AcPMFs, and intended to provide a structural and systematic understanding and potential improvement of bioavailability of PMFs from an absorption point of view. Therefore, our attempt was to disentangle the absorption of PMFs from the following three aspects: solubility, permeability and structural modification. (i) Solubility. The so-called shake-flask method was used in this solubility study. Basically, the substrate was added into two immiscible liquids, octanol/water system—and then the concentration of the substrate in the two liquids was measured separately by chromatographic methods, like HPLC and GC among others(Berthod & Carda-Broch, 2004). In this study, we employed shake-flask method, and used HPLC to determine the distributions of PMFs and their derivatives in octanol and water. (ii) Permeability. As mentioned above, permeability through small intestine is a crucial factor in the absorption process. In the small intestine, the monolayer of epithelial cells serves as a barrier to selectively absorb drugs or nutrients. In the permeability study, Caco-2, the human colon carcinoma cell line, is usually a substitute, which can mimic the function of human differentiated epithelial cells. Caco-2 cells are cultivated onto permeable filters and gradually differentiate into a monolayer with tight junctions and apical (mucosal) side and basolateral (serosal) side during the 21-day incubation. (iii) Structural modification. Acetylation at the 5-postion was proposed to improve the solubility of 5-OHPMFs via acetylation. 5-Acetylated PMFs (5-AcPMFs) can be recognized as prodrugs of 5-OHPMFs and PMFs in the concept of medicinal chemistry, meaning 5-AcPMFs are expected to convert into 5-OHPMFs after administration and portion of 5-OHPMFs are suspiciously methylated in vivo. It was previously found that 5-AcTAN shows a lower cytotoxic effect than TAN and 5-OHTAN in cell studies (Wang et al., 2014). With this in mind, the comparison on bioavailability of PMFs, 5-OHPMFs, and 5-AcPMFs is of particular interest to better understand the difference in their biological effects.
| 2. Reagents and methods | ▴Top |
2.1. Materials
Sinensetin (SIN), nobiletin (NOB), 5-demethylnobiletin (5-OHNOB) and 3,5,6,7,8,3′,4′-heptamethoxyflavone (HeptaMF) were isolated in house from sweet orange peel extracts. Components of 5-demethylsinensetin (5-OHSIN), 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone (5-OHHeptaMF), 5-acetylsinensetin (5-AcSIN), and 5-acetyl-3,6,7,8,3′,4′-hexamethoxyflavone (5-AcHeptaMF) were prepared as described in Section 2.3. HPLC grade acetonitrile, water, and methanol were purchased from Pharmco-AAPER (Brookfield, CT, USA). 1-Octanol (99%) and dimethyl sulfoxide (DMSO, >99.9%) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Caco-2 cell lines were obtained from Rutgers University. Dulbecco’s modified essential medium (DMEM) high glucose with L-glutamine, MEM non-essential amino acids, trypsin-EDTA (0.5%), penicillin-streptomycin, phosphate-buffered saline-pH 7.2 (PBS), hank’s balance salt solution (HBSS), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were purchased from Gibco Life Technologies (Grand Island, NY, USA). Fetal bovine serum was purchased from Biowest (Kansas City, MO, USA). Corning 96-well cell culture plates and 12-transwell plates were purchased from Fisher Scientific (Fairlawn, NJ, USA). The spectrophotometric microtiter plate reader was purchased from BioTek (Winoski, VT, USA).
2.2. Instruments
The glass column (24/40 outer joint, 1 in ID × 12 in E.L., 2 mm Stpk) from Chemglass Life Sciences (Vineland, NJ, USA) was used to prepare the silica gel column. The HPLC system consisted of a Dionex UltiMate 3000 HPLC series (Sunnyvale, CA, USA) including an UltiMate 3000 Pump, an UltiMate 3000 Variable Wavelength Detector, and an UltiMate 3000 Auto-sampler. Chromeleon software was used to perform instrument control and data analysis. Supelco Ascentis® RP-Amide C18 HPLC column (15 cm × 4.6 mm, 3 μm) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The following HPLC program was used: mobile phase consists of water (A) and acetonitrile (B); and the 20 min gradient starts with 40% B, then linearly increases to 55% B in 10 min, and then linearly increases to 70% B in 15 min, finally stays at 80% B at 20 min. Data was collected at wavelength of 214 nm, 254 nm, and 326 nm. The injection volume was set at 5 μL, and the flow rate was 1 mL/min (Wang et al., 2008; Li et al., 2009). Peak areas of the interested compounds were obtained from HPLC, and the concentrations were calculated from standard curves. Data were expressed as mean ± standard error mean (SEM).
