| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 14, June 2021, pages 94-101
Amino acid profiles and in vitro antioxidant properties of cereal-legume flour blends
Elizabeth-Mary Shuluwaa, Akinsola A. Famuwagunb, Dinnah Ahurec, Moses Ukeyimac, Rotimi E. Alukod, David I. Gbenyie, Abraham T. Girgihc, *
aDepartment of Chemistry, Centre for Food Technology, Benue State University, Makurdi, Benue State, Nigeria
bDepartment of Food Science and Technology, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria
cDepartment of Food Science and Technology, University of Agriculture, Makurdi, Benue State, Nigeria
dDepartment of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, Manitoba, Canada R3T 2N2
eDepartment of Food Science and Technology, Federal Polytechnic, Mubi, Adamawa State, Nigeria
*Corresponding author: Abraham Girgih, Department of Food Science and Technology, University of Agriculture, Makurdi, Benue State, Nigeria. Tel: +2347039886210; E-mail: girgihusa@yahoo.com
DOI: 10.31665/JFB.2021.14271
Received: June 13, 2021
Revised received & accepted: June 25, 2020
| Abstract | ▴Top |
Legumes and cereals stand a great chance as remedies to overcome incidences of protein malnutrition and oxidative stress. Cereal grains such as wheat (WT), millet (ML), maize (MZ) and acha (AC) were blended with cowpea (CP), peanut (PN) and soybean (SO) at varying levels to produce four different blends. Protein contents of the cereal-legume blends were determined and found to be higher as the inclusion level of legumes increased in the blends. Amino acid profiles and antioxidant properties of the flour blends were evaluated. Results showed that leucine was the most abundant (6.52–8.45 g/100 g) essential amino acid in the flour blends while total content of essential amino acids increased as the level of legume incorporation increased in the WT/SO (31.3–36.2 g/100 g) and MZ/CP (34.5–37.4 g/100 g). The antioxidant properties showed that MZ50:CP50 exhibited greater ferric reducing antioxidant power while WT70:SO30 and AC50:SO50 had stronger metal chelation activity and ML50:PN50 scavenged the most DPPH radicals when compared to the other flour blends. The results suggest that the composite flours have the potential to be used as ingredients for the formulation of food products with high levels of essential nutrients in addition to antioxidant benefits.
Keywords: Cereals; Legumes; Composite flours; Amino acid composition; Antioxidant properties
| 1. Introduction | ▴Top |
Cereals and legumes occupy an important place in the dietary pattern of sub-Sahara African countries, because they both stand a great chance as remedies to overcome the incidences of malnutrition, especially protein-energy imbalance and other related health challenges. Cereal crops such as maize, wheat, sorghum, millet and acha are grown basically for their grains and serve as sources of energy more than any other crops globally (Emmambux and Taylor, 2013). Wheat is a good source of calories but low in protein and deficient in some amino acids such as lysine and threonine (Bakke and Vickers, 2007). Maize is one of the most cultivated cereals in the world after rice and wheat and is a rich source of minerals, especially magnesium and potassium. Maize also contains trace amounts of lysine and tryptophan, which contributes to its low protein content (Okafor et al., 2018). Acha, a pseudo-cereal grain has highly digestible proteins, but low in minerals. Unlike many other cereal grains, acha has significant amounts of cysteine and methionine, the sulfur-containing amino acids (Anuonye et al., 2010). Millet is one of the cereal grains that has received global attention in recent times as ingredients in traditional food preparations and has also found applications in the beverage industries. Millet is a good source of carbohydrates and as such, a potential source of calories to the body. It has been found to contain high amounts of iron but to be low in protein and lysine (Habiyaremye et al., 2017; Tumwine et al., 2018). A look through the nutritional profiles of cereals indicate that they are either deficient or contain insufficient amounts of protein and/or some essential amino acids, which are vital for proper growth and development. For instance, inadequate amounts of protein and amino acids in infants and young children diets may lead to malfunctioning of the brain with adverse effects on the immune system, which could predispose them to high risk of infections (Habiyaremye et al., 2017). This suggests the need for compositing the cereal flours with protein-rich crops in order to meet the nutritional requirement of the target populace and to improve their health status.
Legumes such as soybean, peanut and cowpea have been used over the years as alternatives to animal proteins, because they are low-cost and within the reach of poor resource population in developing countries. Basically, the protein contents in leguminous crops are in the range of 20–30%, depending on the cultivar and species (Okafor et al., 2018). Cowpea is one of the most popular legume crops in the world and is a chief source of protein in many homes and provides dietary carbohydrates, especially in developing countries. It is a good source of lysine but contains trace amounts of cysteine and methionine (Brennan et al., 2016). Soybean (Glycine max) is a special source of plant protein because in addition to its high-quality protein content, it is considered a complete protein due to the presence of all the nine essential amino acids that the body cannot make, which must be obtained through the diet to support normal growth and development of infants and children. Soybean protein could boost the nutrient density of newly developed food products and provide the indispensable amino acids necessary for protein synthesis in muscles and other tissues (Asif and Acharya, 2013).
