| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 2, June 2018, pages 58-81
Bioactives in seaweeds, algae, and fungi and their role in health promotion
Feriedoon Shahidi*, Md. Jiaur Rahman
Department of Biochemistry, Memorial University of Newfoundland St. John’s, NL, Canada A1B 3X9
*Corresponding author: Feriedoon Shahidi, Department of Biochemistry, Memorial University of Newfoundland St. John’s, NL, Canada A1B 3X9
DOI: 10.31665/JFB.2018.2141
Received: April 3, 2018
Revised received & accepted: May 5, 2018
| Abstract | ▴Top |
Seaweeds, algae, and fungi are rich sources of myriad bioactive compounds. In recent years, special attention has been paid to these secondary metabolites with biological activities that exist in aquatic foods and might render beneficial health effects in humans. Various antioxidant, anticancer, antidiabetic, antiobesity and antimicrobial bioactives have been isolated from seaweeds, algal and fungal species may be used as functional components in foods, food supplement, and as nutraceutical or medicine to promote human health and reduce disease risk. This review provides a cursory account of the health promoting bioactives such as sulfated polysaccharides, various polyphenols, polyunsaturated fatty acids (PUFAs), sterols, proteins and their hydrolysates, bioactive peptides, minerals, vitamins as well as pigments derived from various seaweeds, algal, and fungal species focusing on their potential application and health benefits. In addition, a brief description is provided for the extraction of such bioactives and techniques used to isolate and characterize them.
Keywords: Bioactives; Seaweeds; Algae; Fungi; Health benefits; Application
| 1. Introduction | ▴Top |
Human beings have relied on food as a main resource for nutrients and health. Bioactive compounds are naturally found in plant products and lipid-rich foods in small quantities (Kitts 1994; Kris-Etherton et al. 2002). They exert important effects on health promotion and also in prevention of gastrovascular and chronic diseases in the human body. In recent years, consumption of aquatic foods has increased globally due to their health benefits (Shahidi and Ambigaipalan 2015a). Many aquatic foods are known as functional foods mainly because of their bioactive components such as proteins and peptides, vitamins, minerals, essential fatty acids, polyphenols and carbohydrates which are either absent in the analogous traditional foods or present only in trace amounts (Kumur et al. 2011; Shahidi and Ambigaipalan 2015a). Consequently, concerted efforts have been made to explore and utilize bioactives from marine origin to better understand and enhance the quality of human diet (Kumur et al. 2011). In addition, a need for novel substances for the treatment of human diseases such as cancer, microbial infections, and inflammatory processes has intensified the exploration for new bioactive compounds (Hamed et al. 2015).
The marine world which covers some 70% of the earth surface holds one half of the total global biodiversity. Due to high diversity, the marine world offers a rich natural resource for many biologically active compounds such as sulfated polysaccharides, various polyphenols, polyunsaturated fatty acids (PUFAs), bioactive peptides and proteins, sterols, and pigments (Barrow and Shahidi 2007; Lorden et al. 2011). These marine bioactives are derived from a vast array of sources, including marine seaweeds, algae, fungi, and other aquatic species, all of which contain their own unique set of biomolecules. Bioactives in seaweeds, algae, and fungi can be procured by employing many different processes and methods. The solvent extraction for biologically active compounds includes solid-liquid extraction (SLE), and liquid-liquid extraction. Solvent extraction technique is mainly used in laboratory scale. This technique has several drawbacks like use of a high volume of solvents, low selectivity, low extraction efficiency, long extraction time, solvent residue, and environmental pollution (Ibanez et al. 2012; Kadam et al. 2013; Michalak and Chojnacka 2015; Najafian and Babji 2012). Many novel extraction techniques have been developed and applied for the extraction of biologically active compounds without loss of their activity such as supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), enzyme-assisted extraction (EAE), microwave-assisted extraction (MAE), and pressurized liquid extraction (PLE). These techniques are characterized by a higher extraction yield, shorter extraction time, and better quality of the final extract obtained in a solvent-free environment as a concentrate of biologically active compounds (Ibanez et al. 2012; Jeon et al. 2012; Kadam et al. 2013; Michalak and Chojnacka 2015). However, among all novel techniques, SFE is preferred in the food and pharmaceutical industries because of minimal or no use of organic solvents, faster extraction rate and high yield without loss of bioactivity of the desired compounds (Crampon et al. 2011; Halim et al. 2012; Ibanez et al. 2012; Kadam et al. 2013; Michalak and Chojnacka 2015; Sahena et al. 2009).
Regular consumption of seafood is associated with various beneficial health effects (Hamed et al. 2015; Shahidi and Abuzaytoun 2005; Shahidi et al. 1999). Currently, much attention has been paid on the exploration of new and novel bioactive compounds to treat human diseases such as cancer, microbial infections, and inflammatory ailments. Marine organisms can provide a rich source of such compounds. Many marine compounds such as peptides isolated from fish as well as algal polysaccharides have been reported to have anticancer, anticoagulant, and antihypercholesterolemic activities (Gupta and Abu-Ghannam 2011; Hamed et al. 2015; Himaya and Kim 2015; Holdt and Kraan 2011; Lordan et al. 2011; Michalak and Chojnacka 2015; Shahidi and Ambigaipalan 2015). Marine algal oils contain high amounts of omega-3 fatty acids, whereas seaweeds have potent antioxidants including carotenoids and phenolic compounds (Himaya and Kim 2015; Rasmussen and Morrissey 2007; Shahidi and Ambigaipalan 2015a). Therefore, marine-derived nutrients and non-nutritional bioactive components have excellent potential as functional food ingredients as they possess desirable physiological effects with medicinal characteristics and added health benefits (Barrow and Shahidi 2007; Lorden et al. 2015). This contribution focuses on marine bioactives derived from seaweeds, algae and fungi. In addition, it provides an overview of their potential sources, various methods of production, isolation and characterization. Finally, biological activities and potential application of various marine bioactive compounds as ingredients in functional foods, nutraceuticals, and pharmaceuticals are provided.
| 2. Bioactives in seaweeds, algae and fungi and their biological effects | ▴Top |
Marine organisms are rich sources of biologically active substances and their significance as a natural source of novel bioactives is constantly growing. With marine species covering around half of the total global biodiversity, the oceans and seas offer a huge reservoir of novel bioactives (Aneiros and Garateix 2004; Barrow and Shahidi 2007; Li et al. 2011). Among marine organisms, marine algae and seaweeds are rich sources of bioactives and their value as a source of novel substances has grown rapidly in recent years (Kim and Wijesekara 2010; Wijesekara and Kim 2010; Wijesekara et al. 2010, 2011). Each marine species is unique because of its life under different conditions, such as variation in salinity, pressure, temperature and light (Kulawik et al. 2013; Lordan et al. 2011). Compared to the terrestrial plants and animal-based foods, seaweeds, algae and fungi are rich in certain health-promoting molecules such as polysaccharides, proteins, peptides and polyphenols, pigments, dietary fiber, omega-3 fatty acids, and vitamins with antibacterial, antiviral, and antifungal properties, among others (Hamed et al. 2015; Holdt and Kraan 2011; Kumar et al. 2008b; Shahidi and Ambigaipalan 2015a).
2.1. Bioactive carbohydrates and their role in health and diseases
Carbohydrates are important in the human diet as a source of energy, but they are also used beyond basic nutrition due to their certain structural characteristics. Bioactive carbohydrates have unique structures and bear diagnostic and therapeutic potentials (Yang and Zhang 2009). Many bioactive carbohydrates have been isolated and characterized from marine sources and used as functional food ingredients which possess various biological activities such as antioxidation, anticoagulation, anticancer and antiviral activities (Freitas et al. 2015; Hamed et al. 2015; Holdt and Kraan 2011; Shahidi and Ambigaipalan 2015a).
2.1.1. Bioactive carbohydrates in seaweed and algae
Polysaccharides are polymers of simple sugars (monosaccharides) linked by glycosidic bonds (Kalimuthu and Kim 2015). Recently, many bioactive polysaccharides with desirable functional properties have been discovered from marine algae and seaweeds (Kalimuthu and Kim 2015; Patel 2012). Polysaccharides in seaweeds and algae can be divided into three groups: 1. Structural cell wall polysaccharides 2. Mucopolysaccharides and 3. Storage polysaccharides (Chandini et al. 2008; Kalimuthu and Kim 2015; Murata and Nakazoe 2001). However, the cell wall and storage polysaccharides are species-specific and possess various biological activities.
Seaweeds and algae are rich in sulfated polysaccharides and their biological activities have been reviewed in many studies (Gupta and Abu-Ghannam 2011; Hamed et al. 2015; Holdt and Kraan 2011; Laurienzo 2010). These compounds have high diversity in structural constituents because of differences in molecular weight, disaccharides formation and sulfation which comprise a complex group of macromolecules (Himaya and Kim 2015). Polysaccharides such as agar, alginate, and carrageenans, derived from marine seaweeds, are widely used as thickening and stabilizing agents in gels as well as in foods (Freitas et al. 2015; Rasmussen and Morrissey 2007). Bioactive sulfated polysaccharides found in red algae are mainly galactans (agars, carrageenans) which consist entirely of galactose or modified galactose units. In addition, xylans, floridean starch and porphyrin are mucopolysaccharides found in this type of algae (Holdt and Kraan 2011; Kumar et al. 2008b; Murata and Nakazoe 2001). Brown algae is a well-known source of alginic acid, fucoidan, laminarin (β-1,3 glucan) and sargassan, whereas green algae also serve as a good source of sulfated polysaccharides like ulvans and uronic acids such as glucuronic acid (Gupta and Abu-Ghannam 2011; Hamed et al. 2015; Holdt and Kraan, 2011). These bioactive polysaccharides have been reported to possess anti-inflammatory, antithrombotic, antioxidant, antidiabetic, and anticancer activities and also known to act as modulators of coagulation (Pomin 2009; Wijesekara et al. 2011). The potential sources and biological activities of various sulfated polysaccharides are summarised in Table 1.
 Click to view | Table 1. Bioactive carbohydrates, sources and their biological activities |
Fucoidans and their health benefits
Fucoidan is a sulfated polysaccharide (average MW: 20 kDa) and widely found in the cell wall of brown seaweeds and also several species of brown algae for example mozuku, Kombu, bladderwrack, limu moui, wakame, and hijiki (Berteau and Mulloy 2003; Hamed et al. 2015; Kalimuthu and Kim 2015). Berteau and Mulloy (2003) reported that fucoidan content of brown algal cell wall was more than 40% of dry weight and can easily be extracted using either hot water or an acid solution. It is a complex polysaccharide that consists of α -1, 3-linked sulfated L-fucose as its main sugar unit and sulfate ester groups (Fig. 1). Fucoidan was first isolated from marine brown algae (Kylin 1913). Structurally, fucoidan is a heparin-like molecule and soluble in both water and acid. Acid hydrolysis of fucoidan yields various quantities of D-xylose, D-galactose, and glucuronic acid (Gupta and Abu-Ghannam 2011; Ruperez et al. 2002). However, biological activities of fucoidan depends on the source, molecular weight, composition, and structural heterogeneity (Fitton 2011; Kalimuthu and Kim 2015). Low molecular weight fucoidan (LMWF) has more biological activity than native fucoidan. The biological activity of fucoidans vary with their molecular weight, which is generally classified as low (<10 kDa), medium, or high molecular weight >10,000 kDa (Kalimuthu and Kim 2015). It has been reported that low molecular weight fucoidans have higher anti-tumor activity than high molecular weight heterofucans with low degree of sulfation (Kalimuthu and Kim 2015).
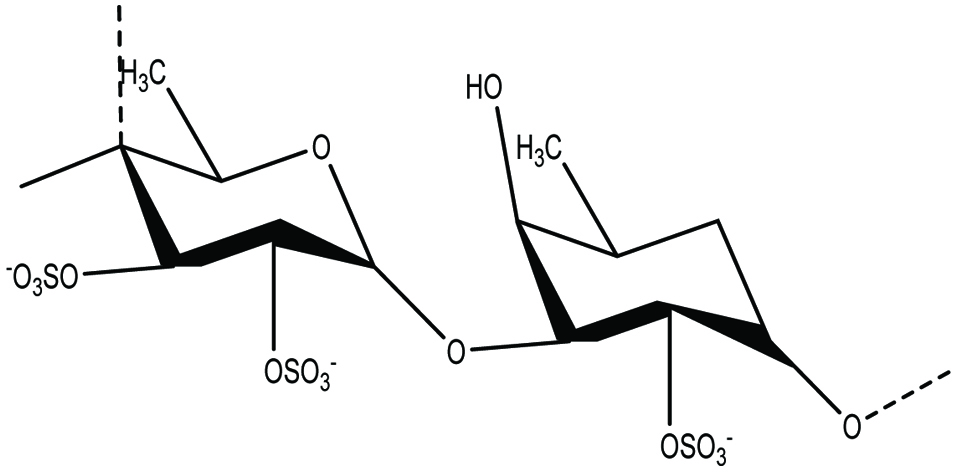 Click for large image | Figure 1. Chemical structure of fucoidan. |
Fucoidan has been reported in many edible seaweed species such as Turbinaria conoides, Fucus vesiculosis, Cladosiphono kamuranus, Laminaria japonica, and Undaria pinnatifida (Chattopadhyay et al. 2010; Himaya and Kim 2015; Luo et al. 2009). Fucoidans have been extensively reviewed for their various physiological and biological activities, such as antiviral, antitumor, anticoagulant, antioxidant, neuroprotective, and antithrombotic, as well as the impact on the inflammatory and immune systems (Ale et al. 2011; Anastyuk et al. 2012; Hamed et al. 2015; Lee et al. 2011, 2012; Luo et al. 2009; Thomas et al. 2010; Wang et al. 2009; Wijesekara et al. 2011). Fucoidan isolated from two brown seaweeds, namely Undaria and Laminaria, exhibited anticoagulant, antiviral and anticancer activities (Chevolot et al. 2001; Kalimuthu and Kim 2015; Zhuang et al. 1995). Fucoidan has stimulating effects on immune system and ability to modify cell surface (Kalimuthu and Kim 2015; Usov et al. 2001). Oral intake of fucoidans has been reported to inhibit viral replication and stimulation of the immune system (innate and adaptive) functions (Hayashi et al. 2008). The mechanism of antiviral activity of fucoidan is to stop viral sorption through inhibiting viral-induced syncytium formation. It has been reported that sulfate group of fucoidan plays an important role in its antiviral activity, whereas (1-3)-linked fucopyranosyl units are very important for the anti-herpetic activity (Kalimuthu and Kim 2015; Mandal et al. 2007). Fucoidan has been reported to possess anti-hyperlipidemic activity and that fucoidan extracted from L. japonica reduced total serum and LDL-cholesterol and triacylglycerols and raised HDL-cholesterol in a hyperlipidemic rat model (Huang et al. 2010; Kalimuthu and Kim 2015).