2.3. Methods of syntheses
2.3.1. Demethylation reaction
As shown in Scheme 1, PMF (SIN or HeptaMF, 1.2 mmole) was dissolved into 150 mL of anhydrous ethyl alcohol, and 20 mL of hydrochloric acid (37%) were gradually added into the solution. This solution was heated and refluxed for about 24 h. The reaction was monitored by HPLC analysis. After the reaction was finished, the solvents were removed in vacuo. Then the residue was re-dissolved in 100 mL ethyl acetate/water (v/v, 1/1). After removing the water layer, the ethyl acetate layer was washed with water three times. The combined water fraction was re-extracted with ethyl acetate to extract remaining PMF. The ethyl acetate fraction was dried by anhydrous sodium sulfate first, and then the solvent was removed in vacuo. The resulted residue was subject to a flash chromatography and gave 5-OHPMFs in >98% purity and 78% yield or higher for 5-OHSIN and 5-OHHeptaMF. After purification, the fraction was evaporated and then freeze-dried. The purity of 5-OHPMFs was analyzed by following the HPLC program stated in Section 2.2. Further identification was carried out by GC-MS and NMR (Li et al., 2006).
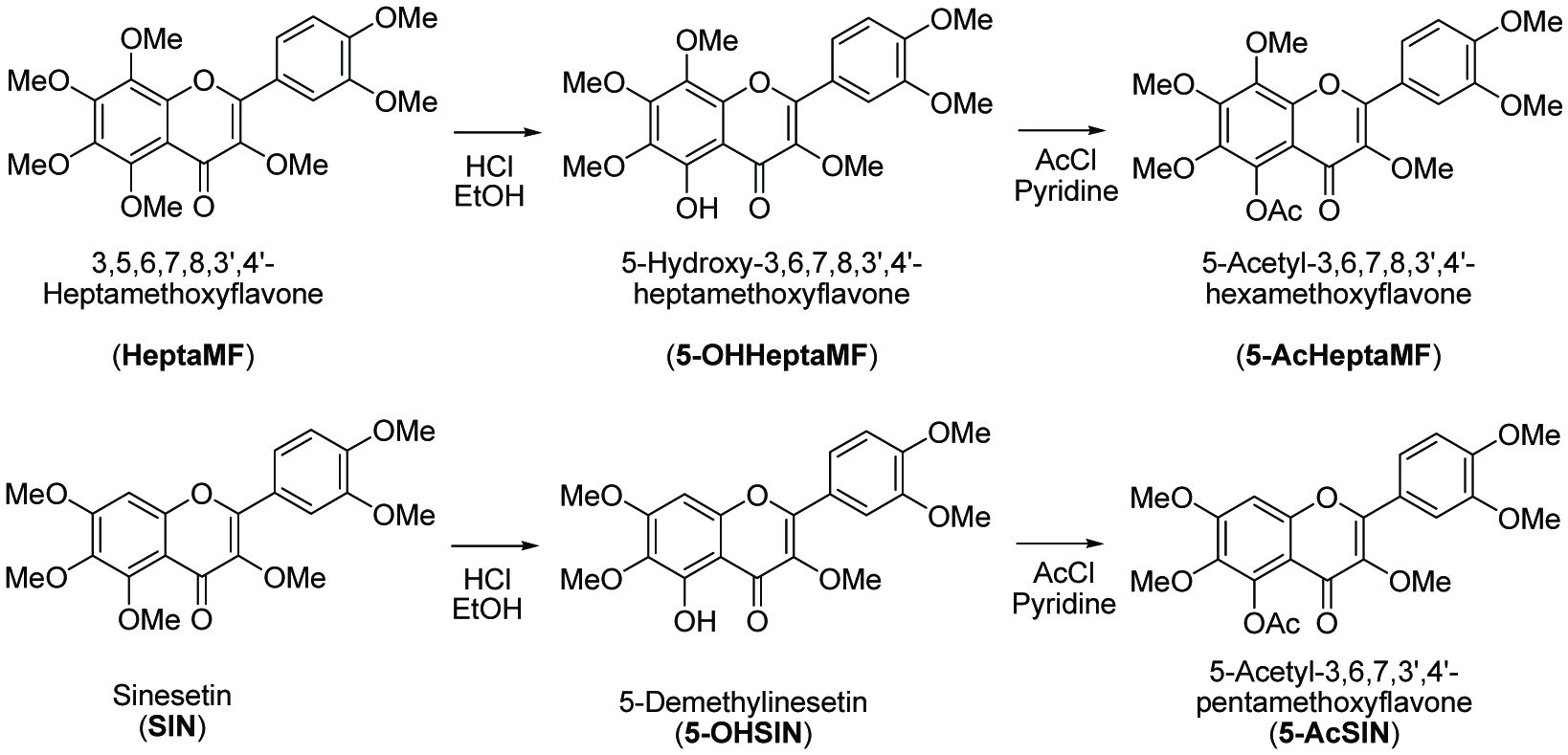 Click for large image | Scheme 1. Structures and preparation of polymethoxyflavones and related acetyl derivatives. |
2.3.2. Acetylation reaction
5-OHPMFs (0.25 mmole) was dissolved in 50 mL of anhydrous dichloromethane, followed by the addition of 10 mL acetyl chloride. After stirring the mixture for 5 min, 3 mL anhydrous pyridine was gradually added into the solution. The resulting solution was stirred under ambient temperature for 24 h. The reaction was monitored by HPLC analysis. After the reaction was finished, the mixture was concentrated in vacuo and the residue was re-dissolved in 100 mL of ethyl acetate/water (v/v, 1/1). After removing the water layer, the ethyl acetate layer was washed with water, 1 M hydrochloric acid, water, and brine, and further dried over anhydrous sodium sulfate. After filtration, the ethyl acetate was concentrated in vacuo. The resulted residue was subject to silica gel flash chromatography to obtain pure 5-AcPMFs with a 98% or above purity and yields of more than 60%. After purification, the fraction was evaporated and then freeze-dried. The purity of 5-AcPMFs was analyzed by following the HPLC program stated in Section 2.2. Further identification was carried out by GC-MS and NMR.