Like cowpea, peanut (Arachis hypogea) is high in protein but limiting in some essential amino acids such as methionine, lysine and tryptophan (Maphosa and Jideani, 2017; Temba et al., 2017). Literature has documented the deficiency of some nutrients in cereals (Saldivar, 2016; Zhu et al., 2007) and this apparent poor nutritional quality of cereal crops needs to be composited with rich protein sources from legumes to achieve a nutritionally balanced diet (Kumari and Sangeetha, 2017). In addition to the balance of amino acids that may probably be achieved through cereal-legume composite flours, it is possible that this strategy will improve the bioactive components of the resulting composite flours. For instance, the cereals and legumes have been identified to contain some bioactive compounds that possess properties such as antidiabetic, antioxidant, antimicrobials, antihypertensive, anticancer (Malaguti et al., 2014; Shahidi and De Camargo, 2019), which may vary depending on the protein content and the amino acids within the composite flours (Maphosa and Jideani, 2017). Several studies have shown that millet grains are rich in bioactive compounds such as polyphenols and proteins (Akanbi et al., 2019) and is among the minor cereals that are underutilized (Okwudili et al., 2017). Fibre rich wheat and barley brans have been reported to possess bioactive ingredients with the capacity to ameliorate oxidative stress and other related morbidities (López-Perea et al., 2019). The antioxidant properties can be effectively measured using methods such as the 1,1-diphenyl-2-picrylhydrazyl (DPPH), hydroxyl and superoxide radical scavenging activities in addition to metal and ferric reducing activities (Adjimani and Asare, 2015). Composite flours with antioxidant properties would in addition to addressing the problem of protein-energy malnutrition also have the potential to reduce the risk of cardiovascular diseases (Bamigbola et al., 2016). Previous studies have shown that different millet types are rich sources of bioactives, phenolic compounds, peptides which possess in vitro antioxidant or antiradical, antidiabetic and antiproliferative activities (Chandrasekara and Shahidi, 2011, 2012). Various cereal and legume flour composites have been formulated for confectionery and bakery products including wheat-water chestnut flour blends Shafi et al. (2017), acha-soybean blend (Anuonye et al., 2010) maize-cowpea and sorghum-cowpea, millet-tigernut (Omoba et al., 2015) with improved protein and amino acid profiles of the resulting flours. Lentils have generated significant interest from food researchers and the ultimate consumers, as excellent dietary sources of legumes that are rich in dietary fiber, carbohydrates, protein, various vitamins, minerals and in addition, they possess several health-beneficial fatty acids (Nwokolo and Smartt, 1996; Yeo and Shahidi, 2015). Epidemiological studies have reported the effects of lentils/legumes in lowering cholesterol and reducing chronic diseases like colon cancer, heart diseases, and type-2 diabetes (Chhabra, 2018; Yeo and Shahidi, 2020). However, there is dearth of information on the influence of cereal-legume compositing on the antioxidant properties of the resulting flours. This study, therefore evaluated the protein content, amino acid profiles and the antioxidant properties of different cereal-legume blends.
| 2. Materials and methods | ▴Top |
2.1. Materials
All the reagents such as, Triton-X 100, sodium phosphate, reduced glutathione (GSH), 95% methanol, Tris-HCl buffer, EDTA, NaOH, HCl, 1,10-phenanthroline, hydrogen peroxide, FeSO4, FeCl3, FeCl2, were of analytical grade and purchased from Sigma (St. Louis, MO, USA). Wheat flour was purchased from a Wurukum market in Makurdi, Benue State, Nigeria. Other cereals (millet, acha and maize) and the legumes (soybean, peanut and cowpea), produced in the 2020 crop season, were purchased from accredited sellers from same market and cleaned prior to utilization.
2.2. Methods
2.2.1. Preparation of defatted soybean and peanut protein meals
Soybean and peanut seeds were preconditioned as appropriate and processed into defatted meals according to the methods described by Gbadamosi and Famuwagun (2016) through unit operations involving roasting, dehulling, winnowing, milling into whole full-fat flours, defatting the flours using acetone and followed by air drying the residue cakes overnight in a fumehood. The dried defatted soybean and peanut cakes residue were finally ground into defatted soy and peanut protein meals and used in compositing the cereals according to the following ratios: Wheat (100% WT), 90% wheat + 10% soybean (90WT:10SO), 70% Wheat + 30% Soybean (70WT:30SO), 50% Wheat + 50% Soybean (50WT:50SO); 90% Millet + 10% Peanut (90ML:10PN), 70% Millet + 30% Peanut (70ML:30PN), 50% Millet + 50% Peanut (50ML:50PN); 90% Acha + 10% Soybean (90AC:10SO), 70% Acha +30% Soybean (70AC:30SO), 50% Acha +50% Soybean (50AC:50SO) and 90% Maize + 10% Cowpea (90MZ:10CP), 70% Maize + 30% Cowpea (70MZ:30CP), 50% Maize + 50% Cowpea (50MZ:50CP).