Several literature reports have shown that fucoidan has anti-tumor effects, but its mode of action is not fully understood. Aisa et al. (2005) reported that fucoidan prevents proliferation and induces apoptosis of human lymphoma HS-Sultan cells. Ermakova et al. (2011) reported that fucoidan extracted from brown seaweeds such as Eclonia cava, Sargassum hornery, and Costaria costata inhibited human melanoma and colon cancer cells.
A native fucoidan isolated from Fucus evanescens was able to inhibit the growth of human malignant melanoma cancer cell (SK-MEL-28 and SK-MEL-5). Cumashi et al. (2007) reported that fucoidans strongly blocked MDA-MB-231 breast carcinoma cell adhesion and implications in tumor metastasis and the extract of fucoidan used was derived from various brown seaweeds such as L. saccharina, L. digitata, F. serratus, F. distichus and F. vesiculosus. Fucoidan has also been reported to inhibit the proliferation of prostate cancer (PC-3) cells (Boo et al. 2013). Zhang et al. (2013) reported that low molecular weight fucoidan (LMWF) induced apoptosis through various mitochondrial mediated pathways in MDA-MB-231 breast cancer cells and also played related roles of Ca2+ homeostasis, mitochondrial dysfunction and caspase initiation. Fucoidan from the marine alga Cladosiphon okamuranus (Phaeophyceae) has been shown to inhibit dengue virus type 2 infection (Hidari et al. 2013), and found that it interacts directly with envelope glycoprotein on the virus. Thus, fucoidan could be a good drug for prevention of the dengue virus.
Agar and its health benefits
Agar is a water-soluble long-chain polysaccharide (Fig. 2) mainly found in red algae. It consists of agarose and agaropectin and widely used in the manufacture of gelatin. Agar content in Gracilaria species can reach 31% (Holdt and Kraan 2011). Agar has been isolated and characterised from several of seaweeds such as Gracilaria sp., Gracilaria cornea, Gracilaria dominguensis, Gigartina sp. and Gelidium sp. (FAO 2008; Fernandez et al. 1989; Freile-Pelegrin and Robledo 1997; Holdt and Kraan 2011; Mouradi-Givernaud et al. 1992). Agar is active in decreasing blood sugar concentration and exerts an anti-aggregation effect on red blood cells, and displays antioxidant, antitumor as well as antiviral activities (Holdt and Kraan 2011; Murata and Nakazoe 2001). It also shows activity against α-glucosidase and absorption effect of UV rays (Chen et al. 2005, Murata and Nakazoe 2001). In Japan, it is a typical and traditional food and is used for cooking and in Japanese style confectionary. In addition, in medical application, agar is used in the manufacture of capsules for treatment of various diseases and also as a medium for cell cultures biotechnology and microbiology.
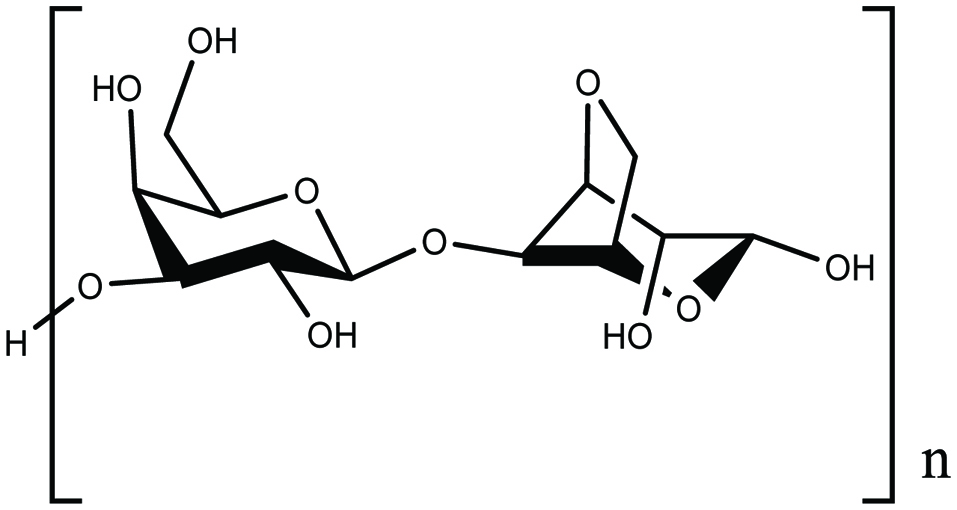 Click for large image | Figure 2. Chemical structure of agar. |
Anti-tumor activity has been reported in Gracilaria sp. an agar-type polysaccharide from cold water. It has also been shown that agar content varies seasonally from 26 to 42% in Gelidium spp. (Holdt and Kraan 2011; Jeon et al. 2005). Agaro-oligosaccharides also suppress the production of a pro-inflammatory cytokine and an enzyme associated with the production of nitric oxide (Enoki et al. 2003; Holdt and Kraan 2011).
Carrageenan and its health benefits
Carrageenans are a group of linear sulfated polysaccharide of D-galactose and 3, 6-anhydro-D-galactose (Fig. 3). Carrageenans can be categorized into three general forms, namly kappa, lambda, and iota, having strong gelling properties (Holdt and Kraan 2011; Rasmussen and Morrissey 2007). They are water soluble and form highly viscous solutions and remain stable over a wide pH range (Holdt and Kraan 2011). In general, carrageenans are derived from some red algal species such Chondrus crispus, Eucheuma cottonii, Kappaphycus alverezii, Kappaphycus striatam, Gigartina skottsbergii (Caceres et al. 2000; Carlucci et al. 1997; Rodrigueza and Montano 2007; Shanmugam and Mody 2000; Zhou et al. 2006a). It has been reported that marine algae Chondrus crispus and Kappaphycus sp. contain up to 71 and 88% of carrageenan, respectively (Chopin et al. 1999; Holdt and Kraan 2011; Rodrigueza and Montano 2007). There are food applications of carrageenans (E-407) and are widely used in canned foods, salad dressings, bakery fillings, dessert mousses, ice creams, instant desserts and canned pet foods (Holdt and Kraan 2011). The industrial applications of purified extracts of carrageenans include clarification of beer, wine and honey (Holdt and Kraan 2011). From a human health perspective, carrageenans have been reported to possess anti-tumor and antiviral properties (Holdt and Kraan 2011; Vlieghe et al. 2002; Zhou et al. 2006b). Irish moss or carrageen (C. crispus and Mastocarpus stellatus) is used in Ireland to make traditional medicinal teas and cough medicines to cure cold, bronchitis and chronic cough (Holdt and Kraan 2011). In addition, carrageenan is used to cure bowel problems such as diarrhoea, constipation and dysentery (Holdt and Kraan 2011). It is also reported that it is useful for dislodging mucus and has antiviral properties and used as suspension agent and stabiliser in other drugs, lotions and medicinal creams (Holdt and Kraan 2011). In addition, it serves as a good anticoagulant in blood products and control of stomach ulcers working as internal poultices (Holdt and Kraan 2011; Morrissey et al. 2001). Carrageenan gels from C. crispus may block the transmission of the HIV virus and also other STD viruses such as gonorrhoea, genital warts and the herpes simplex virus (HSV) (Caceres et al. 2000; Carlucci et al. 1997; Holdt and Kraan 2011; Luescher-Mattli 2003; Shanmugam and Mody 2000; Witvrouw and DeClercq 1997).
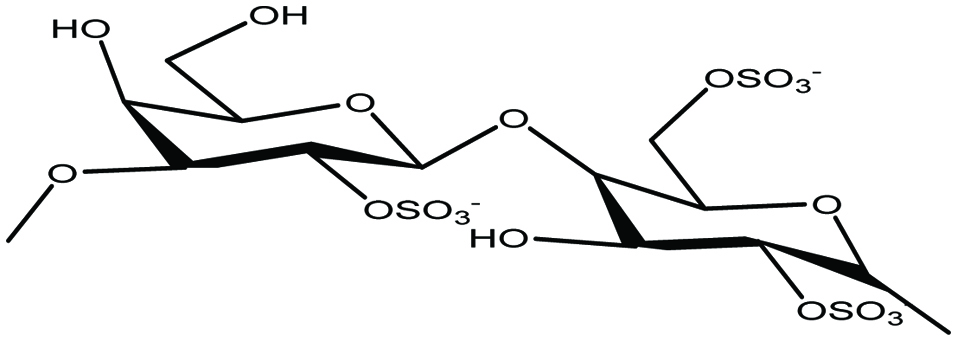 Click for large image | Figure 3. Chemical structure of carrageenan. |
It inhibits herpes simplex virus and HIV-1 infectivity was about 1,000-fold higher than the IC50 observed for genital HPVs in vitro (Luescher-Mattli 2003; Witvrouw and DeClercq 1997). The basis of the anticoagulant activity of carrageenan appears to be an anti-thrombotic property. λ-Carrageenan showed a greater anti-thrombotic activity than κ-carrageenan, probably due to its higher sulfate content. Similar results were obtained with λ-carrageenan of Phyllophora brodiaei which gave the highest blood anticoagulant activity (Holdt and Kraan 2011; Sen et al. 1994). In addition, the hypoglycemic effect of carrageenan may prove useful in the prevention and management of metabolic conditions such as diabetes (Dumelod et al. 1999; Holdt and Kraan 2011).
Laminarin and its health benefits
Laminarin, the second source of glucan and a major polysaccharide, has been studied in brown algae for its various bioactivities. Laminarin is a water-soluble polysaccharide composed of (1,3)-β-D-glucan with β (1,6) branching and contains 20–25 glucose units (Fig. 4) (Gupta and Abu-Ghannam 2011). It has been identified as a potential regulator of intestinal metabolism through its sound effects on intestinal pH level, mucus structure, and short chain fatty acid production (Deville et al. 2007; Freitas et al. 2015; Hamed et al. 2015; Holdt and Kraan 2011). Laminarin has been reported as a tumor-inhibiting agent in the form of surgical dusting powder and, as an anticoagulant in the form of a sulphate ester (Holdt and Kraan 2011; Miao et al. 1999). In addition, several functions of laminarin such as antibacterial, protection against severe irradiation, boosting the immune system by increasing the B cells and helper T cells, lowering systolic blood pressure and reducing cholesterol levels in serum (Hoffmane et al. 1995; Holdt and Kraan 2011), lowering of total cholesterol and free cholesterol levels, lowering of liver triacylglycerol and phospholipid (Holdt and Kraan 2011; Miao et al. 1999; Renn et al. 1994a, b) have been documented.
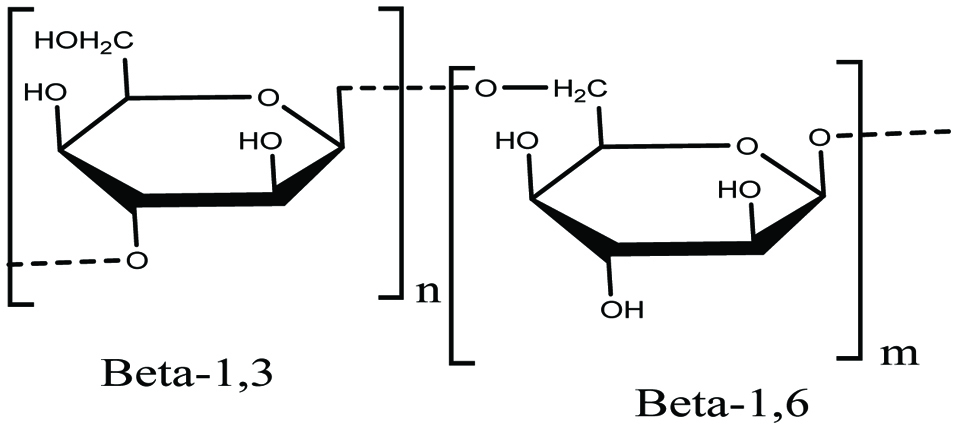 Click for large image | Figure 4. Chemical structure of lamarinin. |
Alginate and its health benefits
Alginic acid or alginate is a linear sulfated polysaccharide composed of 1,4-linked β-D-mannuronic and α-L-guluronic acid residues arranged in structural chain (Fig. 5) (Andrade et al. 2004; Gupta and Abu-Ghannam 2011). Alginate extracted from brown algae, mainly in the form of sodium and calcium alginate, has wide applications in the food and pharmaceutical industries because of its ability to form highly viscous solutions and to chelate metal ions (Gupta and Abu-Ghannam 2011). It has been reported that alginic acid plays a key role in human heath by decreasing the concentration of cholesterol, exerting an anti-hypertension effect and also preventing the absorption of toxic chemical substances (Holdt and Kraan 2011; Kim and Lee 2008; Murata and Nakazoe 2001; Nishide and Uchida 2003).
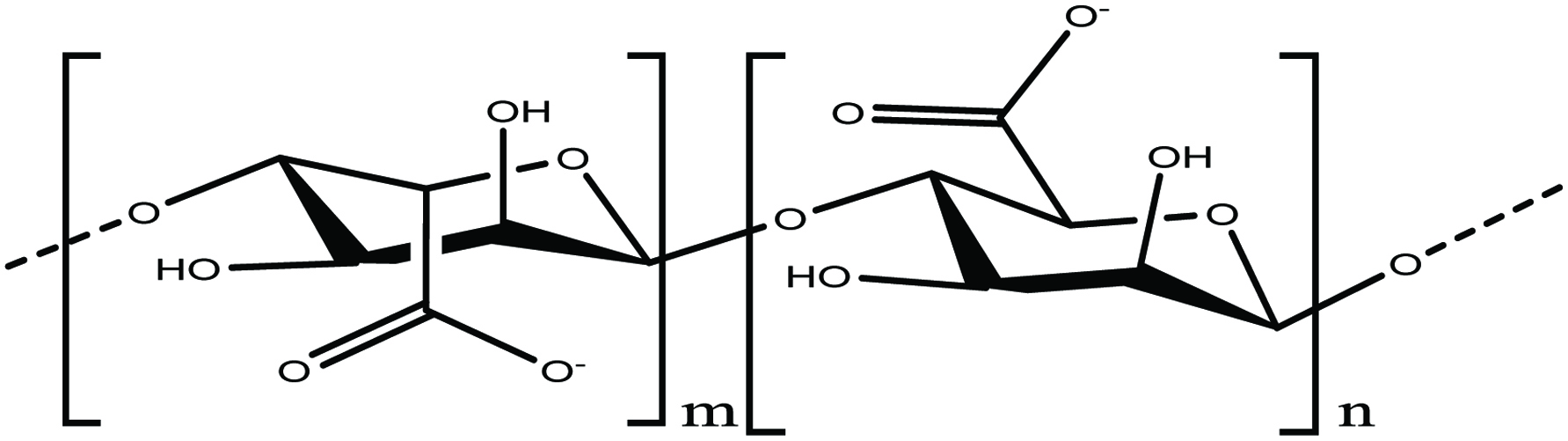 Click for large image | Figure 5. Chemical structure of alginate. |
Ulvan and its health benefits
Ulvans are highly branched, water-soluble sulfated polysaccharides containing rhamnose, uronic acid and xylose as their main monomeric sugars (Fig. 6). The potential sources of marine Ulvans are Ulva lactuca, Ulva rigida, and Monostroma sp. (Lahaye and Robic 2007; Nagaoka et al. 2003). Ulvans also serve as a potential source of iduronic acid that is used in the synthesis of heparin fragment analogues with anti-thrombotic activities (Lahaye and Robic 2007). It has been reported that Ulvan can be used for the treatment of gastric ulcers, and acts as anti-influenza (Lahaye and Robic 2007; Nagaoka et al. 2003).