2.4. Solubility measurement
Two PMFs and their derivatives, SIN, 5-OH-SIN, 5-AcSIN, HeptaMF, 5-OHHeptaMF, and 5-AcHeptaMF, respectively, were prepared into 10 mg/mL solutions in DMSO. Then the solutions were diluted 100 times into a mixture of water/octanol (v/v, 1:1). The obtained mixtures were vortexed, and then left until equilibrated. Both the water and octanol layers were analyzed by following the HPLC program stated in Section 2.2 (Table 1).
 Click to view | Table 1. Partition coefficient (log P) of PMFs and their derivatives |
2.5. Permeability measurement
2.5.1. Cell Culture
Caco-2 cells (passage 53–63) were maintained in DMEM medium containing 10% FBS, 1% PS, and 1% non-essential amino acids. Cells were incubated in 5% CO2 with 95% relative humidity at 37 °C. Cells were detached by trypsin-EDTA treatment after reaching 80% confluence.
2.5.2. MTT Assay
The screening process started with PMF concentration of 50 μM, and further went down to a concentration of 20 μM. To begin with, the PMF solutions were freshly prepared in DMSO and diluted into DMEM medium with the final DMSO concentration of less than 0.1%. Caco-2 cells were seeded into 96-well plates with a density of 1 × 105 cells/well for 24 hours. Subsequently, cells were treated with 0.2 mL of DMEM medium containing targeted concentration of PMF samples. The control group was treated with 0.2 mL of DMEM medium without PMFs added. After 24 hours, the DMEM medium was discarded, and the cells were washed with PBS. Subsequently, 0.2 mL 0.5 mg/mL MTT solution in DMEM medium was added into each well, and the cells were incubated for 4 hours. Finally, MTT solution was discarded, and the formazan crystals were extracted with 0.1 mL DMSO. Light absorption at 560 nm was measured by the spectrophotometric microtiter plate reader. Each PMF at each concentration was repeated six times. Data were expressed as mean ± standard error mean (SEM). Statistical significance of mean difference between two groups was determined by student t-test. Significance levels of p < 0.05 and p < 0.001 were applied.
2.5.3. Caco-2 cell monolayer transport assay
Cells of 3 × 106 were seeded onto the permeable inserts (0.4 μm pore size and 12 mm diameter) of 12-transwell plates. The medium was changed every other day for 21 days to induce the differentiation of Caco-2 monolayer. The monolayer integrity was confirmed by measuring transepithelial electrical resistance (TEER) value at 37 °C. Monolayers with TEER values lower than 165 Ω cm-2 were discarded.
At the beginning of the transport experiment, cells were washed twice with pre-warmed HBSS solution and then incubated for 20 min at 37 °C. For aptical (AP) to basolateral (BL) transport, 0.45 mL HBSS containing 20 μM PMF samples was added to the AP side, meanwhile 1.2 mL blank HBSS was added to the BL side. Similarly, for BL to AP transport, 1.25 mL HBSS containing 20 μM PMF samples was added to the BL side, and 0.4 mL blank HBSS was added to the AP side. For both, 0.05 mL solution was taken at 0 min from the donor chamber to confirm the initial concentration. The monolayers were incubated at 37 °C, and sample solutions (0.6 mL for AP-BL and 0.2 mL for BL-AP) were taken at 20, 40, 60, 80, and 100 min from the receiver chambers and immediately re-added the same amount of blank HBSS solution to maintain the volume of 1.2 mL for AP-BL and 0.4 mL for BL-AP.
2.5.4. Sample analysis
Each sample collected from the transport experiment was added with 0.1 mL of 20 μM NOB or 5-OHNOB in HBSS as internal standard, and extracted with 0.6 mL ethyl acetate three times. The ethyl acetate layer was combined and evaporated in oven at 40 °C. Then 0.1 mL methanol was used to reconstitute the residue. The HPLC program used is stated in Section 2.2. The concentration of each sample was calculated from its correlated standard curve. Data were expressed as mean ± standard error mean (SEM).
| 3. Results and discussion | ▴Top |
3.1. Preparation of 5-OHPMFs and 5-AcPMFs
The demethylation reaction and acetylation reaction generated high yields of products. 5-OHPMFs are present in natural orange peel but in trace amounts. 5-AcPMFs are not present in natural orange peel at all. With chemical synthesis, we are able to obtain large amounts of 5-OHPMFs and 5-AcPMFs in order to study on their properties. The yield of each reaction was more than 60%. The final purities of the PMF derivatives were all above 98% by HPLC analysis.