2.2.2. Protein content and amino acid composition
Total protein content was determined using the modified Lowry method (Markwell et al., 1987) after samples were treated with 0.1 M NaOH to solubilize the proteins. The amino acid profile of each sample was determined according to the established methods described by Girgih et al. (2011) using a HPLC system after hydrolysis with 6 M HCl. The cysteine and methionine contents were determined after performic acid oxidation while the tryptophan content was determined after alkaline hydrolysis.
2.2.3. Determination of antioxidant properties
2.2.3.1. DPPH radical scavenging activity (DRSA)
The DRSA of each flour mix was determined using the method described by Onuh et al. (2014) with slight modifications for a 96-well flat bottom microplate. Samples were dissolved in 0.1 M sodium phosphate buffer, pH 7.0 containing 1% (v/v) Triton-X 100, to an assay concentration of 1.0 mg/mL. Prior to obtaining the desired assay concentration, the sample mixtures were vortexed for 3 min to obtain optimal dissolution, then centrifuged and the supernatant used for the analysis. DPPH was dissolved in 95% methanol to assay concentration of 100 µM. A 100 µL aliquot of each sample was mixed with 100 µL of the DPPH radical solution in a 96-well plate and incubated at room temperature in the dark for 30 min. The buffer was used in the blank assay while 1.0 mg/mL reduced glutathione (GSH) served as the positive control. Absorbance was measured at 517 nm using a spectrophotometer and the percentage DRSA was determined using the following equation:
2.2.3.2. Superoxide radical scavenging activity (SRSA)
The method described by Xie et al. (2008) was used to determine SRSA of the sample mix. Samples (1.5 mg/mL assay concentration) were each dissolved in 50 mM Tris–HCl buffer, pH 8.3 containing 1 mM EDTA followed by the transfer of 80 µL into a clear bottom microplate well while 80 µL of buffer was added to the blank well. This was followed by addition of 40 µL 1.5 mM pyrogallol (dissolved in 10 mM HCl) into each well in the dark and the rate of reaction was measured immediately at room temperature as absorbance (Abs) change over a period 4 min (1 min interval) using a microplate reader at a wavelength of 420 nm. The SRSA was calculated using the following equation:
2.2.3.3. Hydroxyl radical scavenging activity (HRSA)
The HRSA of samples was determined using the method described by Ajibola et al. (2011) with slight modifications. Samples, prepared to assay concentration of 1 mg/mL and 3 mM 1,10-phenanthroline were separately dissolved in 0.1 M phosphate buffer (pH 7.4) while 3 mM FeSO4 and 0.01% (w/v) hydrogen peroxide were each separately dissolved in distilled water. The mixture was kept at room temperature for 1 h and then centrifuged (3500 x g) for 30 min. Fifty microliters (50 µL) of the samples or GSH standard were first added to a 96-well plate followed by 50 µL each of the 1,10-phenanthroline and FeSO4. To initiate the Fenton reaction in the wells, 50 µL of hydrogen peroxide was added to the mixture and the covered plates incubated at 37 °C for 1 h with constant shaking. The blank consisted of 50 µL phosphate buffer instead of the protein sample. Absorbance of the colored reaction mixtures was measured at 10 min intervals for 1 h in a microplate reader at a wavelength of 536 nm. The reaction rate (ΔA/min) was then used to calculate the HRSA value as follows:
2.2.3.4. Ferric reducing antioxidant power (FRAP)
The FRAP of each sample was determined using the method of (Benzie and Strain, 1996), which was slightly modified as follows. FRAP working reagent was prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tri-(2-pyridyl)-1,3,5-triazine, and 20 mM FeCl3 in the ratio of 5:1:1, respectively to obtain a straw-colored solution, and the temperature of the mixture raised to 37 °C. Samples were dissolved in distilled water to an assay concentration of 1.0 mg/mL. Into a clear 96-well microplate, 40 µL of samples and 200 µL of FRAP reagent were added and absorbance read at 593 nm. Iron II sulfate heptahydrate (FeSO4·7H2O) at 0.025–0.25 mM was used as standard. Iron reducing activity of the samples was determined from the standard curve and the results expressed as Fe2+ (mM).
2.2.3.5. Metal (iron) chelation activity (MCA)
The MCA of the samples was determined according to a previous method (Xie et al., 2008), which was modified as follows. Briefly, samples were prepared to give assay concentrations of 1 mg/mL in distilled water. A 1 mL aliquot of the sample solution or blank (distilled water) was mixed with 50 µL of 2 mM FeCl2 and 1.85 mL double distilled water in a reaction tube. This was followed by the addition of 100 µL of 5 mM Ferrozine. The mixture was vortexed thoroughly and incubated at room temperature for 10 min. After incubation, a 200 µL aliquot of the reaction mixture was transferred into a 96-well plate and absorbance values of both the blank (Ab) and samples (As) were measured at 562 nm using microplate reader. The metal chelating activity was calculated as follows:
2.2.4. Statistical analysis
The cereal-legume blends were prepared in duplicates and analyses performed in triplicates. The results were subjected to analysis of variance using SPSS version 18.0. The statistical significance of differences (p < 0.05) between mean values were determined using the Duncan’s multiple range test.