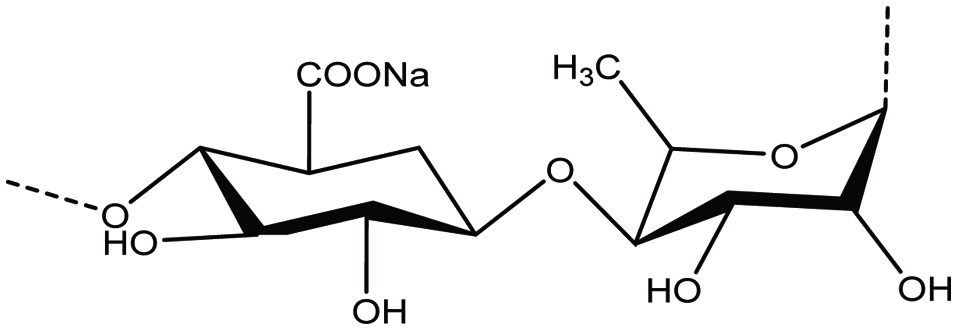 Click for large image | Figure 6. Chemical structure of ulvan. |
Porphyran and its health benefits
Porphyran is a sulfated polysaccharide and the main component of the red algae Porphyra; it is active as antioxidant and anticoagulant (Hamed et al. 2015). β- 1,3-Glucan is another sulfated polysaccharide that has been reported from Chlorella. It is active an as immunostimulator, a free radical scavenger and thus reduces blood lipids (Hamed et al. 2015; Spolaore et al. 2006). It has been reported that polysaccharides extracted from microalgae (Porphyridium and Nostac flegelliforme) exert biological activities against Herpes simplex virus (HSV-1 and HSV-2) (Kanekiyo et al. 2007; Vo and Kim 2010).
Chitin, chitosan and chitosan oligosaccharides (COS) and their health benefits
Chitin, a long-chain polymer of an N-acetyl-D-glucosamine, is a naturally abundant polysaccharide and is found in various marine species. It is the most abundant biopolymer in nature having unique structures and multidimensional properties after cellulose (Jeon et al. 2000; Rasmussen and Morrissey 2007; Shahidi and Abuzaytoun 2005; Shahidi and Ambigaipalan 2015a). Its N- acetyl -D-glucosamine residues are linked by β-(1-4) bonds (Fig. 7) (Rasmussen and Morrissey 2007; Shahidi and Abuzaytoun 2005; Shahidi et al. 1999). In both terrestrial and aquatic organisms, chitin is a major structural part in their hard-outer coatings (Barrow and Shahidi 2007; Rasmussen and Morrissey 2007). Chitin is mainly found in shells of crab, shrimp, lobster, and crayfish as a component of their exoskeleton. In addition, chitin is distributed in the cell wall of some marine green algae (Chlorella sp.), protozoa, as well as fungi (Zygomycetes) (Barrow and Shahidi 2007; Jeon et al. 2000; Rasmussen and Morrissey 2007; Shahidi et al. 1992, 1999).
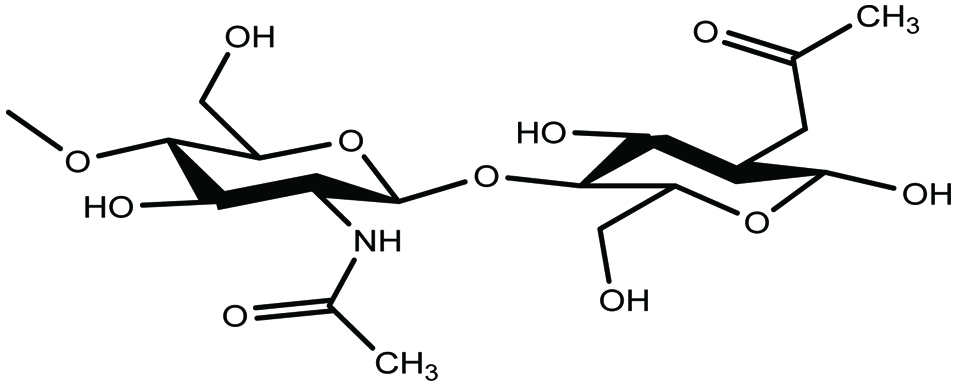 Click for large image | Figure 7. Chemical structure of chitin. |
Partially alkaline deacetylation of chitin produces a variety of derivatives, such as chitosan, chitosan oligosaccharides (COS) and glucosamine. Because of their increased water solubility, these chitosan derivatives are more effective as nutraceutical and functional food ingredients than the fully acetylated, insoluble form of chitin (Barrow and Shahidi 2007; Rasmussen and Morrissey 2007; Shahidi and Abuzaytoun 2005; Shahidi and Ambigaipalan 2015a; Jeon et al. 2000; Shahidi et al. 1992; 1999).
Chitosan is a linear copolymer containing a mixture of both D-glucosamine and N- acetyl -D-glucosamine monomers that are linked by β-(1-4) bonds (Fig. 8) (Barrow and Shahidi 2007; Jeon et al. 2000; Rasmussen and Morrissey 2007; Shahidi and Abuzaytoun 2005; Shahidi et al. 1992, 1999). It is basically a partially deacetylated form of chitin (Barrow and Shahidi 2007; Shahidi and Abuzaytoun 2005;). The amino groups of chitosan carry a positive charge and are soluble in acidic solutions (Rasmussen and Morrissey 2007). Because of its inherent properties and its ability to interact with a range of biomolecules, chitosan exhibits a number of health benefits. Several biological activities have been described for chitosan and chitooligosaccharides, such as antioxidant, antimicrobial, and an immune system booster, inhibition of angiotensin-Iconverting enzyme, anticancer, hypoglycemic, hypocholesterolemic, and anticoagulant activities (Rasmussen and Morrissey 2007; Freitas et al. 2015; Jeon et al. 2000; Shahidi and Abuzaytoun 2005; Wijesekara and Kim 2010; Shahidi and Ambigaipalan 2015a; Shahidi et al. 1992, 1999a, b). Chitosan can adsorb humic acid in the gastrointestinal tract. Consequently, it could be used as a bioactive dietary fiber for the inhibition of blackfoot disease (Chen et al. 2011; Freitas et al. 2015). In addition, many biological activities have been reported for low molecular weight (LMW) chitosans including reduction in total cholesterol, low density lipoprotein (LDL) and liver triacylglycerol levels in rats fed high-fat diets (Ibanez et al. 2012; Jeon et al. 2000; Shahidi and Ambigaipalan 2015a; Shahidi et al. 1992, 1999; Zhang et al. 2012). Furthermore, it has been reported that acid-soluble chitosan with 99% deacetylation was more effective in inhibiting bacterial growth than water-soluble chitosan (Jung et al. 2010; Jeon et al. 2000; Shahidi et al. 1992, 1999). It should be noted that chitin, chitosan and related products do not exist much in seaweeds, and algal species and hence this topic has only been covered in a cursory manner in this contribution. However, chitin is can be found in the cell wall of some marine green algae (Chlorella sp.), protozoa, as well as fungi (Zygomycetes) (Barrow and Shahidi, 2007; Jeon et al. 2000; Rasmussen and Morrissey 2007; Shahidi et al. 1992, 1999).
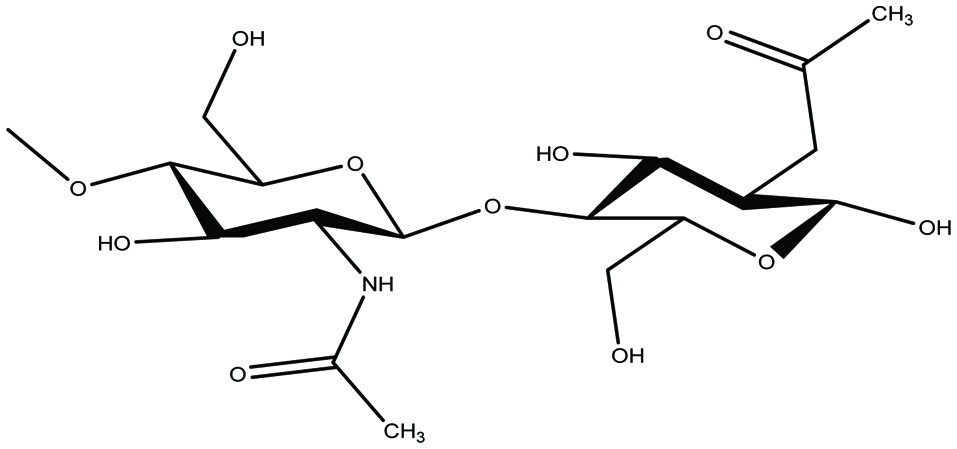 Click for large image | Figure 8. Chemical structure of chitosan. |
2.2. Bioactive phenolics and polyphenolics
The interest in antioxidant-rich food and dietary supplements with antioxidant potential has recently increased with the hope of keeping the body healthy and free from diseases (Sen and Chakraborty 2011). Antioxidants can play their protective role against free radicals by different mechanisms such as HAT (hydrogen atom transfer), SET (single electron transfer) or by binding or inactivating metal ions that prevent the generation of reactive oxygen species (ROS), among others (Shahidi 2000, 2004; Shahidi and Ambigaipalan 2015b; Shahidi and Naczk 1995; Shahidi et al. 1992, 2008; Rahman et al. 2017, 2018a, 2018b). The role of phenolic compounds as antioxidant and activity against cancer, coronary heart disease (CHD), neurological deterioration, inflammatory problems, and aging is well documented (Castro-Puyana et al. 2013; Shahidi 2000, 2004; Shahidi and Ambigaipalan 2015a; Shahidi and Naczk 1995; Shahidi et al. 1992, 2008; Wollgast and Anklam 2000). Currently, antioxidants are used in various products manufactured by the food and pharmaceutical industries. Because of the important role of antioxidants in food preservation and health promotion, special attention has been paid to their extraction from novel resources (Herrero et al. 2006; Shahidi and Ambigaipalan 2015a). However, the application of synthetic antioxidants in food or medicine has been restricted because of their possible side effects that may pose safety risks to humans (Shahidi 2000, 2004; Shahidi and Ambigaipalan 2015a; Shahidi et al. 1992, 2008; Vadlapudi 2012). Therefore, marine seaweeds, fungi and algal species as well as other aquatic species might provide a new natural rich source of possibly safe antioxidants.
2.2.1. Bioactive phenolic and polyphenolics in seaweeds and algae
Polyphenols are a large group of secondary metabolites found in plant materials associated with multiple phenol structural units. Basically, they serve as potential antioxidants against ultraviolet (UV) radiation and oxidative stress (Shahidi 2000, 2004; Shahidi and Ambigaipalan 2015b; Shahidi et al. 1992, 2008). Plant phenolics can be classified as simple phenols, phenolic acids (both benzoic and cinnamic acid derivatives), coumarins, flavonoids, stilbenes, hydrolysable and condensed tannins, lignans, and lignins (Naczk and Shahidi 2004).
Seaweeds are a rich source of phenolic compounds. However, marine algae have also been described as a potential source of secondary metabolites with antioxidant activities, such as tocopherols (Yuan et al. 2005), carotenoids, polyphenols (phlorotannins) (Lee et al. 2013; Michalak and Chojnacka 2015; Ragan and Glombitza 1986), catechins (Michalak and Chojnacka 2015; Yoshie et al. 2000;) flavonoids, tannins, and lignans (Gupta and Abu-Ghannam 2011; Michalak and Chojnacka 2015). Various phenolic compounds such as stypodial, isoepitaondiol, taondiol, sagaol, sagaquinone, stypoldione have been identified and quantified from brawn algae T. atomaria (Fig. 9) (Nahas et al. 2007) and terpenoids from Cystoseria sp. (Foti et al. 1994). Marine algae Halimeda sp. has been reported as being a good source of polyphenols such as catechin, epicatechin etc. and flavonoids from Palmaria palmate have also been noted (Yuan et al. 2005).