3.2. Solubility
3.2.1. Solubility measurement
SIN and HeptaMF
The HPLC results tell that PMFs and their derivatives generally have very low aqueous solubility, meaning nearly all the compounds distributed in the octanol layer. SIN had a peak area of 186.68 mV.s in octanol layer, but 7.27 mV.s in water layer, both detected at 214 nm wavelength. This indicates that SIN was 26 times more distributed in octanol than in water. HeptaMF has an even worse water solubility, for which the peak area is 136.96 mV.s in octanol, but only 1.41 mV.s in water, meaning HeptaMF was 97 times more distributed in octanol than in water. This result is actually quite reasonable because HeptaMF contains two more methoxy groups than SIN.
5-OHSIN or 5-OHHeptaMF
There were hardy any of 5-OHSIN or 5-OHHeptaMF distributed in water, which reveals 5-OHPMFs probably have the poorest aqueous solubility among OHPMFs. One interesting observation on 5-OHPMFs is that, with the presence of octanol, the HPLC retention time was changed. 5-OHPMFs are expected to be more polar than PMFs due to the presence of more hydroxy group. However, the elution orders on normal phase silica gel column and C18 reverse phase HPLC suggest that 5-OHPMFs would be more non-polar. For example, from the silica gel column, 5-OHNOB is eluted before NOB; on the contrary, 5-OHNOB comes out later than NOB on the reverse phase HPLC system. This is due to the formation of stable six-member ring from the intramolecular hydrogen bond between the hydrogen on 5-hydroxy group and the oxygen on the 4-carbonyl group (Li et al., 2006). Octanol has a unique property, which is hydrophobic on the body but hydrophilic at the tail of the molecule. When octanol is introduced to 5-OHPMFs, the hydrophobic body will bind to PMFs, while the hydrophilic tail tends to bind to the oxygen on the 4-carbonyl group. This results in the breakage of the hydrogen bond between the hydrogen on 5-hydroxy group and the oxygen on the 4-carbonyl group. Thus, 5-OHPMFs dissolved in octanol have different retention profiles than their regular solutions, which are prepared in methanol. HPLC data of 5-OHSIN show a retention time of 11.29 min when it is in octanol, however, the retention time changed back to 11.82 min when diluted the octanol fraction 100 times into methanol, which is a regular profile. Similarly, 5-OHHeptaMF, when in octanol, had a retention time of 11.49 min, whereas switched back to 14.41 min when diluted into methanol. These results mean the hydrogen bond between the hydrogen on 5-hydroxy group and the oxygen on the 4-carbonyl group was broken in octanol, and reformed when octanol was minimized or removed. In other words, octanol actually changes their hydrophilic affinity.
5-AcSIN and 5-AcHeptaMF
These molecules are less aqueous soluble than their PMF counterpart. 5-AcSIN distributed over 70 times in octanol than in water, and 5-AcHeptaMF distributed over 121 times in octanol than in water.
3.2.2. Partition coefficient (log P)
When a solute is added into a mixture of two immiscible liquids, the solute has a preference to distribute itself into the two liquids; when it reaches equilibrium, the ratio of the two concentrations is called partition coefficient. The octanol-water partition coefficient is calculated via the following equation:
The partition coefficient (log P) for SIN and HeptaMF are then calculated as 1.76 and 1.97, respectively, which reflects a similar fact as HPLC chromatogram indicating that HeptaMF is more lipophilic than SIN. Unfortunately, no partition coefficient was calculated for 5-OHSIN and 5-OHHeptaMF since there was no peak detected in the water layer at all. The detected peak of 5-AcSIN and 5-AcHeptaMF in water layer was too low and already under the reliable range of standard curve. Therefore, their partition coefficient was not calculated as well.
In summary, the overall solubility of PMFs and their derivatives are very low. This shake-flask solubility test roughly tells us that the hydrophilic affinity of the tested PMFs is: SIN > 5-OHSIN> 5-AcSIN, HeptaMF > 5-OHHeptaMF > 5-AcHeptaMF, and SIN > HeptaMF. The logarithm of partition coefficient, log P, for SIN and HeptaMF are less than 5, which meets the Lipinski rule of five, indicating they qualify to be a potential oral drug. However, it is difficult to judge the 5-hydroxyl and 5-acetyl derivatives since their partition coefficient data is missing. It was interesting to observe the hydrogen bond between the hydrogen on 5-hydroxyl group and the oxygen on the 4-carbonyl group break and re-form under different conditions. Since fatty acids have the same structural property as octanol—hydrophobilc body and hydrophilic tail—5-OHPMFs might undergo the same change when mixed with fatty acids. Therefore, the hydrophilic affinity of 5-OHPMFs is not as simple as predicted from in vitro study because our diet is made up of tens of hundreds of components, which could potentially change the form of administered compounds.