| 3. Results and discussion | ▴Top |
3.1. Protein content of cereal-legume flour blends
The protein content of the whole wheat (100% WT) was 13.0% with 13.5–20.3% increases in the protein content of wheat-soybean flour blends as a result of soybean flour addition (Table 1). Increases in the amount of soybean flour in acha/soybean flour blends may be responsible for the observed 15.1, 19.8 and 25.0% increases in the protein content of the blends at 10, 30 and 50% inclusion of soybean flour, respectively. In a similar manner, as the amount of peanut flour increased from 10 to 50% in millet/peanut blends, there were increases in the protein content from 14.0 to 19.5%. For addition of cowpea to maize flour, there were similar increases in the protein content by 6.9-82.3% when the amount of cowpea was raised from 10 to 50% in the blends. Overall, the results showed that acha/soybean blends resulted in the highest protein content (15.1–25.0%) while the least was associated with millet/peanut blends (14.0–19.5%). The differences in the protein contents of the cereal-legume blends may be attributed to variations in the protein content of the starting material (individual cereals and legumes) before blending. Previous reports on wheat/soybean flour (Ndife et al., 2011), flaxseed/wheat flour (Kaur et al., 2017), acha/pigeon pea (Olagunju et al., 2018) and acha/soybean (Ayo and Kajo, 2016) blends also showed increases in the protein contents of the composite flours as the inclusion level of legumes increased.
 Click to view | Table 1. : Protein content of cereal-legume flour blends* |
3.2. Amino acid composition
Proteins are essential in the body because they are involved in various chemical processes, which sustain life and they serve as body structure building blocks for all living things in addition to acting as catalytic enzymes and modulators of the body system pathways (Wu, 2016). The extent at which proteins perform these functions is based on the content of amino acids, which is the building block of proteins. The amino acid composition of cereal-legume composite flours is shown in Table 2. The contents of aspartic/asparagine and glutamic/glutamine increased as the level of legume inclusion increased from 10–30% in the cereal-legume composite flours. The glutamic acid contents were in greater amounts in the composite flour blends when compared to aspartic acid content in same samples. The sample that contained wheat/soybean blends had the highest glutamic and aspartic acid contents. Though tryptophan appeared to be the limiting amino acid in all the composite flours, its content was further reduced as the percentage of soybean and peanut increased in the mixtures. The methionine content of the flour mixtures also decreased as the soybean and cowpea component of the flour mix increased in all the composite flours. These reductions are consistent with the low contents of tryptophan in soybean and peanut and the low methionine levels in soybean and cowpea. This is in contrast to previous studies that reported increases in the methionine content of acha/soybean (Anuonye et al., 2010), and co-fermented maize/cowpea and sorghum/cowpea flour blends (Ndife et al., 2011). However, inclusion of peanut led to slight increases in the sulfur containing amino acids of the composite flours. The flour blend that contained 90:10 maize/cowpea ratio had the highest methionine content (3.04 g/100 g). The phenylalanine contents of the flour mixes were enhanced as the content of legume flours increased. The phenylalanine was higher in maize/cowpea (4.81–5.43 g/100 g) compared to millet/peanut flours (4.20–4.73 g/100 g), which had the lowest. Basically, the contents of the essential amino acid (EAA) were higher in the composite flours as the level of soybean and cowpea increased but not peanut. The major essential amino acid in this study was leucine (6.39–8.45 g/100 g) while the least was tryptophan (0.70–1.44 g/100 g) across the composite flours. In a similar manner, maize/cowpea (50MZ:50CP) flour blend in this study exhibited greater amounts of aromatic (9.82%), essential (37.35%), positively charged (16.10%) and branched chain (17.25%) amino acids. The 90:10 maize-cowpea mixture had the highest hydrophobic amino acids (52.78%), while negatively charged amino acids were most abundant (44.69%) in WT90:10SO flour and sulfur containing amino acids highest (4.4%) in each of 50MIL:50PN and 90MZ:10CP.