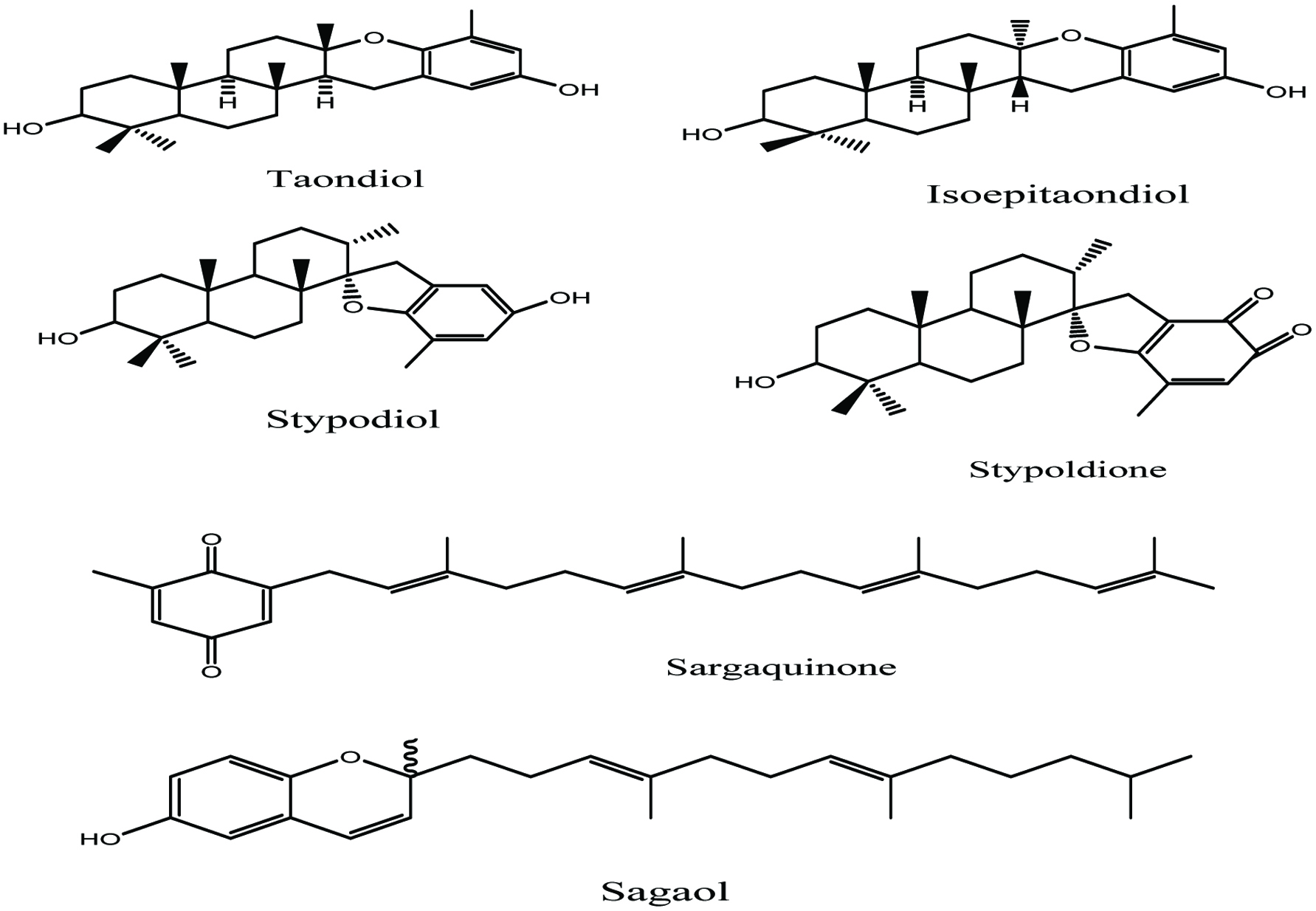 Click for large image | Figure 9. Isolated secondary metabolites from marine Alga T. atomaria (Nahas et al. 2007). |
2.2.2. Phlorotannins in marine seaweeds and algae
Phlorotannins are a series of phloroglucinol (1,3,5- trihydroxybenzene)-based polyphenols widely found in marine brown algae cell walls (Hu et al. 2016). Phlorotannins are well-known to be synthesised by the acetate-malonate pathway and finally converted into hydrophilic polymers of phloroglucinol monomers with a wide range of molecular weights varying from 126 to 650 Da (Hu et al. 2016; Vo and Kim 2010). They help brown algae to survive under stress conditions and escape from herbivores. They are diverse in structure and polymerisation and constitute an extremely heterogeneous group of molecules displaying a wide range of potential biological activities (Burtin 2003; Holdt and Kraan 2011). In marine seaweeds, phlorotannins are found in physodes which are cytoplasmic vesicles surrounded by bound membrane, and the secretion of phlorotannins in seaweeds occurs due to the fusion of physodes with cell membranes (Bartsch et al. 2008; Li et al. 2009; Luder and Clayton 2004).
In marine algae, pholorotannins can be categorized into four subclasses 1. Fucols (with a phenyl group), 2. Phlorethols (Contain ether bonds), 3. Fucophlorethols (Contain both ether and phenyl link) and Eckols (with a dibenzodioxin link) (Himaya and Kim 2015; Jeon et al. 2015: Li et al. 2011). Many brown algal species such as Ecklonia spp., Undaria pinnatifida, Sargassum thunbergii, Ishigeo kamurae, and Laminaria japonica are reported to contain bioactive phlorotannins (Jeon et al. 2015; Jung et al. 2008; Heo et al. 2009; Li et al. 2011; Okada et al. 2004). The well-studied phlorotannins derived from brown algae Ecklonia sp. are phloroglucinol, eckol, dieckol, 6,6-bieckol, phlorofucofuroeckol A and also pholorotannins such as dioxinodehydroeckol, 7-phloroeckol isolated from Eisenia bicyclis, and diphlorethohydroxycarmalol from Ishige okamurae (Fig. 10) (Lee and Jeon 2013; Michalak and Chojnacka 2015).
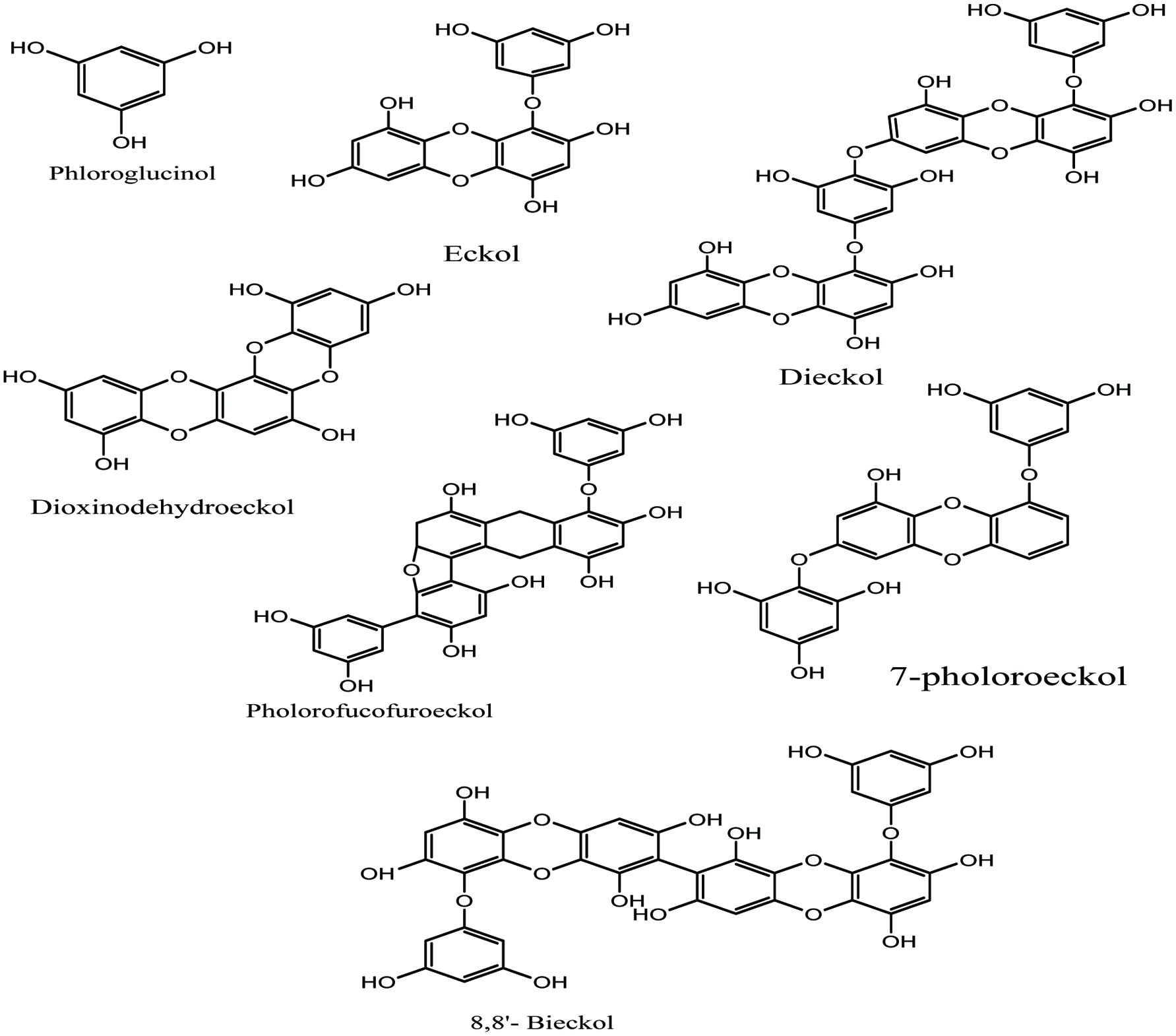 Click for large image | Figure 10. Isolated pholorotanins derivatives from various brown algae. |
2.2.3. Role of bioactive polyphenols in health promotion
Polyphenols are known as natural antioxidants because of their activities in scavenging free radicals and acting in radiation protection, antidiabetic and antibiotic effects (Holdt and Kraan 2011; Shahidi 2000, 2004; Shahidi and Ambigaipalan 2015b; Shahidi and Naczk 1995; Shahidi et al. 1992, 2008). Some polyphenols also exert protective effects against cancer, cardiovascular disease, arthritis and autoimmune disorders by assisting to protect tissues against oxidative stress (Holdt and Kraan 2011; Kang et al. 2003; Shahidi 2000, 2004; Shahidi and Ambigaipalan 2015b; Shahidi and Naczk 1995; Shahidi et al. 1992, 2008). Furthermore, polyphenols are reported to act as antibacterial agents (e.g. anti-Staphylococcus activity) and have anti-allergic effect and anti-inflammatory activity together with other comprehensive therapeutic perspectives (Holdt and Kraan 2011; Li et al. 2009; Shahidi 2000, 2004; Shahidi and Ambigaipalan 2015b; Shahidi and Naczk 1995; Shahidi et al. 1992, 2008).
2.2.4. Phlorotannins and their role in health promotion
Phlorotannins are polyphenolic compounds that have been reported in many brown algal families and possess antioxidant activity (Hamed et al. 2015; Lordan et al. 2011). They are known to play an important role in protecting from ultraviolet (UV) radiation and oxidative stress and exhibit many other biological activities (Hamed et al. 2015; Himaya and Kim 2015). A variety of phlorotannins have been studied for their biological activities in human health promotion and disease risk reduction, as described below.
Antioxidant activity
Pholorotannins as polyphenolic compounds possessing strong biological activity and as such are derived from marine brown algae. They exert strong antioxidant activities against free radical-mediated oxidative damage (Heo et al. 2005; Kang et al. 2003, 2004; Shibata et al. 2008). Li et al. (2009) reported that in the lineloic acid model system, the phlorotannins extract of marine brown alga, Ecklonia cava, exhibited strong potential against 1,1-diphenyl 1,2-picrylhydrazyl (DPPH), hydroxyl, superoxide, and peroxyl radicals in vitro, using electron spin resonance(ESR) technique. Siriwardhana et al. (2005) reported that phlorotannins extract derived from H. fusiformis showed potential as radical scavenger, thus serving as a viable source of natural antioxidative compounds. Furthermore, several phlorotannins which were purified from brown seaweeds such as E. cava, E. kurome, E. bicyclis, and H. fusiformis, are found to exhibit potent antioxidant activities and show protective effects against hydrogen peroxide-induced cell damage (Kang et al. 2005a, b, 2006). In addition, Shibata et al. (2008) reported that eckol, phlorofucofuroeckol A, dieckol, and 8,8″-bieckol show a potent inhibition of phospholipid peroxidation at 1µM in a liposome system and these phlorotannins have significant radical scavenging activities against superoxide and DPPH radicals compared to ascorbic acid and α-tocopherol.
Anti-obesity activity
Phlorotannins are active compounds that prevent obesity and obesity-related disorders mediated by multiple mechanisms. Several studies have demonstrated that phlorotannins may prevent fat absorption through the inhibition of pancreatic lipase. Eckol has been isolated and characterized from marine brown alga E. bicyclis and showed its anti-diabetic role as inhibitory activity against glycation and α-amylase enzyme (Himaya and Kim 2015; Okada et al. 2004). The extract of brown alga E. cava due to eckol and dieckol has been shown inhibitory effect on melanogenesis and defensive properties against photooxidative stress prompted by UV-B radiation (Heo et al. 2009; Himaya and Kim 2015). Both dioxinodehydroeckol and 7-phloroeckol were isolated from E. cava. They are potential anticancer drugs for human breast cancer and apoptosis (Herrero et al. 2006; Himaya and Kim 2015).
Health benefits of the extract of marine brown Alga T. atomaria
It has been reported that the extract of the brown Alga T. atomaria exhibit many biological activities such as anticancer effect because of the metabolite stypodiol (Mayer and Lehmann 2000) and inhibition of microtubule in in vitro studies because of the presence of stypoldione (Jacobs et al. 1985; Nihash et al. 2007). Stypoldione has also been shown to exhibit a relatively low toxicity and in prolonging the survival of mice injected with tumor cells (Nihash et al. 2007; O’Brien et al. 1984). The extract of brown Alga T. atomaria showed antifungal activity because of the metabolite compound taondiol (Nihash et al. 2007; Wessels et al. 1999), and insecticidal activity due to the compound isoepi-taondiol (Gerwick and Fenical 1981; Nihash et al. 2007). In addition, hydroxysargaquinone, a metabolite also found in brown Alga T. atomaria, exhibited cytotoxicity against P-388 lymphocytic cells (Nihash et al. 2007).
2.3. Bioactive carotenoids
Carotenoids are the most abundant pigment group of compounds in nature and occur in the free form as well as in the form of esters, glycosides, sulfates and carotenoproteins (Matsuno 2001; Shahidi et al. 1998). They are yellow to red isoprenoid polyene pigments with a 40-carbon polyene chain, which offers unique molecular structures (Himaya and Kim 2015; Shahidi et al. 1998). On the basis of structure, carotenoids are divided into two major classes of carotenes, which are hydrocarbons containing no oxygen, such as α-, β-, and γ-carotenes, as well as lycopene, and oxygenated derivatives, known as xanthophylls, such as fucoxanthin, cantaxanthin, astaxanthin, cryptoxanthin, lutein, and zeaxanthin (Del Campo et al. 2000; Guedes et al. 2011; Himaya and Kim 2015; Shahidi et al. 1998). Marine seaweeds, algae, fungi, and aquatic species contain various carotenoids that show structural diversity (Maoka 2011; Matsuno 2001; Shahidi et al. 1998;). Many carotenoids have been identified from marine resources and their pharmaceutical potential activities studied such as β-carotene, lycopene, zeaxanthin, fucoxanthin, astaxanthin, cantaxanthin, lutein, and violaxanthin (Himaya and Kim 2015; Jaswir et al. 2011; Plaza et al. 2009; Shahidi et al. 1998). These carotenoids have been reported as potent antioxidants and their activities are well described because of structural heterogeneity which has the ability to absorb the energy of oxidative radical species (Guerin et al. 2003; Himaya and Kim 2015; Shahidi et al. 1998). Because of antioxidative and immune modulatory influence of carotenoids, they are active agents against oxidative stress and chronic inflammation such as cardiovascular disease, cancer, eye disorders, rheumatoid arthritis, and some neurodegenerative diseases (Abe et al. 2007; Himaya and Kim 2015; Shahidi et al. 1998; Vílchez et al. 2011).