3.3. MTT assay
The aim of MTT assay in this study was to determine the proper dosage for PMFs and their derivatives, at which the concentration was applied in the Caco-2 monolayer transport experiment. The ultimate goal was to find one concentration, at which all the six PMFs and their derivatives are not harmful to the Caco-2 cells (Duan et al., 2014).
The relative cell viability was calculated by using the following equation:
The viability of the control group was considered 100%. By screening down from 50 μM to 20 μM (Figure 2), it was found that 20 μM was the best concentration to select. At 20 μM concentration, the viability of SIN and its two derivatives was over 100%, meaning no detectable toxicity. Some compounds, such as 5-OHHeptaMF had a viability below 80% when the concentration was higher than 20 μM. Cell viability was above 95% for all the PMF samples at 20 μM concentration (Figure 1), meaning PMFs are not cytotoxic at this concentration. Thus, 20 μM was chosen as the dosage for the Caco-2 monolayer transport experiment. Except for achieving this purpose, MTT assay also gave indication on the inhibitory effects of PMFs and their derivatives on human colon cancer cells.
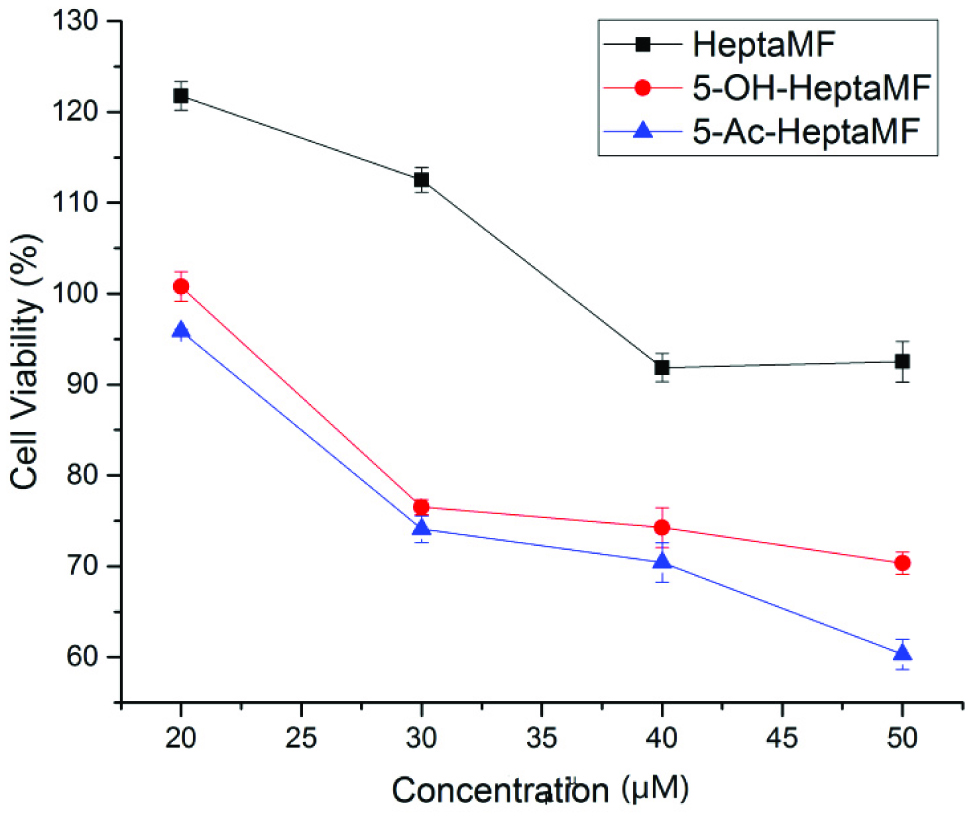 Click for large image | Figure 2. Inhibitory effects of HeptaMF, 5-OHHeptaMF, and 5-AcHeptaMF on Caco-2 cells. |
We have found that as concentration increases, the relative cell viability decreases regardless of which compound is considered. As illustrated in Figure 2, the highest dosage used in this study was 50 μM, which gave 92.52, 70.34, and 60.31% viable cells after treated with HeptaMF, 5-OHHeptaMF, and 5-AcHeptaMF, respectively. At p < 0.001 level, cell viability treated with 5-OHHeptaMF and 5-AcHeptaMF was significantly different from the control group, while it was not significantly different when treated with HeptaMF. This result reveals that 5-OHHeptaMF and 5-AcHeptaMF are more potent than HeptaMF in the inhibition of human colon cancer cells. In addition, cell viability treated with 5-AcHeptaMF is significantly greater than that with 5-OHHeptaMF (p < 0.05), Therefore, with the addition of an acetyl group, the anti-cancer property is enhanced. Generally speaking, 5-AcHeptaMF had the best inhibitory effect, followed by 5-OHHeptaMF and HeptaMF.