 Click to view | Table 2. : Amino acid composition (g/100 g) of cereal-legume flour blends* |
3.3. Antioxidant properties
3.3.1. DPPH radical scavenging activity
The DRSA is one of the methods often used to screen the antioxidant properties of food materials (Moukette et al., 2015). The method involves a change in purple colour of the DPPH radical to colourless on encountering an antioxidant compound. The DRSA of the cereal-legume composite flour blends are shown in Figure 1. The composite flour blend that contained 50% millet/peanut exhibited significantly greater (p < 0.05) DRSA (43.7%) when compared to other composite flour blends (4.70–22.3%) but lower than GSH (69.0 %). The higher DRSA of 50/50 millet/peanut composite flour could be due to the higher levels of sulfur-containing amino acids, which enhance electron donation to the DPPH radical. The DRSA of wheat flour (4.7 %) was increased from 8.33 to 17.7% (an increase of 77.2–276.6%) after blending with soybean meal at 10–50%. Similar patterns of increased DRSA were observed when acha flour was composited with equivalent proportions of soybean meal additions. The DRSA of millet flour was enhanced on compositing with peanut and this increases in the radical scavenging activities was directly proportional to the level of peanut meal in the blends. Addition of cowpea to maize flours resulted in greater DRSA, which was similarly directly proportional to the percentage of cowpea in the flour blends. Generally, addition of leguminous seed flours such as the soybeans, cowpea and peanut improved the radical scavenging activities. Previous works on wheat/chestnut (Shafi et al., 2017) and wheat/flaxseed (Kaur et al., 2017) flour blends also reported increased DRSA, which were attributed to the phenolic compounds present in chestnut and flaxseed. This claim was supported by Amarowicz and Pegg (2008) that leguminous seeds are recognized as sources of antiradical agents due to the presence of phenolic acids and their derivatives, such as the flavanols, flavan-3-ols, anthocyanins/anthocyanidins, condensed tannins/proanthocyanidins and tocopherols. Itagi and Singh (2012) evaluated the antioxidant activities of multigrain composite mixtures and reported their DRSA to range from ∼76–86%. The authors suggested that the presence of compounds like amino acids in the mixtures could enhance antioxidant capacity through synergistic interactions with the polyphenols present in the food matrix to produce a higher antioxidant capacity. The DRSA of mixed cereal/legume grains in this study are significantly (p < 0.05) lower than those reported by (Itagi and Singh, 2012). The DRSA for wheat/legume composite flours ranged from 5.27–11.3% in comparison to wheat alone (3.74%), indicating the adding legumes to cereals for development of baked products or confectioneries is advantageous as this improved the DRSA by 40.9–202.1%. The presence of phytochemicals could also be partly responsible for the increased DRSA of the legume-enriched composite flours observed in this work. However, the enriched composite flours exhibited varied DPPH scavenging activities, indicating possible differences in the content of phytochemicals in the legumes seed flours.
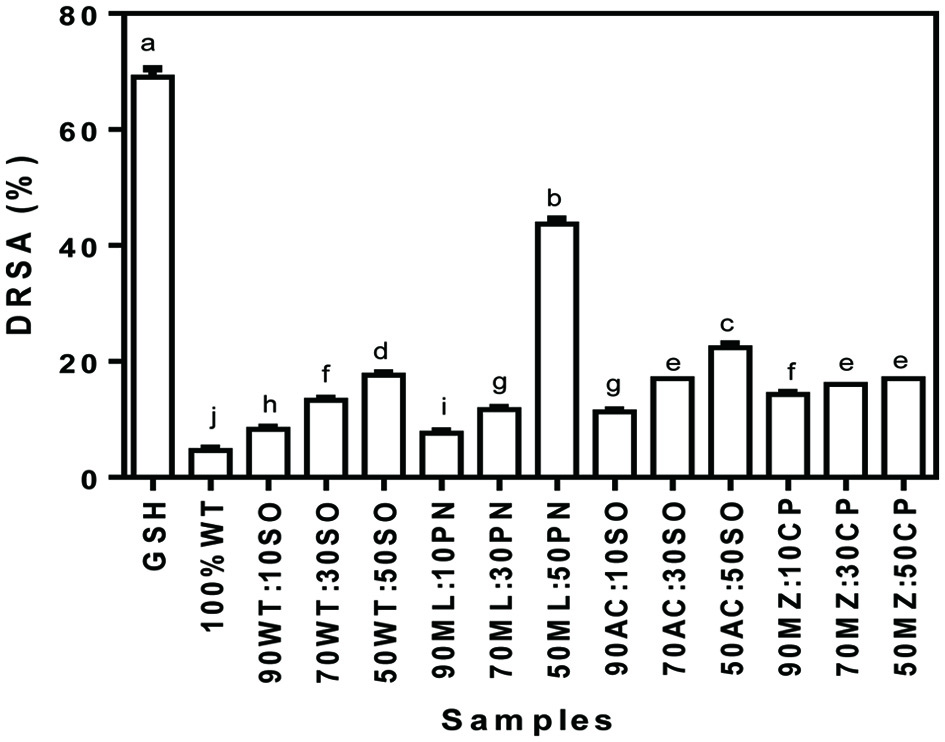 Click for large image | Figure 1. DPPH radical scavenging activity (DRSA) of cereal-legume flour blends. WT, Wheat flour; SO, Soybean meal; ML, Millet flour; PN, Peanut meal; AC, Acha flour; MZ, Maize flour; CP, Cowpea flour |
3.3.2. Superoxide radical scavenging activities
Superoxide radicals have been a major concern over the years because they are precursors to many deadly reactive species radicals such as the hydroxyl and hydrogen peroxides. These toxic reactive species have affinity for body’s vital cell components such as the DNA, protein and lipids, hence the need to scavenge the radical precursor and its by-products to protect human health. As shown in Figure 2, with the exception of acha/soybean (90:10) flour blends, all the composite flour exhibited significantly (p < 0.05) higher SRSA when compared with GSH (37.3%). The cereal/legume composite flour mixtures in this study had lower SRSA, which ranged from 35–50.7% when compared to that of 100% wheat flour (56.7%). Millet/peanut showed greater SRSA at each level of legume inclusion, followed by maize/cowpea composite flours. Just like the DRSA, there was an increase in the SRSA as the level of legume crops increased in the composite flours. The increase in the SRSA may be attributable to the presence of phenolics in the legumes as previously suggested and condensed tannins associated with the reduction of oxidative stress. Zou et al. (2016) showed through structure-function analysis that Lys, Leu and Pro may enhance superoxide scavenging activity of peptides by contributing to the high overall superoxide radical scavenging activities. Addition of legume flours led to increased levels of Lys and Leu (Table 2), which could have contributed to the enhanced SRSA when compared to the cereal flours alone.