2.3.1. Bioactive carotenoids in seaweeds and algae
Carotenoids are linear polyenes and their functions include both light energy harvesters and antioxidants that inactivate ROS formed upon contact with light and air (Holdt and Kraan 2011; Von Elbe and Schwartz 1996). It has been reported that carotenoids protect cells from oxidative stress by quenching singlet oxygen damage with various mechanisms (Christaki et al. 2013). Some carotenoids also function as vitamins and perform many biological functions as hormones, antioxidants, and regulators of cell and tissue growth and mediators of cell signalling (Holdt and Kraan 2011). Dietary carotenoids act as provitamin-A, which is converted into vitamin A that bears nutritional and therapeutic role in human health (Hamed et al. 2011). Algal carotenoids as antioxidants have been reported to play a vital role in preventing human pathologies linked to oxidative stress.
Seaweeds are potential sources of natural carotenoids. Green seaweeds are known to produce many carotenoids including β-carotene, violaxanthin, lutein, neoxanthin and zeaxanthin, whereas red algae produces mainly α- and β-carotenes, lutein and zeaxanthin. Brown algae are rich sources of β-carotene, violaxanthin and fucoxanthin (Holdt and Kraan 2011). Marine animal are also good sources of various carotenoids. Marine sponges contain bastaxanthins and fucoxanthins like carotenoids and are therefore brightly colored (Maoka 2011; Matsuno 2001; Rogers and Molinski 2005). Astaxanthin has been reported in some jelly fish and sea anemones. Mollusks contain various carotenoids like lutein, zeaxanthin, fucoxanthin and beta-carotene (Maoka 2011; Matsuno 2001). Major bioactive carotenoids, sources and their health benefits are summarized in Table 2. Oysters, clams, scallops and mussels contain carotenoids because of eating microalgae and modifying algae carotenoids. In oysters and clams, the carotenoid fucoxanthin is found. Astaxanthin is the major carotenoid reported in crustaceans and is seen in their blue, purple and yellow colors. Many fish accumulate carotenoids, but salmon, rainbow trout and Arctic char accumulate astaxanthin in their muscle. Beta-carotene and lutein have been reported in dolphins (Maoka 2011; Matsuno 2001).
 Click to view | Table 2. : Bioactive carotenoids and their health benefits |
2.3.2. Bioactive carotenoids in health and diseases
Microalgal pigments are non-toxic and exhibit biological activity in a wide range of applications, including prevention of acute and chronic coronary syndromes, atherosclerosis, rheumatoid arthritis, muscular dystrophy, cataract and neurological disorders. They are also recommended for protection of the skin and eyes against UV radiation (Mimouni et al. 2012; Sies and Stahl 2004).
β-carotene and its health benefits
β-Carotene (Fig. 11) is one of the most abundant colorants in nature. Green unicellular marine algae Dunaliella salina has been identified as one of the most promising sources of β-carotene with 14% of dry weight (Himaya and Kim 2015; Metting Jr, 1996). The content of β-carotene (with provitamin A activity) ranges from 36 to 4,500 mg/kg (or ppm) of dry weight with Porphyra and its content in seaweeds may vary because of life cycle and harvesting time (Holdt and Kraan 2011). It has been reported that β-carotene could prevent the onset of lung cancer (Astorg 1997; Holdt and Kraan 2011). A diet rich in carotenoids such as β-carotene, and lycopene reduces the risk of cardiovascular disease, cancers and carotenoids lutein, and zeaxanthin, which diminish ophthalmological diseases (Holdt and Kraan 2011). Microalgal-derived β-carotene has been shown to be biologically more active compared to the synthetic β-carotene (Hamed et al. 2015) and can be used as a natural food additive (Hamed et al. 2015). As a major natural colorant, β-carotene has been used in a wide range of food and drinks with the purpose of enhancing their quality (Hamed et al. 2015). β-Carotene could reduce the action of UV rays on premature aging in the skin because of its antioxidant properties (Godic et al. 2014; Hamed et al. 2015).
 Click for large image | Figure 11. Chemical structure of beta-carotene. |
Fucoxanthin and its health benefits
Fucoxanthin is a major carotenoid found in brown seaweeds and algae. It has a unique structure with an unusual allenic bond and a 5, 6 monoepoxide (Fig. 12); it is crucial in effective light harvesting and utilisation (Jung et al. 2012; Shahidi et al. 1998). Fucoxanthin is well known in lowering body fat in obese subjects, thus reducing the risk of obesity. In brown seaweed, the content of fucoxanthin varies from 172 to 720 mg/kg dry weight but its content was more in F. serratus (Holdt and Kraan 2011). Fucoxanthin, isolated from seaweeds such as Undaria pinnatifida, Hijikia fusiformis, Sargassum fulvellum and Laminaria japonica, has been reported to diminish obesity by preventing intestinal lipase activity (Freitas et al. 2015; Matsumoto et al. 2010) as well as having antiangiogenic and antiviral properties (Cornish and Garbary 2010; Freitas et al. 2015). It has also been reported that fucoxanthin possesses antioxidant, antidiabetic, antiphotoaging, and anticancer activities (D’Orazio et al. 2012). In addition, fucoxanthin has been shown to reduce the blood glucose levels and enhance insulin resistance by controlling the secretion of cytokine from white adipose tissue (Miyashita et al. 2011; Shahidi and Ambigaipalan 2015a). Fucoxanthin and its metabolites such as fucoxanthinol and halocynthiaxanthin, isolated and characterized from the sea squirt H. roretzi, were reported to prevent human breast cancer cells (MCF-7), the growth of human leukemia cells (HL-60), and Caco-2 human colon cancer cells (Miyashita et al. 2011; Shahidi and Ambigaipalan 2015a). Furthermore, marine fucoxanthin showed its biological activity against obesity and related metabolic conditions (Miyashita 2014; Shahidi and Ambigaipalan 2015a). Fucoxanthin has anti-allergic activity which was recently reported using a rodent mast cells model (Mimouni et al. 2012; Sakai et al. 2009). It could also limit the risk of obesity (Maeda et al. 2007; Mimouni et al. 2012).
 Click for large image | Figure 12. Chemical structure of fucoxanthin. |
Astaxanthin and its health benefits
Astaxanthin is an abundant carotenoid found in marine microalgae Haematococcus pluvialis (Fig. 13). These algae contain the highest amount of astaxanthin, approximately 1.5–3% (Himaya and Kim 2015). Astaxanthin is also found in marine aquatic species such as salmonid fish and crustaceans and exists in the free, monoester and diester forms, the latter being dominant in crustaceans. Astaxanthin has various biological functions related to preventing cancer, diabetes, heart and gastrovascular diseases (Chew et al. 1998; Chuyen and Eun 2017; Ikeuchi et al. 2007; Matsumoto et al. 2010; Park et al. 2010; Tanaka et al. 1994; Yoshida et al. 2010). It has been reported that astaxanthin has anti-proliferative effects on the mouse urinary bladder; its effect was greater than that of canthaxanthin in an in vivo study (Chuyen and Eun 2017; Tanaka et al. 1994). In an in vivo investigation, astaxanthin was reported to diminish the incidence and multiplicity of neoplasms in the large intestine of rats and also inhibited the growth of aberrant crypt foci and reduced cell proliferation (Chuyen and Eun 2017; Tanaka et al. 1995). Astaxanthin showed an inhibitory effect on the growth of tumor by inhibiting the AH109A cell invasion (Kozuki et al. 2000). It has been reported that astaxanthin inhibits the growth of human prostate cancer cells induced by androgens (Chuyen and Eun 2017; Sharoni et al. 2002). It also showed an obvious decrease in body weight, adipose tissue, liver triacylglycerol, liver weight, plasma triacylglycerol, and total cholesterol levels in obese mice fed a high-fat diet supplemented with astaxanthin (30 mg/kg) (Chuyen and Eun 2017; Ikeuchi et al. 2007). In addition, astaxanthin exhibited significant reduction in plasma levels of triacylglycerols, WAT (white adipose tissue) size, fasting blood glucose, non-esterified fatty acids, and an increase in high-density lipoprotein cholesterol (Chuyen and Eun, 2017; Hussein et al. 2007). Anti-obesity and anti-diabetic effects of astaxanthin (6 mg/kg per day) have been exhibited through improved insulin sensitivity by activation of insulin receptor-β and by decreases in the secretion of oxidative stress and pro-inflammatory cytokine (Arunkumar et al. 2012; Chuyen and Eun 2017). Astaxanthin also lowered blood pressure (Nakao et al. 2010) and showed preventive effects against atherosclerosis by prolonging oxidation lag time of low-density lipoprotein cholesterol (Chuyen and Eun 2017).
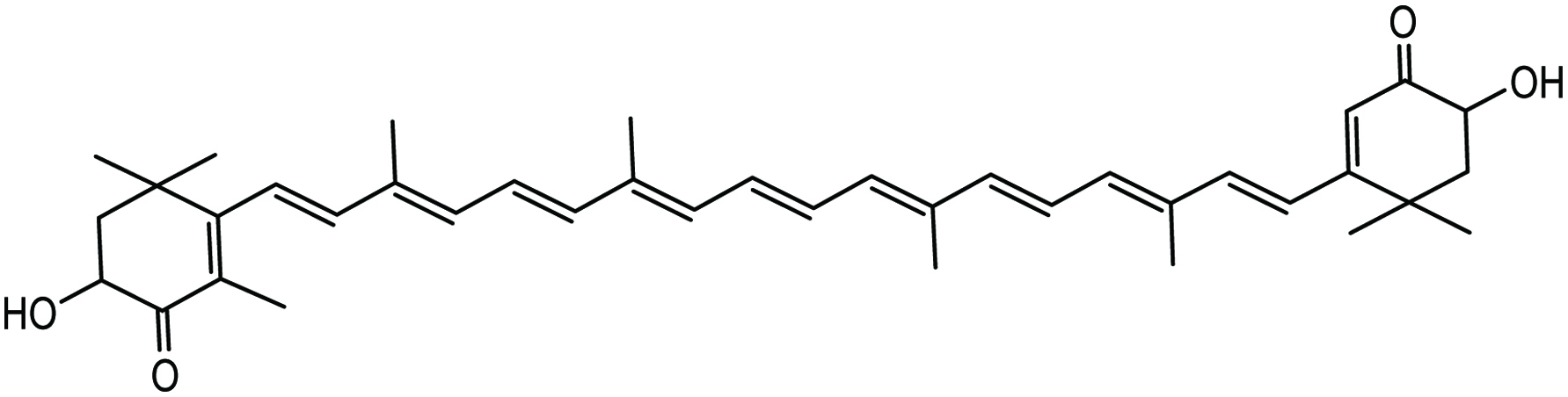 Click for large image | Figure 13. Chemical structure of astaxanthin. |
Canthaxanthin and its health benefits
Canthaxanthin (Fig. 14) is a carotenoid found in marine algae and crustaceans and is known to have considerable antioxidant, anti-cancer, antidiabetic, and antiobesity properties. Marine algae such Chlorella vulgaris, Haematococcus pluvialis, Coelastrella striolata var. multistriata all serve as potential sources of canthaxanthin (Abe et al. 2007). It has been reported that canthaxanthin has anti-proliferative effects on mouse urinary bladder (Chuyen and Eun 2017; Tanaka et al. 1994) and diminished the incidence and multiplicity of neoplasms in the large intestine of rats and also inhibited the growth of aberrant crypt foci and reduced cell proliferation (Chuyen and Eun 2017; Tanaka et al. 1995). Canthaxanthin showed an inhibitory effect on the growth of tumor inhibiting AH109A cell invasion; its effect was greater than that of β-carotene, astaxanthin, β-cryptoxanthin, lycopene, α-carotene, zeaxanthin, and lutein (Kozuki et al. 2000).
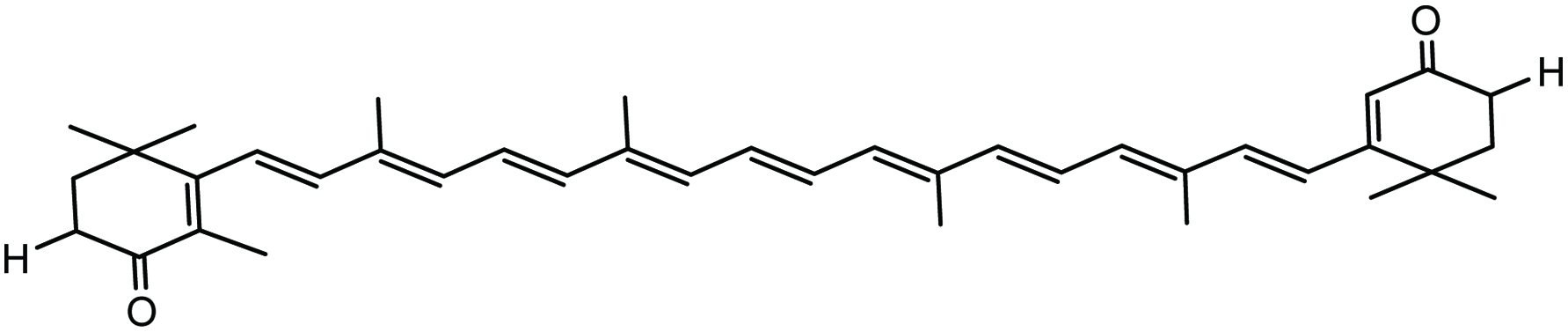 Click for large image | Figure 14. Chemical structure of canthaxanthin. |
Lutein and health benefits
Lutein is a highly polar carotenoid with hydroxyl groups on its cyclic ring structure (Fig. 15) and as a result it bears a higher antioxidative potential. It could be effectively harvested and used in applications to promote health and protect against chronic disease conditions (Himaya and Kim 2015). Lutein is an abundant xanthophyll in green microalgae. Generally, it is stored in the macula of the human retina and as such protects the eyes from oxidative stress, and acts as a filter for the blue light involved in macular degeneration and age-related cataract (Alves-Rodrigues and Shao 2004; Hashimoto et al. 2012; Mimouni et al. 2012).
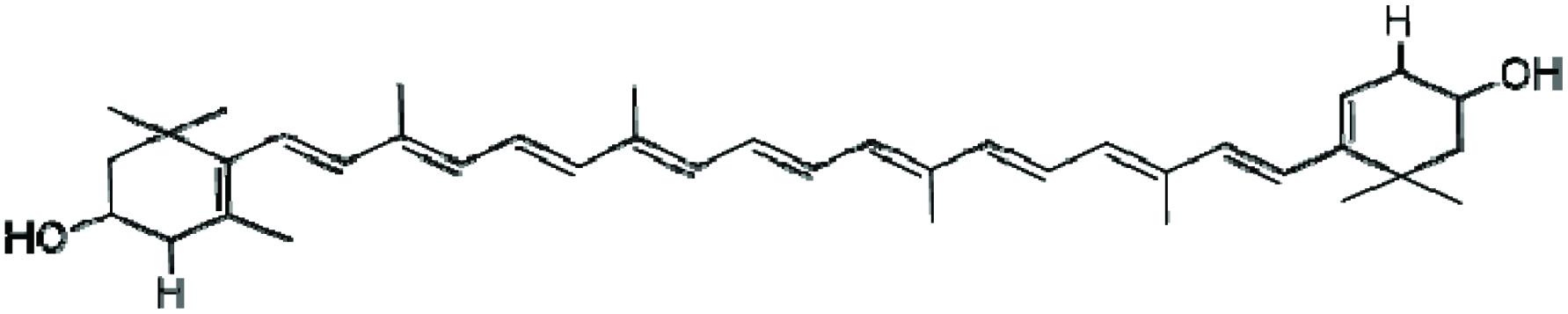 Click for large image | Figure 15. Chemical structure of lutein. |
Zeaxanthin and its health benefits
Zeaxanthin (Fig. 16) is also found in marine algae and is known to play a vital role, like lutein, in absorbing damaging blue and near-ultraviolet light to protect the macula lutea from light-associated damage (Chuyen and Eun 2017; Krinsky and Johnson 2005).