3.4. Permeability assessment via Caco-2 cell monolayer transport model
Caco-2 cells are the human colon carcinoma cell line. They are widely used in the prediction of drug permeability since they have enterocyte-like properties after forming a monolayer. Similar to epithelial cells in small intestine, Caco-2 monolayer gives tight junctions, mucosal/apical side, and serosal/basolateral side, along with various metabolic enzymes and transporters, such as sulfotransferase (Satoh et al., 2000) and P-glycoprotein (Hunter et al., 1993), respectively. Therefore, Caco-2 monolayer would definitely be a powerful tool to unravel the mystery of a drug being transported in and out. In this study, we investigated the permeability property of total six PMFs and their derivatives, namely SIN, 5-OHSIN, 5-AcSIN, HeptaMF, 5-OHHeptaMF, and 5-AcHeptaMF.
In order to systemically compare the permeability properties of PMFs and their derivatives, Caco-2 monolayer transport experiment was carried out on SIN, 5OH-SIN, 5-AcSIN, HeptaMF, 5-OHHeptaMF, and 5-AcHeptaMF, and the experiment was conducted for both apical to basolateral (AP-BL) and basolateral to apical (BL-AP) direction. In order to present the amount of compound permeated, we calculated permeability rate (%) using this following equation:
Data were expressed as mean ± SEM. Figure 3 shows the permeability rate of all the tested compounds at each time point. There are several trends that are summarized below:
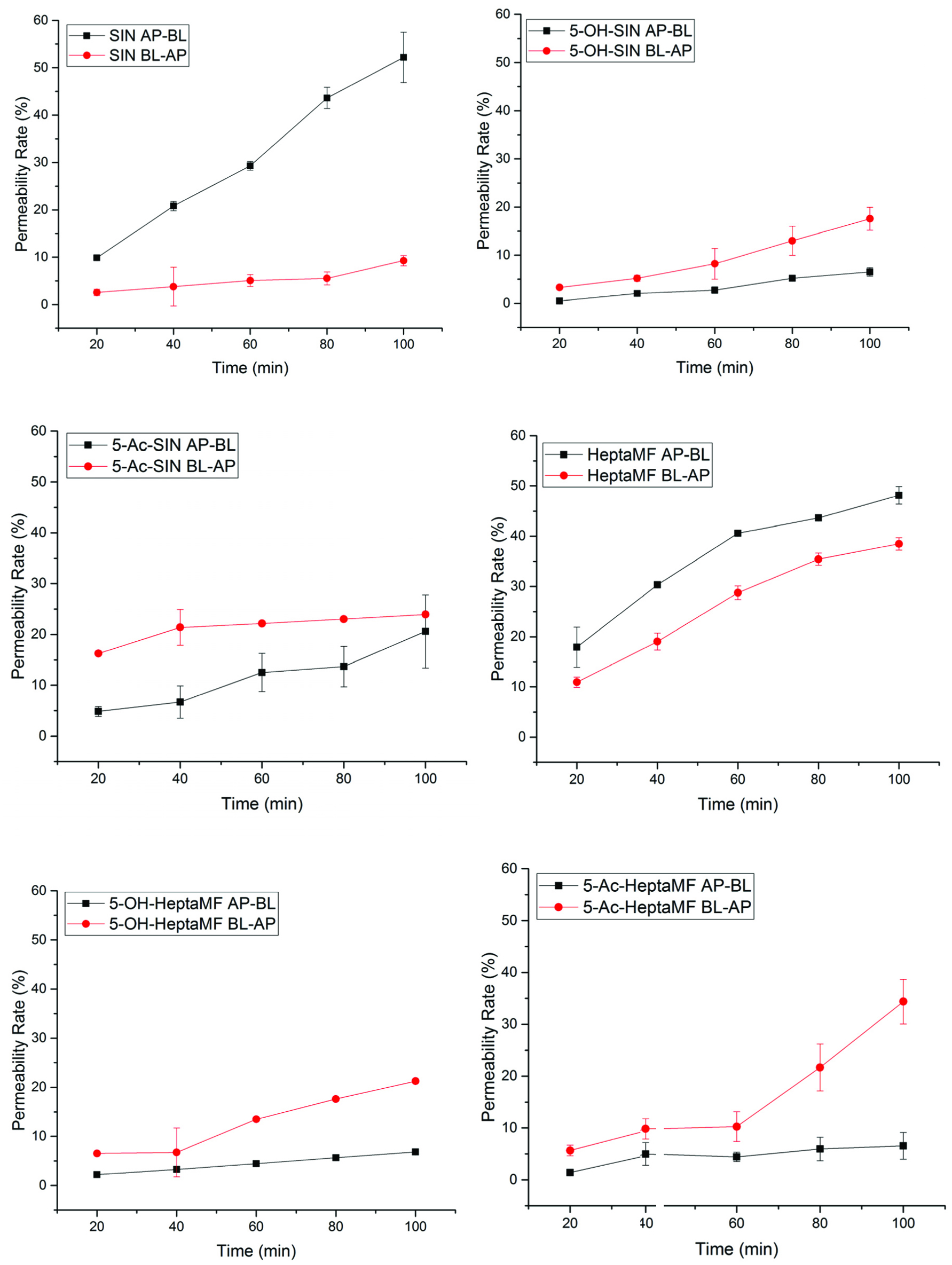 Click for large image | Figure 3. Permeability rates of PMFs and their derivatives as a function of time. |
3.4.1. PMFs had better permeability than their derivatives in the AP-BL direction
At 100 min, SIN had 48.78% permeated from AP to BL, while 5-OHSIN and 5-AcSIN had permeated 6.54 and 27.70%, respectively. Similarly, HeptaMF had 45.89% permeated at 100 min from AP to BL, but only 6.84% for 5-OHHeptaMF and 6.56% for 5-AcHeptaMF. This means it is easier for PMFs to enter the systemic circulation than their 5-hydroxyl and 5-acetyl derivatives.