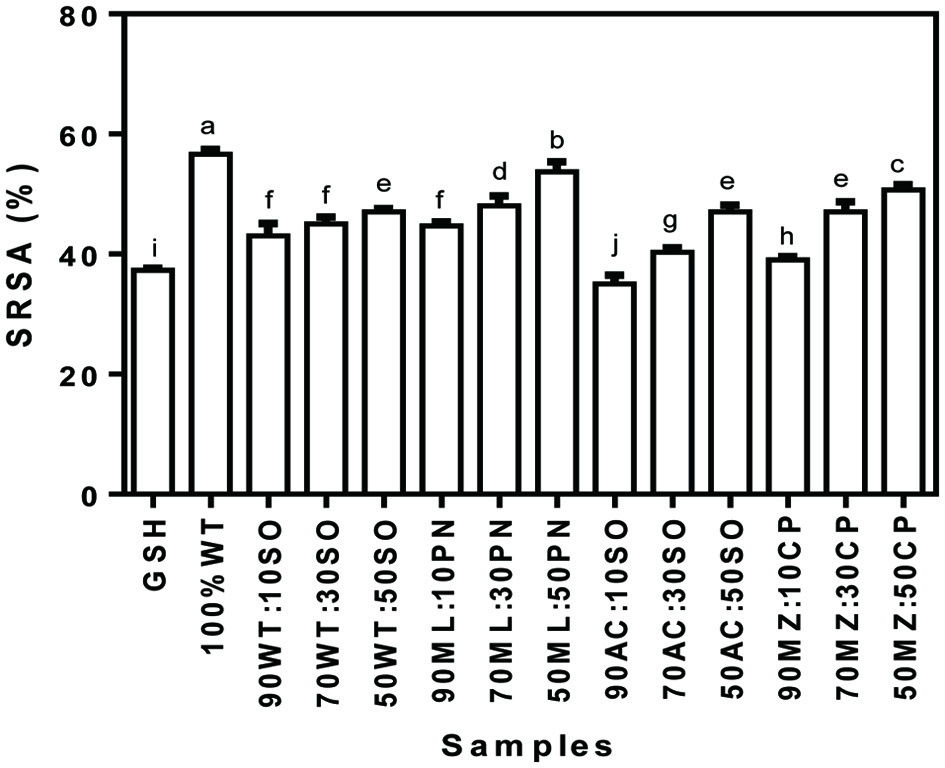 Click for large image | Figure 2. Superoxide radical scavenging activity (SRSA) of cereal-legume flour blends. WT, Wheat flour; SO, Soybean meal; ML, Millet flour; PN, Peanut meal; AC, Acha flour; MZ, Maize flour; CP, Cowpea flour |
3.3.3. Hydroxyl radical scavenging activity
Figure 3 shows the HRSA of cereal-legume composite flours. It was observed that unlike the SRSA, there were no significant (p > 0.05) differences in the HRSA of wheat/soybean flour blends, irrespective of the variation of soybean in the blends. Also, there were no significant differences (p > 0.05) in the flour blends that contained acha flour at 10–30% soybean flours (85.3 and 88.0%) but the blend that contained 50% soybean (91.3%) exhibited significantly (p < 0.05) higher HRSA than the samples that contained lower soybean levels. The sample that contained lowest percentage (10%) in the millet/peanut flour blends exhibited greater HRSA (87.7%) compared with samples that contained >10% (84.3 and 84.3%) peanut in millet/peanut flour blends. The pattern of the result was also similar for the maize/cowpea flour blends. Among the maize/cowpea composite flours, the blend that contained 30% cowpea showed greater HRSA (94.3%) when compared with other blends that contained 10 and 50% cowpea. Among all the flour blends, the sample that contained 30% cowpea in the maize/cowpea flour blends exhibited the highest HRSA. The HRSA of all the flour blends were >50% suggesting that they are good hydroxyl radical scavengers. Siddeeg et al. (2015) reported the HRSA of seniat seeds protein hydrolysate fractions at high concentration of 3 mg/mL to be 68.5, 60.1 and 57.3% for glutelin, albumin, and globulin, respectively, which are lower than reported for most of the cereal/legume flour blends in the present study.