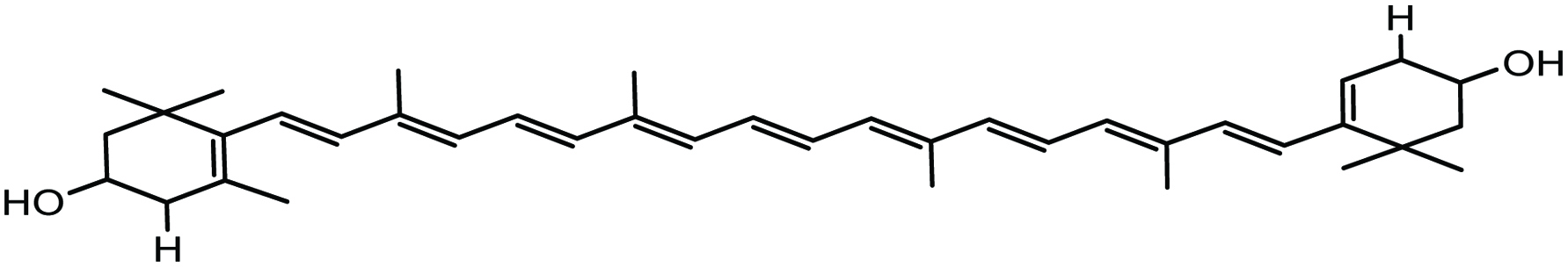 Click for large image | Figure 16. Chemical structure of zeaxanthin. |
Other carotenoids and their health benefits
Other carotenoids have also been identified in marine sources; these include violaxanthin, halocynthiaxanthin, echinenone, and tunaxanthin. In addition, many biological functions of these carotenoids have been reported, such as protective role against inflammation and cardiovascular diseases, anti-cancer, anti-obesity, and anti-diabetic activities (Chuyen and Eun 2017).
2.4. Bioactive lipids
Marine fish and algae are recognised as a vital source of bioactive lipids with a high proportion of polyunsaturated fatty acids (PUFAs), which have been reported to be effective in inhibiting or treating several diseases (Andrich et al. 2005; Shahidi 2007). Basically, marine-based long-chain PUFAs (LC-PUFAs) contain 20 or more carbons with two or more double bonds from the methyl (omega) end group of the molecule. These fatty acids have received special attention as functional food ingredients due to their multiple activities (Rasmussen and Morrissey 2007; Shahidi 2007). Because of metabolic links, PUFAs can be classified into two families: 1. linoleic acid family which are known as ω-6 essential fatty acids and 2. α-linolenic acid family which are ω-3 essentials fatty acids (Shahidi 2007). Basically, most marine lipids consist of several LC PUFAs with ω-3 fatty acids as essential components and also monounsaturated fatty acids (MUFAs). Among ω-3 fatty acids found in marine lipids are eicosapentaenoic acid (EPA; 20:5 n-3), docosahexaenoic acid (DHA; 22:6 n-3), and docosapentaenoic acid (DPA; 22:5 n-3), with precursor α-linolenic acid (ALA; 18:3 n-3) (Holdt and Kraan 2011). It has been reported that dietary ω-3 PUFAs show their protective effect against atheroscloretic heart diseases, among others (Dyerberg and Jorgensen 1982; Lagarde et al. 1986; Mimouni et al. 2012; Rousseau et al. 2003; Sakai et al. 1990; Shahidi 2007).
2.4.1. Bioactive lipids in marine algae
Marine fish species and algae are the key producers of ω-3 PUFAs. In general, marine algae synthesize ω-3 PUFAs during metabolism whereas fish usually accumulate them via food chain and eating various algae (Mimouni et al. 2012). The main ω-3 PUFA in many seaweeds is EPA which consists of as high as 50% of the total fatty acid content (Dawczynski et al. 2007; Holdt and Kraan 2011; Murata and Nakazoe 2001). Red and brown algae are rich in ω-3 fatty acids such as EPA, α-linolenic acid and ω-6 fatty acids such as arachidonic acid (AA) and α-linoleic acid, along with relatively high levels of oleic and palmitic acids (Fig. 17) (Dawczynski et al. 2007; Freitas et al. 2015, Holdt and Kraan 2011). Green seaweeds such as Ulva pertusa have been reported to be rich sources of hexadecatetraenoic (16:4 n-3), oleic (ω-9), and palmitic acids (Floreto et al. 1993; Freitas et al. 2015). As an algal source of DHA, brown alga Schizochytrium sp. has 40% DHA, and 17% DPA. Meanwhile 40–50% of DHA is found in green alga Ulkenia sp. and red alga Crypthecodinium cohnii (Mimouni et al. 2012; Spolaore et al. 2006). As a rich source of omega-3 fatty acid octadecatetraenoic acid (18:4 n-3), two brown algae, namely Laminaria ochroleuca and Undaria pinnatifida have also been reported (Hamed et al. 2015; Sanchez-Machado et al. 2004). In addition, marine fungi such as phycomycetes are a good source of omega-3 fatty acids. Among all types of marine fungus Mortierella genus, has been reported to yield high levels of either γ-linolenic acid (GLA; 18:3 n-6) acid, AA, or EPA (Hamed et al. 2015; Yap and Chen 2001).
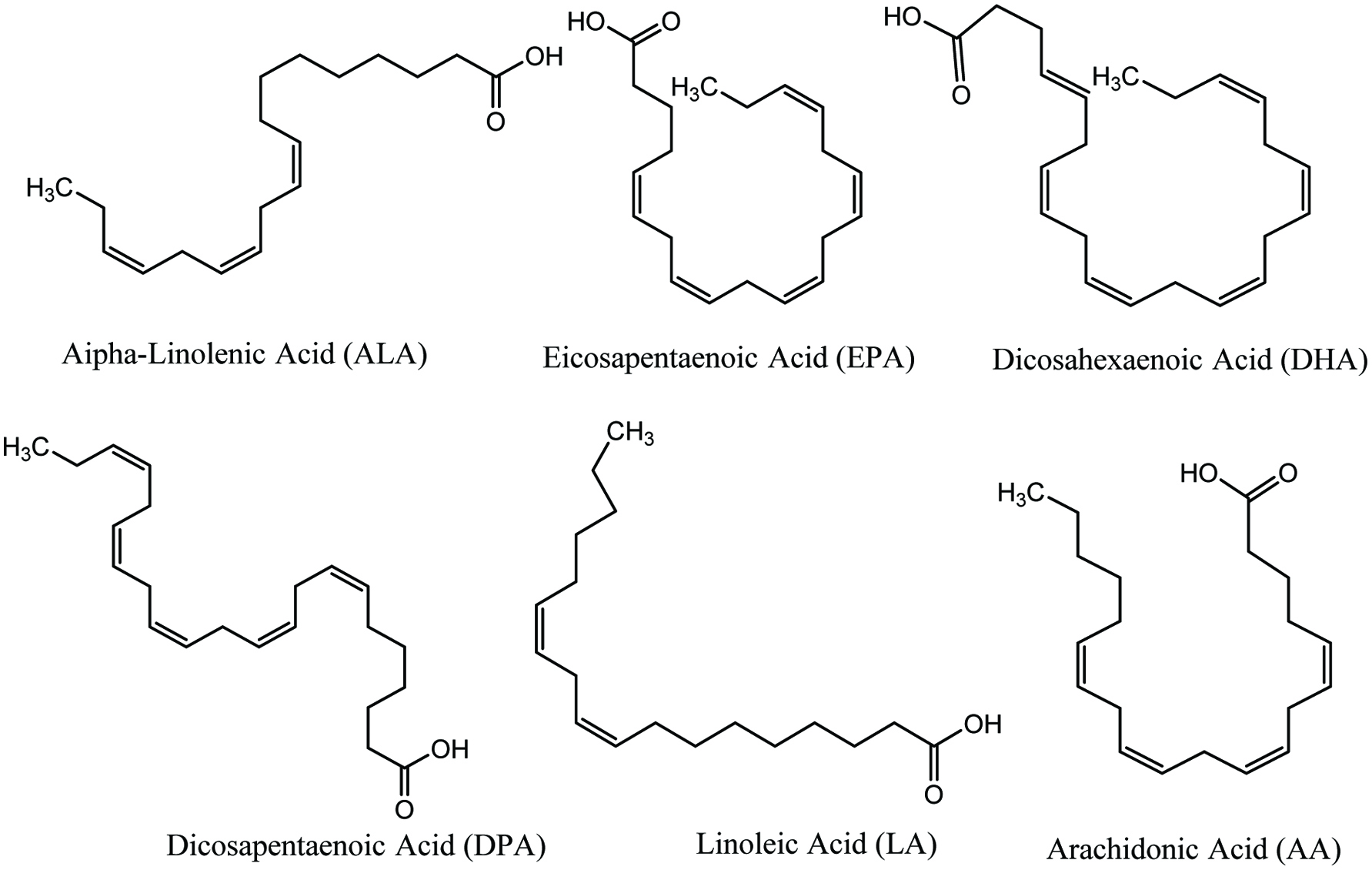 Click for large image | Figure 17. Isolated bioactive lipids from seaweeds and marine algae. |
2.4.2. Bioactive lipids in health and diseases
Marine algae are good sources of functional lipids with a myriad of biological activities and are important in health promotion and disease risk reduction. Because of environmental variation, marine algae serve as rich sources of ω-3 PUFAs, including DHA and EPA but the content and composition of their oils depend on the species, season and location of the harvest (Hamed et al. 2015).
Seafood lipid fraction consists mainly of PUFAs that are essential for human health (Berquin et al. 2007; Chan and Cho 2009; Hamed et al. 2015; Shahidi and Ambigaipalan 2015a, 2018; Zheng et al. 2013). Humans cannot synthesize omega-3 (ω-3) fatty acids because the precursor for omega-3 fatty acids, α-linolenic acid (ALA) needs to be acquired from dietary sources (Himaya and Kim 2015; Shahidi 2007, 2011; Shahidi and Ambigaipalan 2018). However, humans can produce EPA and DPA from ALA albeit to only 1–4% (Shahidi 2007, 2011). Both in vitro and in vivo studies have shown that ω-3 fatty acids affect blood lipid profiles, composition of membrane lipid, cardiovascular health, cell signaling cascades, eicosanoid biosynthesis, and gene expression (Shahidi 2007, 2011, 2015; Shahidi and Ambigaipalan 2015a, 2018; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006). Results of epidemiological studies have shown that the intake of omega-3 fatty acids from natural sources or supplements may have a vast array of health promoting benefits such as prevention of coronary heart disease, atherosclerosis, hypertriglyceridemia, blood platelet aggregation, type-II diabetes, general inflammation, cysticfibrosis, hypertension, oculardiseases, arthritis, and everalcarcinomas (Himaya and Kim 2015; Lavie et al. 2009; Shahidi 2015; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006; Shahidi and Wanasundara 1998; Wu and Bechtel 2008). PUFAs are widely consumed from different seafood sources or supplements in order to prevent or treat heart disease and other ailments (Shahidi 2007, 2011; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006).
Marine oils and their omega-3 fatty acids provide the best example of a functional food ingredient and nutraceutical that may serve as a continuum for their perceived and demonstrated health benefits. Thus, they may prevent ailments caused by their deficiency, such as those encountered in the body, and secondly, they aid in reducing disease risk, and thirdly they act as therapeutic agents in treating certain diseases or conditions (Shahidi 2007, 2011, 2015; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006). As principal long-chain ω-3 fatty acids, EPA and DHA have been found in marine algal oil and have a wide range of biological effects (Guschina and Harwood 2006; Lebeau and Robert 2003; Mimouni et al. 2012; Shahidi 2007, 2011; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006) Both EPA and DHA have shown activity on lipoprotein metabolism, coagulation, lowering blood pressure and also platelet and endothelial function (Mimouni et al. 2012; Rousseau et al. 2003; Shahidi 2007, 2011, 2015; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006). EPA plays a vital role as a precursor of several lipid regulators involved in the cellular metabolism and also plays many roles in biological membranes (Shahidi 2007, 2011, 2015; Mimouni et al. 2012). In addition, the effect of ω-3 fatty acids may depend, at least to some extent, on the presence of underlying disorders such as dyslipidemia, hypertension, diabetes mellitus, and vascular diseases (Shahidi 2007, 2011, 2015; Mimouni et al. 2012). DHA is the most abundant PUFAs and a major component of the brain, retina of the eye and heart muscle (Shahidi 2007, 2011, 2015; Shahidi and Ambigaipalan 2015a, 2018; Mimouni et al. 2012). DHA is naturally present in breast milk, together with DPA, and is reported as being important for normal brain and eye development of the fetus and also for cardiovascular health (Horrocks and Yeo 1999; Mimouni et al. 2012; Shahidi 2007, 2011; Shahidi and Ambigaipalan 2015a, 2018; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006).
A large body of literature provides information about the health benefits of omega-3 fatty acids, mainly those arising from EPA and DHA that serve as building blocks of cells in vital organs, particularly those with electrical activity such as the brain, the heart, and the eye, and also are important for normal growth and development (Shahidi 2007; Shahidi and Miraliakbari 2004; 2005). Therefore, adequate intake of omega-3 fatty acids by pregnant and lactating women is encouraged in order to address the need of the fetus or the infant for these essential components that are necessary for the development of their essential organs. Whereas most studies have examined the health benefits of EPA and DHA, research on DPA is rather limited because this fatty acid occurs in minor amounts in seafood and marine oils. However, DPA is found in relatively high abundance in blubber of marine mammals and some algal oils; it is also present in breast milk and it may play a major role in health promotion and disease risk reduction (Mimouni et al. 2012; Shahidi 2007, 2011; Shahidi and Finley 2001; Shahidi and Mialialiakbari 2004, 2005, 2006). It is worth noting that ALA supplementation increased the concentration of ALA, EPA, and DPA (but not DHA) in breast milk lipids (Shahidi 2007, 2011; Shahidi and Finley 2001; Shahidi and Miraliakbari 2004, 2005, 2006). Some studies have clearly indicated that infant formulas devoid of omega-3 fatty acids may adversely affect the intelligence quotient, verbal skill, and general performance of the infants when compared with mother’s milk that is rich in omega-3 fatty acids.