3.4.2. PMFs had absorption over efflux, while the derivatives had efflux over absorption
At 100 min, SIN had 39.51% more absorbed than pumped out; however, 5-OHSIN and 5-AcSIN had 11.03 and 3.35% more pumped out than absorbed, respectively. Similarly, HeptaMF had 3.26% more absorbed than pumped out, while 14.41 and 27.81% more pumped out for 5-OHHeptaMF and 5-AcHeptaMF, respectively. It was indicated that PMFs are more likely taken into the system; on the contrary, their 5-hydroxyl and 5-acetyl derivatives tend to be pumped out very fast from the blood circulation system.
3.4.3. SIN vs. HeptaMF
There is no big difference between SIN and HeptaMF on the absorption direction. However, by comparing the efflux direction, SIN had only 9.27% pumped out, while HeptaMF had 42.64%. It tells that SIN can be easily absorbed but hard to leave the system; by contrast, it is easy for HeptaMF to enter as well as leave the systemic circulation.
3.4.4. 5-Acetyl PMFs as pro-drugs successfully improved permeability of 5-hydroxylPMFs
5-Hydroxyl PMFs were reported to have more potent biological activities than their PMF counterparts especially anti-inflammation and anti-cancer cancer (Lai et al., 2007). The idea of synthesizing 5-acetyl PMFs was to deliver 5-hydroxy PMFs into the system and better perform the biological functions. 5-AcSIN had a permeability rate of 20.59% (AP-BL) and 23.94% (BL-AP), which were greater than 6.54% (AP-BL) and 17.57% (BL-AP) for 5-OHSIN. Furthermore, 5-AcHeptaMF had 6.56% (AP-BL) and 34.37% (BL-AP) transported, while 5-OHHeptaMF had 6.84% (AP-BL) and 21.26% (BL-AP) transported. However, the permeability rates of 5-AcSIN and 5-AcHeptaMF were underestimated since metabolic changes were not taken into consideration here. Details on metabolism are discussed in the following section.
3.5. Apparent permeability coefficient and efflux ratio
The Apparent permeability coefficient (Papp, cm s−1) measures the amount of drug transported per time:
Efflux ratio (EfR) is used to compare the drug absorption and efflux:
Generally, a compound has good permeability when Papp > 100 × 10−7 cm/s, and efflux liability shows up when EfR > 3 (Li et al., 2008). By looking through the data presented in Table 2, it was found that they all have good permeability except for SIN at the efflux direction (Papp = 47 × 10−7 cm/s). In addition, SIN at the absorption direction, 5-AcSIN, and HeptaMF at both directions have much higher Papp than 100 × 10−7 cm/s, indicating they have excellent permeability. EfR values for all the tested compounds are below 3, which means no efflux liability for those compounds.
 Click to view | Table 2. Apparent permeability coefficient and efflux ratio of tested PMFs and their derivatives |
3.6. Metabolism
Various enzymes were previously reported being present in the Caco-2 cell monolayer, which can metabolize certain compounds while they are transporting through the monolayer. For example, methylation and sulfation were observed on green tea catechins from the Caco-2 monolayer transport experiment (Zhang et al., 2004). In this study, we observed deacetylation from the transport of 5-AcSIN and 5-AcHeptaMF from the HPLC analysis. Deacetylation was found on 5-AcSIN from both AP-to-BL and BL-to-AP direction, but only detected from AP-to-BL transport for 5-AcHeptaMF. Therefore, in Figure 3, the permeability rates of 5-AcSIN and 5-AcHeptaMF were underestimated. After adjusting the amount deacetylated, Figure 4 gives the total amount of 5-AcSIN and 5-AcHeptaMF permeated in this test.