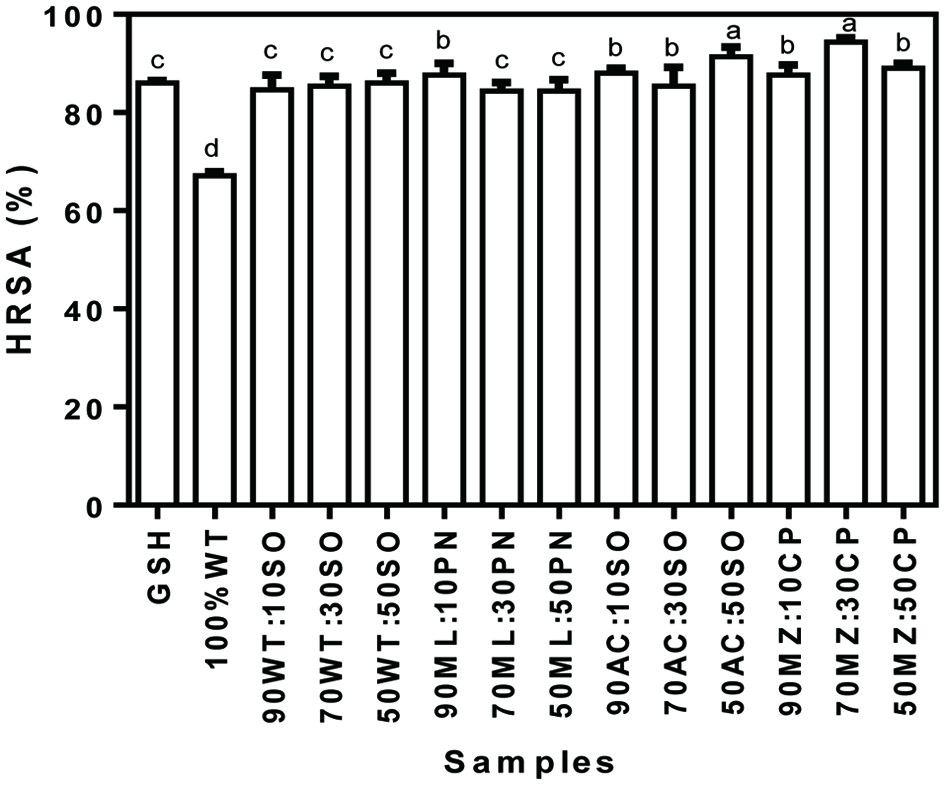 Click for large image | Figure 3. Hydroxyl radical scavenging activity (HRSA) of cereal-legume flour blends. WT, Wheat flour; SO, Soybean meal; ML, Millet flour; PN, Peanut meal; AC, Acha flour; MZ, Maize flour; CP, Cowpea flour |
3.3.4. Ferric reducing antioxidant power
FRAP measures the potential of an antioxidant material to break a chain reaction through its hydrogen atom donating ability. As indicated in Figure 4, all the composite flours exhibited greater FRAP (0.06–0.45) when compared with the FRAP of GSH (0.04). There were increases in the FRAP of the composite flours as the content of the legume flours increased in the mixture from 10–50%. Among the composite flour blends, the maize/cowpea flour mix exhibited greater range of FRAP (0.16–0.45) activities when compared with other blends, which ranged from 0.06–0.42. Conversely, wheat/soybean (0.06–0.21) flour blends had the least FRAP activities at all the levels of soybean incorporation. The pattern of FRAP results agrees with that of the DRSA in this study. Earlier reports by Shafi et al. (2016) also reported increases in the FRAP content of composite flours of wheat/chestnut as the content of chestnut flour increased, which is consistent with the data reported from this work.
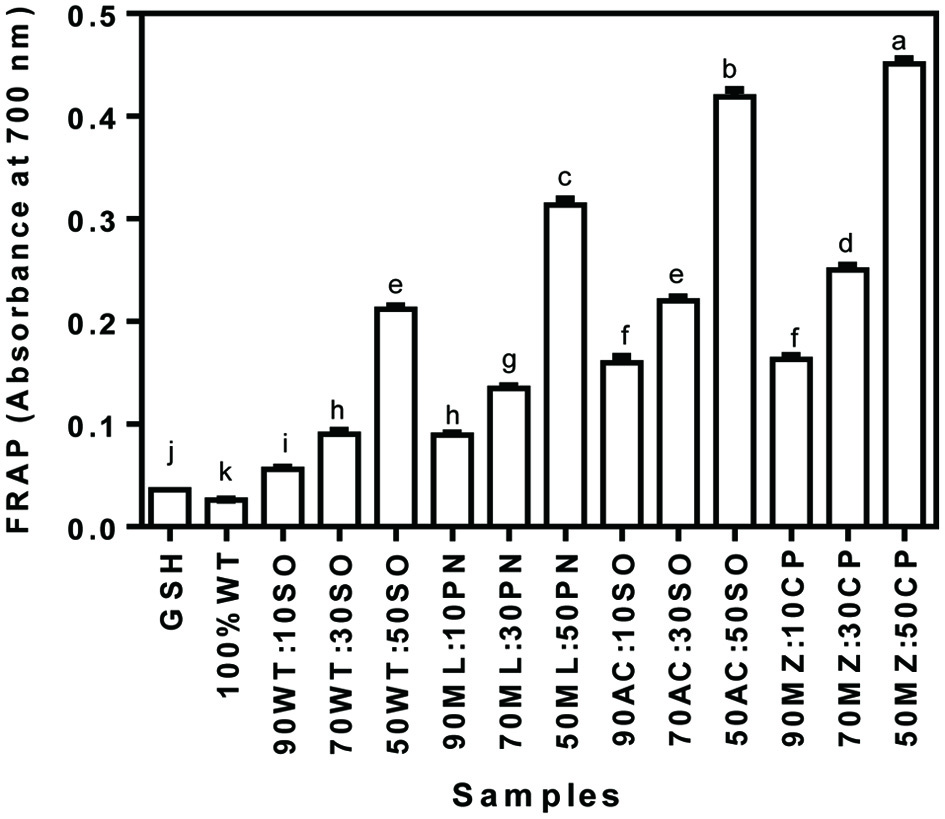 Click for large image | Figure 4. Ferric reducing antioxidant power (FRAP) of cereal-legume flour blends. WT, Wheat flour; SO, Soybean meal; ML, Millet flour; PN, Peanut meal; AC, Acha flour; MZ, Maize flour; CP, Cowpea flour |
3.3.5. Metal chelation activity