2.5. Bioactive sterols and their role in human health promotion
Sterols are an important class of compounds that are produced by eukaryotes as well as by some bacteria (Hamed et al. 2015). Marine seaweeds, algae and fungi may contain substantial amounts of sterols. In marine algae, phytosterols are an important group and have been reported for their particular biological activities including antioxidative effects (Lee et al. 2003; Xiao et al. 2013) anti-cancer properties (Awad et al. 2003; Bradford and Awad 2007; Rao and Janezic 1992; Xiao et al. 2013), anti-inflammatory (Akihisa et al. 2007; Xiao et al. 2013) and anti-diabetic activities (García-Llatas et al. 2011; Xiao et al. 2013). It has been reported that sterols have the ability to reduce “bad” low-density lipoprotein cholesterol (LDL-C) levels and could also reduce the risk of heart disease by prevention and reduction of hypercholesterolemia (Kim and Van Ta 2011).
In red algae, the main sterol is cholesterol. Some species of red algae also contain demosterols and 22-dehydrocholestrols. A red alga, Peyssonnelia sp., has been shown to have activity in human cancer cell lines. Two sterol glycosides isolated from this alga showed significant cytotoxicity in human breast cancer MDA-MB-468 cells and in human lung cancer cell line A549 with IC50 of 0.71 and 0.86 mM, respectively (Jeon et al. 2015; Lin et al. 2010). Fucosterol has been reported as a major sterol in brown algae. Some brown algae also contain traces of cholesterol and biosynthetic precusors of fucosterol. Fucosterol isolated and characterized form brown algae Pelvitia siliquosa showed antioxidant and antidiabetic activities (Jeon et al. 2015; Lee et al. 2003, 2004). In addition, saringosterol a derivative of fucosterol, isolated from the brown algae Lessonia nigrescens has been reported to prevent the growth of Mycobacterium tuberculosis (Jeon et al. 2015; Wachter et al. 2001). Saringosterol could be a good candidate for inhibiting tuberculosis. Green algae have been reported to serve as a potential source of many bioactive sterols such chondrillasterol, poriferasterol, 28-isofucosterol, ergosterol and cholesterol, 24-methylcholesterol and sargasterol (Fig. 18) (Bhakuni et al. 2005; Sanchez-Machado et al. 2004a). It has been reported that sterols from some edible marine algae, such as Himanthalia elongate, Undaria pinnatifida, Phorphyra sp., Chondus crispus, Cystoseira sp., Ulva spp., display activity in reducing the total and LDL-C level. In addition, marine sponges are rich sources of sterols. The marine sponge Petrosia weinbergi was identified as a good source of isofucosterol, and clionasterol with antiviral activities (Giner et al. 1999; Jeon et al. 2015). The starfish Linckia laevigata has been reported to contain a new sterol, named linckoside L1, a polyhydroxylated steroid glycoside that can potentially be used as a drug for neurodegenerative diseases (Jeon et al. 2015; Kicha et al. 2007). Each bioactive sterol shows a wide range of bioactivities in the marine ecology. Thus, bioactive sterols may contribute to templates or drug candidate for future therapeutic applications.
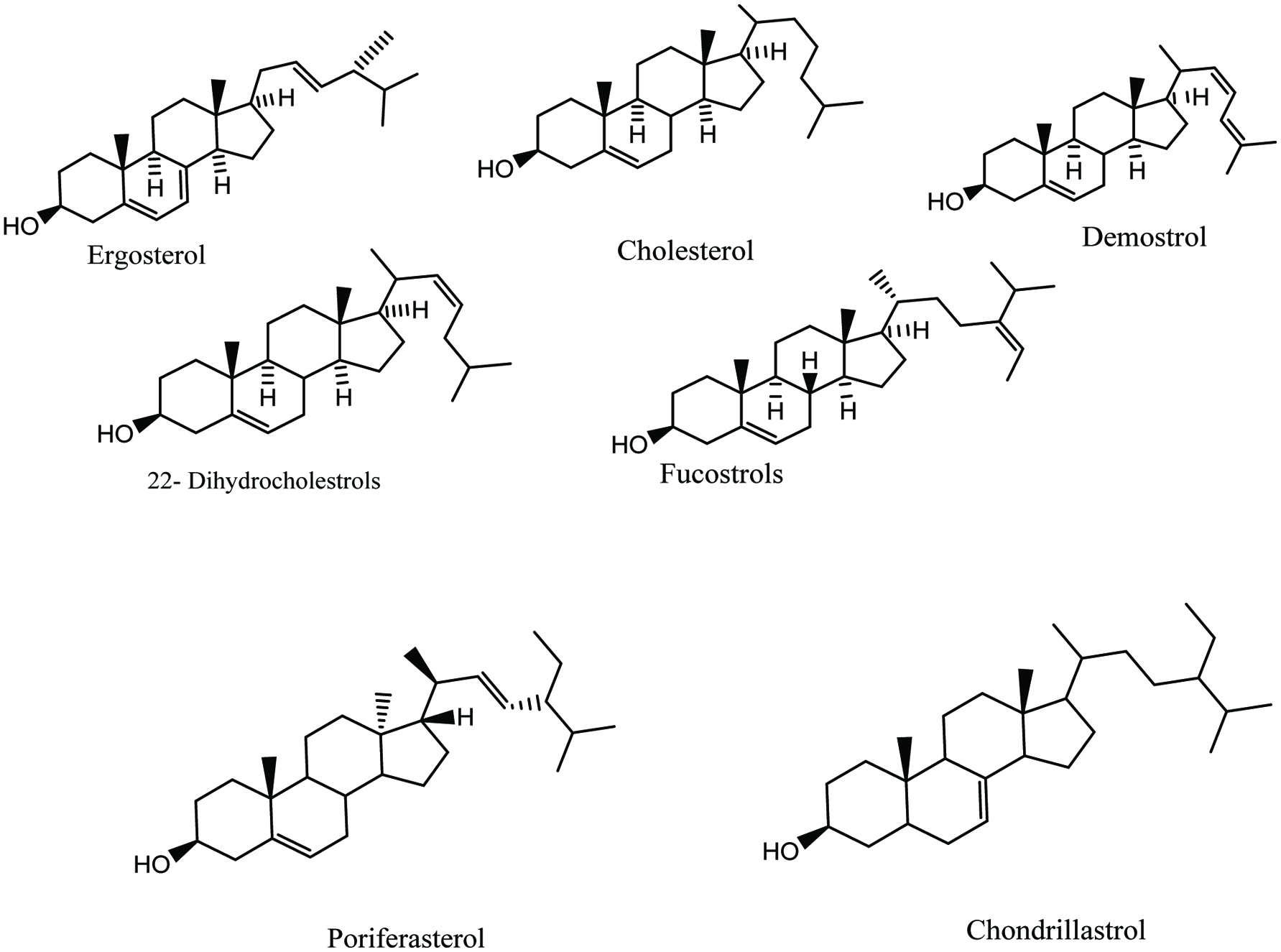 Click for large image | Figure 18. Isolated phytosterol from marine seaweeds and algae. |
The sterols from marine algae are non-toxic and have the ability to reduce blood cholesterol level. They are also reported to reduce the tendency to form fatty liver and excessive fat deposition in the heart diseases (Jeon et al. 2015; Kicha et al. 2007).
2.6. Bioactive proteins/peptides and their health benefits
Biologically active peptides and proteins have been isolated and characterized not only from marine animals, but also from various seaweeds, algae and fungi. Screening of medicinal potential of seaweed and algae for various proteins and peptides exhibiting bioactive properties has been carried out (Aneiros and Garateix 2004; Devadasan et al. 1994). Though there is little information in the literature in comparison to marine animals, some useful bioactive proteins such as lectins and phycobiliproteins (PBP) have been studied for many seaweeds and marine algae.
2.6.1. Bioactive lectins
Lectins are glycoproteins abundant in marine algae and seaweeds with many biological function and have proven to be useful in the field of clinical diagnosis and other health applications (Aneiros and Garateix 2004; Holdt and Kraan 2011). They are a structurally diverse group of carbohydrate binding proteins of non-immune origin found and analysed in a wide range of marine organisms (Holdt and Kraan 2011; Hori et al. 2000). Bioactive lectins are found and characterized in some macroalgal species such as Ulva sp., Eucheuma spp. and Gracilaria sp. (Holdt and Kraan 2011). Lectins isolated and purified from the green marine algae Codium fragile subsp. tomentosoides have been produced and sold commercially for health promotion (Holdt and Kraan 2011; Smit 2004). Basically, lectins can interact with specific glycan structures linked to soluble and membrane-bound glycoconjugates. It is these protein-carbohydrate interactions that allow lectin to be involved in many biological activities such as host-pathogen interactions, cell-cell communication, induction of apoptosis, cancer metastasis and differentiation, as well as recognising and binding of carbohydrates (Holdt and Kraan 2011; Hori et al. 2000). It has been reported that lectin is active in increasing the agglutination of blood cells (erythrocytes) and is also useful in the detection of disease related alterations of glycan synthesis, including infectious agents such as bacteria, viruses, fungi and parasites (Bird et al. 1993; Cardozo et al. 2007; Holdt and Kraan 2011; Murata and Nakazoe 2001). In addition, many other bioactive properties have been exhibited by marine algal lectins, for example antibiotic, mitogenic, anti-inflammatory, cytotoxic, anti-adhesion anti-nociceptive, and anti-HIV activities (Bird et al. 1993; Holdt and Kraan 2011; Mori et al. 2005; Smit 2004). Lectins are derived from three marine Eucheuma species such as E. serra, E. amakusaensis and E. cottonii and have strong mitogenic activity in mouse and human lymphocytes. A marine glycoprotein E. serra agglutinin (ESA) isolated from E. serra exhibited a cytotoxic effect on numerous cancer cell lines, for example colon cancer Colo201 cells and cervical cancer HeLa (Holdt and Kraan 2011; Kawakubo et al. 1997, 1999; Sugahara et al. 2001). Hori et al. (2007) reported that ESA-2, a lectin derived from E. serra, blocked colonic carcinogenesis in mice after oral feeding and exhibited growth inhibition of 35 human cancer cell lines. This phycolectin was also reported to inhibit adenosine diphosphate and collagen-induced human platelet aggregation in a dose-dependent manner (Holdt and Kraan 2011).
Antibacterial lectins have also been isolated from E. serra and Galaxaura marginata (Liao et al. 2003). These carbohydrate-binding proteins exhibit antibacterial activity against the fish pathogen Vibrio vulnificus. Aggregation and immobilisation of V. vulnificus cells were observed following incubation of the cells with glycoprotein molecules because lectins recognise and bind to complementary carbohydrates or glycoproteins on either the cell walls or plasma membranes of the associated bacteria cells. As a result, the microorganisms are immobilised and further growth and multiplication is prevented (Liao et al. 2003). Mucin-binding agglutinin and isoagglutinin glycoproteins isolated from two different species of red algae possessed anti-inflammatory activity and showed mitogenic activity on mouse spleen lymphocytes, and they inhibited the growth of mouse leukaemia cells L1210 and mouse FM3A tumor cells (Bitencourt et al. 2008; Holdt and Kraan 2011; Hori et al. 1988).
2.6.2. Bioactive phycobiliproteins (PBP)
Phycobiliproteins are oligomeric proteins, built up from two chromophore-bearing polypeptides (Aneiros and Garateix, 2004). Phycobiliproteins are water-soluble compounds and form special type of particles named phycobilisomes on the surface of the thylakoids before being set in the membranes. Phycobiliproteins consist of pigmented phycobilins, which are linear tetrapyrroles (Holdt and Kraan 2011). They are divided into three main groups: 1. Phycoerythrins (PEs) with a red pigment joined to the protein molecule, 2. Phycocianins with a blue pigment and 3. Alophycocianins, all of them absorb light at different wavelengths of the spectrum (Aneiros and Garateix 2004; Holdt and Kraan 2011; Sidler et al. 1989). Generally, phycobiliproteins play a vital role in the photosynthetic process of blue-green, red and cryptophytic algae (Aneiros and Garateix 2004; Holdt and Kraan 2011). Phycobiliproteins, especially phycoerythrin, are the major part of the red algal cell proteins and present at 1.2 and 0.5% of dry weight in P. palmata and Gracilaria tikvahiae, respectively (Holdt and Kraan 2011; Wang et al. 2002). Currently phycobiliproteins are being used as natural colorants in food and cosmetic applications, including chewing gums and dairy products, in addition to lipsticks and eyeliners (Sekar and Chandramohan 2008). Two Japanese (Tokyo) companies market phycobiliprotein phycocyanin as a natural food colorant (Holdt and Kraan 2011). In addition, phycobiliproteins are naturally fluorescent “in vivo” and “in vitro” molecules. Among all phycobiliproteins, phycoerythrin is the most widely used fluorescent probe in many fluorescent immunoassays, fluorescent immunohistochemistry and other methodologies (Aneiros and Garateix 2004; Glazer 1994; Johnson and Holborow 1986). Phycobiliproteins have been reported to have various biological activities such as antioxidant, anti-inflammatory, hepatoprotective, hypocholesterolemic, neuroprotective, anti-tumor, liver protecting, antiviral, atherosclerosis treatment, serum lipid-reducing and lipase inhibition activities (Holdt and Kraan 2011; Sekar and Chandramohan 2008). It has been reported that phycocyanin c and phycoerythrin exhibit potent antioxidant and anti-inflammatory activities in both in vivo and in vitro studies (Bhat and Madyastha 2000; Gonzalez et al. 1999; Mimouni et al. 2012).
2.7. Bioactive pigments and their role in health promotion
Color plays a very important role in the general acceptability of a food and food products. The color of a seafood is the first characteristic that is directly related to the ensuing acceptance or rejection of it by the consumer (Shahidi et al. 1998). Marine algae and seaweed are rich sources of bioactive pigments and their potential role in health promotion and disease reduction is documented in the literature (Hamed et al. 2015; Himaya and Kim 2015; Holdt and Kraan 2011; Mimouni et al. 2012; Shahidi et al. 1998). Basically, three major classes of photosynthetic pigments occur in marine algae, namely chlorophylls and derivatives, cartenoids (carotenes and xanthophylls) and phycobilins; all together, they represent hundreds of molecules (Mimouni et al. 2012; Rao and Rao 2007).