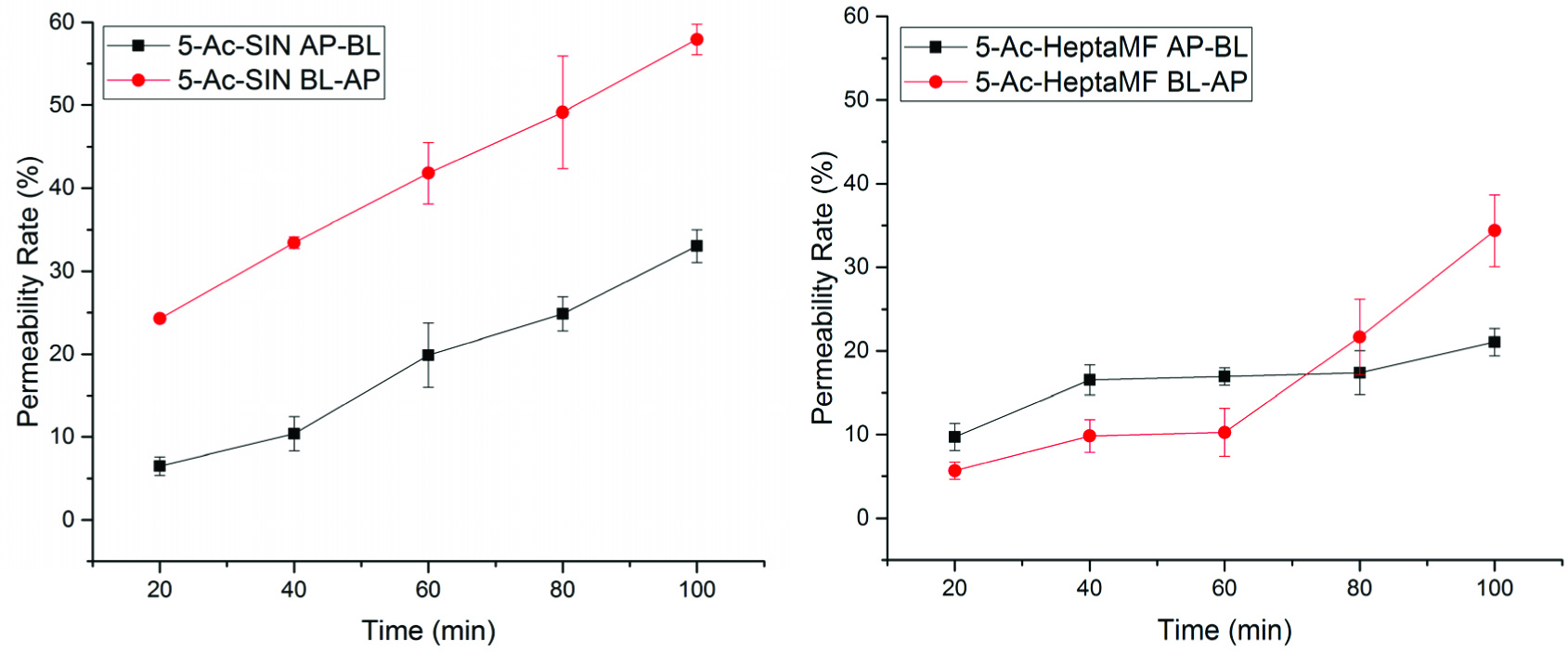 Click for large image | Figure 4. Total permeability rates of 5-AcSIN and 5-AcHeptaMF with the amount of deacetylation adjusted. |
For 5-AcSIN at 100 min, 33.03% (AP-BL) and 57.94% (BL-AP) permeated, which is much more than calculated before adjusting the amount of deacetylated (20.59 and 23.94%, respectively). Similarly, HeptaMF had 45.89% permeated at 100 min from AP to BL, but only 6.84% for 5-OH-HeptaMF and 6.56% for 5-Ac-HeptaMF, indicating that it is easier for PMFs to enter the systemic circulation than their 5-hydroxyl and 5-acetyl derivatives.
| 4. Conclusion | ▴Top |
Polymethoxyflavones have well-known biological functions, including anti-cancer, anti-inflammation, and antioxidants among others. It will benefit if we can fully understand bioavailability of this unique group of compounds. In order to make a contribution to research on the bioavailability of PMFs, this study systemically compared the permeability of PMFs, 5-hydroxyl-PMFs, and 5-acetylPMFs by applying the Caco-2 cell monolayer transport model.
Overall, PMFs and their tested derivatives exhibited good permeability. The idea of pro-drug should definitely be promoted since 5-Ac-PMFs have better permeability than 5-OH-PMFs. Metabolism, especially deacetylation, was observed in this research. Transport pathway of PMFs was not investigated, but it is highly needed in order to grasp the whole picture of their bioavailability. Furthermore, in-vivo studies are of great interest to test the accuracy of this Caco-2 cell monolayer transport model.
| References | ▴Top |