Most of the marine algae are photoautotrophs. Consequently, their chloroplasts are rich in pigmented molecules, for example tetrapyrroles and carotenoids (Mimouni et al. 2012). These molecules are able to absorb light because of their characteristic conjugated double bonds and show various biological activities. Each photosynthesizing microalga contains at least a tetrapyrrole ring as in chlorophyll a (Mimouni et al. 2012). In red algae, chlorophyll a is complemented by the open tetrapyrrole phycoerythrin, phycocyanin and allophycocyanin, but green and brown algae contain a different type of chlorophyll molecule (Mimouni et al. 2012). The set of light harvesting molecules is accompanied with numerous carotenoids (Mimouni et al. 2012). The diatom Haslea ostrearia synthesizes and excretes a hydrosoluble blue pigment named marrenine, accountable for green color of oyster gills (Lebeau & Robert 2003; Mimouni et al. 2012). This pigment exhibits an antiproliferative effect on lung cancer model (Carbonnelle et al. 1998; Mimouni et al. 2012) and has potential antiviral and anticoagulant properties (Berge et al. 1999; Mimouni et al. 2012).
The potential of microalgal pigments to offer molecules of therapeutic value has received renewed interest because of their high structural diversity and the possibility to pharmacomodulate these molecules (Mimouni et al. 2012; Shahidi et al. 1998). Because of their lability and difficult purification, the biological activity of most molecules remains unknown. In the literature, many studies designed to identify and purify bioactive molecules from marine algae have led to the isolation of pigments. At very low concentrations, these purified pigments usually have a high activity on pharmacological and cellular effectors (Mimouni et al. 2012). As the potential sources and health benefits of bioactive pigments such as carotenoids and phycobilins discussed earlier, therefore in this section, the sources and health and therapeutic potential of bioactive chlorophylls and their derivatives are discussed in detail.
2.7.1. Bioactive chlorophylls, chlorophyll derivatives and their health benefits
Chlorophylls are green lipid-soluble pigments found in all algae, higher plants and cyanobacteria that do photosynthesis (Holdt and Kraan 2011). Generally, chlorophyll a content is 565–2,000 mg/kg on a dry weight basis in the brown algae (Holdt and Kraan 2011). Chlorophyll plays an important role in the reaction center of the thylakoid, light-harvesting structures wherein photosynthesis is carried out (Holdt and Kraan 2011; Rasmussen and Morrissey 2007).
In processed vegetables, chlorophyll is converted to pheophytin, pyropheophytin and pheophorbide. These derivatives exhibit antimutagenic effect and have important function in cancer prevention (Holdt and Kraan 2011). It has been reported that pheophorbide is more active than pheophytin in the cellular uptake and inhibition of myeloma cell multiplicity. On the other hand, pheophytin is more cytostatic/cytotoxic than pheophorbide (Chernomorsky et al. 1999; Holdt and Kraan 2011).
Oxidative stress is a major cause of inflammatory effect involved in many diseases, such as cancer, neurodegenerative and cardiovascular diseases, and diabetes (Shahidi and Ambigaipalan 2015b). The antioxidant and anti-inflammatory activities of microalga pigments are extensively studied and shown in many in vitro free radical scavenging assays and in vivo studies (Chew et al. 1998; Chuyen and Eun 2017; Ikeuchi et al. 2007; Matsumoto et al. 2010; Mimouni et al. 2012; Miyashita 2014; Miyashita et al. 2011; Park et al. 2010; Tanaka et al. 1994; Yoshida et al. 2010). Due to their antioxidant and anti-inflammatory activities, many marine algal pigments have shown neuroprotective effects in cultured rat cerebellar neurons, and hepatoprotective effects in hepatocytes grown in vitro (e.g., phycocyanin, phycoerythrin) (Mimouni et al. 2012; Sekar and Chandramohan 2008). In addition, some studies have demonstrated antiviral and antifungal activities for several pigments (Sekar and Chandramohan 2008).
Due to the presence of porphyrin ring and the ability of to transfer electrons, chlorophylls and their deriviatives exhibit antioxidant activity in the dark (Mimouni et al. 2012). It has been shown that metallo-derivatives of chlorophyll a have a better antiradical capacity than that of their corresponding metal-free chlorophyll-derivatives such as chlorins, pheophytins, and pyropheophytins (Mimouni et al. 2012). In addition, Lanfer-Marquez et al. (2005) reported that protoporphyrin methyl ester and its magnesium chelated derivative, as well as pheophorbide b and pheophytin b, have strong antioxidant activity. The high antioxidant activity of pheophorbide b, compared to pheophorbide a, is due to presence of the aldehyde group, which may also be critical for this activity (Schoefs 2002). It has also been reported that both metal-free and metallo-chlorophyll derivatives have antimutagenic activities, as demonstrated using a bacterial mutagenesis assay (Ferruzzi and Blakeslee 2007; Ferruzzi et al. 2002). Microalgal pigments may restore drug sensitivity or reverse multi-drug resistance in cancer cells, as some of them inhibit or down-regulate drug efflux pumps (Mimouni et al., 2012). Tang et al. (2007) showed a substantial decrease of P-glycoprotein expression in R-HepG2 cells, at both transcriptional and translational levels that was observed when cells were treated with pheophorbide a. Furthermore, the physicochemical characteristics of chlorophylls and chlorophylls catabolites make them suitable for use as fluorescent probes for cellular and tissular analysis (e.g., cell sorting, flow cytometry, histofluorescence, binding assays, ROS detection, cytofluorescence, labeling of pathological and apoptotic cells, etc.) (Mimmouni et al., 2011).
2.8. Bioactive minerals and vitamins
Seaweeds are a rich dietary source of a number of essential minerals. The major essential mineral abundant in seaweeds include iodine, potassium, iron, luorine, sodium, calcium, magnesium, zinc, copper, chlorine, sulfur, phosphorus, cobalt, manganese, selenium, bromine, and arsenic (Cerna 2011; Kalasariya et al. 2016). The best example of the human nutritional benefits of seaweed minerals is for iodine and iron, which is abundant in marine algae (Wells et al. 2017). However, nutritional mineral composition of seaweeds may differ because of seasonal, geographic, and taxonomic variations (Holdt and Kraan 2011; Jensen 1993; Wells et al. 2017). To support the above statement, Indonesian algae (e.g. green, brown, and red algae) contain high levels of potassium, calcium, and sodium, but significantly lower levels of iron and zinc than was reported in some major algal varieties which are found in the Japanese ocean such as yropia (as Porphyra) yezoensis, Ulva (Enteromorpha) intestinalis, and Sargassum (Hijikia) fusiformis (Takeshi et al. 2005; Wells et al. 2017).
It is well documented that seaweed consumption reduces the rate of goiter and other thyroid disorders (Holdt and Kraan 2011; Wells et al. 2017). In humans, lack of Iodine causes hypothyroidism (Miyai et al. 2008). It is therefore important to note that supplementation of iodine in daily diet is crucial for human health promotion. Seaweeds are a good nutritional source for iodine and some kelps such as Laminaria spp., Saccharina spp. have high level of iodine, and salts that are available commercially as kelp powder provide a vital source of this nutrient. Brown algae are well studied for their high level of iodine, but lower levels have also been reported in some varieties (e.g. the kelps of Undaria (wakame) and in Alaria (Atlantic wakame) that are comparable to red seaweeds in their iodine content (e.g. Dulse (Palmaria palmata)) (Holdt and Kraan 2011; MacArtain et al. 2007; Schiener et al. 2015; Wells et al. 2017). Overconsumption of some brown sea vegetables such as Laminaria; Saccharina can be unhealthy because of their high iodine content (Miyai et al. 2008; Teas et al. 2004b; Wells et al. 2017). The benefits of seaweeds consumption as a dietary source of iodine by both humans and animals for thyroid health is a well-established phenomenon (Holdt and Kraan 2011). A well-known sea vegetable, L. digitata is widely used as a health supplement for myxoedema and for the treatment of goitre (Holdt and Kraan 2011; Müssig 2009). U. pinnatifida is a microalga that has been reported as anti-tumorogenic in many studies (Funahashi et al. 1999; Holdt and Kraan 2011; Maruyama et al. 2003; Takahashi et al. 2000). This Asian kelp species or its equivalent iodine content inhibited tumorogenesis in rats with carcinogen-induced mammary tumors, although the mechanism of action involved is not understood (Funahashi et al. 2001). Seaweeds and marine algae serve as a great dietary source of. The iron content in these marine organisms may vary because of harvesting season and other environmental factors (Garcia-Casal et al. 2007, 2009; Wells et al. 2017). The major seaweeds and algae with a content high level of iron are Ulva spp., Sargassum spp., Porphyra spp., and Gracilariopsis spp (Wells et al. 2017).
Seaweeds and algae are rich in natural vitamins. These vitamins are essential for health promotion and disease risk reduction because most of them cannot be synthesized directly in sufficient quantities in the human body and so instead must obtain from the diet (Wells et al. 2017). Most common vitamin-deficiency disorders are known as pellagra (niacin), pernicious anemia (cobalamin, vitamin B12), beriberi (deficiency in thiamine, vitamin B1), and scurvy (ascorbic acid, vitamin C) (Martin et al. 2011; Stabler and Allen 2004; Wells et al. 2017). Vitamins serve as precursors for essential enzyme cofactors and are needed for essential metabolic functions (Wells et al. 2017). Several seaweeds and microalgae such as laver (Porphyra umbilicalis), sea spaghetti (Himanthalia elongata), and Gracilaria changii contain sufficient levels of vitamin C, almost equivalent to the amount found in fruits and vegetables such as tomatoes and lettuce (Ferraces-Casais et al. 2012; Norziah and Ching 2000; Wells et al. 2017). Hernandez-Carmona et al. (2009) reported that the vitamin C content was 34.4 mg per 100 g dry wight for the brown seaweed Eisenia arborea which is equal to that of mandarin oranges. Various sea vegetables also are a good source of B-group vitamins (particularly B1, B12), as well as the lipophilic vitamin A (derived from β-carotene) and vitamin E (tocopherol) (Wells et al. 2017). The amount of β-carotene (pro-vitamin A) reported in the sea vegetables such as Codium fragile and Gracilaria chilensis was higher than that in carrots (Ortiz et al. 2009; Wells et al. 2017). Several algal species such as Tetraselmis suecica, Isochrysis galbana, Dunaliella tertiolecta, and Chlorella stigmatophora were reported to serve as being rich in lipid-soluble (A and E) and B-group vitamins [including vitamins B1, B2 (riboflavin), B6 (pyridoxal), and B12] (Fabregas and Herrero 1990; Wells et al. 2017). Vitamin E includes α-, β- and δ-tocopherols (Holdt and Kraan 2011; Kumar et al. 2008b). However, the concentrations of several vitamins, including E, B1, and β-carotene in seaweeds and marine algae may vary because of their origin, varieties, harvesting time and other environmental factors.
| 3. Bioactives in marine fungi and their potential in health and diseases | ▴Top |
Limited information is available on marine fungi and their bioactives like sulfated polysaccharides, polyphenols, carotenoids, pigments, protein and peptides as well as bioactive lipids. Here, some insight is provided on marine fungi and their secondary metabolites especially terpenoids with special interest on their biological potential.
Most of the fungal species in marine environment are considered to serve as potential producers of biologically active secondary metabolites. Many reports have been published on the isolation and development of marine fungal metabolites (Bajpai et al. 2014; Liberra and Lindequist 1995; Namikoshi et al. 2001; Pietra 1997). Marine environment offers an enormous and versatile treasure of filamentous fungi with significant sources of novel fungal metabolites, resulting in the emergence of new areas of multidisciplinary research (Bajpai et al. 2014; Namikoshi et al. 2001). Currently marine derived fungal secondary metabolites, especially terpenoids, have attracted several applications in medical and therapeutic areas with a diverse range of biological value to serve as novel biomolecules in the treatment of chronic and severe diseases including AIDS, cancer, Alzheimer’s disease and arthritis which are very critical to cure (Bajpai et al. 2014; Namikoshi et al. 2001).Thus, studies on marine fungi and their potential bioactive compounds are gaining substantial prominence.
Marine fungal species are rich sources of structurally diversified terpenoids such as monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes, steroids, and tetraterpenes (Kim and Li 2012). Metroterpenoids are most commonly isolated from fungi and marine organisms, however bacteria and higher plants also produce mixed biosynthesized products (Kim and Li 2012). Mixed polyketide-terpenoid-derived fungal natural products are the largest class of metroterpenoids, while those derived from orsellinic acid or its mono- and dimethyl derivatives are the most frequently isolated meroterpenoids from fungi, especially Penicillium and Aspergillus spp (Kim and Li 2012).
Marine fungi produce a range of bioactive terpenoids including trichothecanes, the protoilludane group, cyathins, fussicoccanes, and many triterpenoids. Huang et al. (2006) reported that marine fungus, Tryblidiopycnis sp is a potential source of a chlorinated monoterpene. Sesquiterpenes have been reported from three marine fungal species, namely Dendryphiella sp., Penicillium sp., and Hansfordia sp. (Kim and Li 2012; Schneider et al. 1997). A marine-derived fungus Fusarium sp. has been reported to secrete sesterterpenes as its secondary metabolites (Renner et al. 1998). Furthermore, triterpenes have been isolated from Phomopsis sp. (Kim and Li 2012; Li et al. 2008). In addition, steroids have been reported in some marine-derived fungi, such as Rhizopus sp., Gymnascella sp., and Penicillium sp. (Ebel 2010; Kim and Li 2012). Ebel (2010) reported that marine fungal-derived terpenoids such as neurosporaxanthin, β-carotene, γ-carotene, and torulene have potential antioxidant activity which was analysed by various “in vitro” methods such as DPPH, lipid peroxide inhibition, ferric reducing antioxidant power, nitric oxide scavenging, ABTS radical scavenging, and superoxide radical and hydroxyl radical scavenging assays. Many studies have reported terpenoids derived from marine fungi with antioxidant potential that have antiproliferative activity in several human cancer cell lines “in vitro”, as well as inhibitory activity on tumor growth in mice (Ebel 2010; Kim and Li 2012; Gross and König 2006). In addition, they have antimetastatic activity by blocking the interactions between cancer cells and the basement membrane (Gross and König 2006). Many species of marine-derived fungi contain significant quantities of complex structural terpenoids that have been shown to inhibit the proliferation of pathogenic microorganisms (Gross and König 2006).
| 4. Conclusions and future direction | ▴Top |
Marine world offers a vast resource of bioactive molecules that are important for human health promotion and disease risk reduction. They can be used in many fields such as food, drug, and cosmetic industries. Marine products can easily be used to develop functional foods since they are widely available and have the ability to prevent certain diseases or to cure some. Sea offers an enormous resource for finding novel compounds and it is considered as the largest remaining reservoir of natural molecules that may be used as functional ingredients in the food and pharmaceutical industries. Therefore, extraction, identification and quantification of marine bioactives and assessment of their biological potential in both “in vitro” and “in vivo” would be of much scientific as well as economic value that warrant further studies.
| References | ▴Top |