Ferrous ions have been recognized to participate actively in Haber-Weiss reaction, whose product, such as the superoxide radical results in the formation of hazardous hydroxyl radicals (Xie et al., 2008). Therefore, chelation of ferrous ion (Fe2+) will decrease the amount of these ions that can participate in the reaction thereby reducing the formation of the damaging hydroxyl radicals. Unlike the FRAP of the samples, the MCA of the samples took a different pattern as shown in Figure 5. With the exception of acha/soybean flour blends, the MCA of other flour blends followed a similar pattern. For instance, blends that contained 30% legume flour incorporation exhibited the highest MCA when compared with 10 and 50% legume flour incorporation. In all, the blend that contained 50% soybean flour in the wheat/soybean mixture exhibited the least MCA while the highest was obtained in the flour blend that contained 30% soybean in wheat/soybean flour mixture.
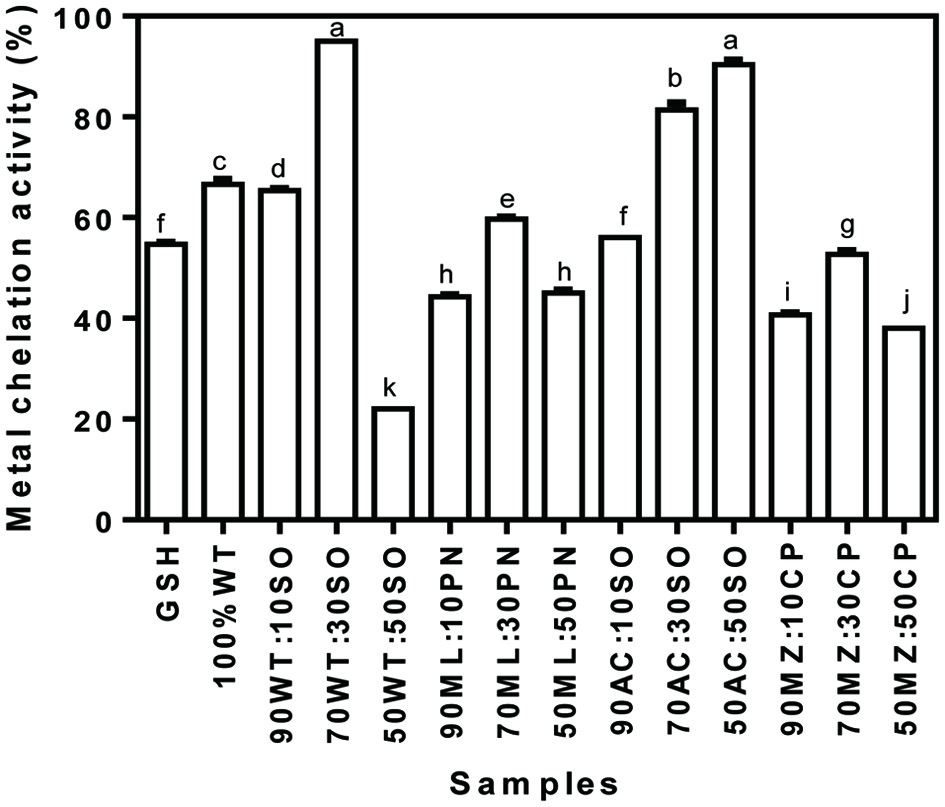 Click for large image | Figure 5. Metal chelation activity of cereal-legume flour blends. WT, Wheat flour; SO, Soybean meal; ML, Millet flour; PN, Peanut meal; AC, Acha flour; MZ, Maize flour; CP, Cowpea flour |
| 4. Conclusions | ▴Top |
Protein content of the cereal/legume composite flours were enhanced as the level of addition of legumes increased with the highest level achieved in the acha/soybean flour blends. The results showed significant improvements in the amino acid content and antioxidant properties of cereal flour upon blending with legumes. The antioxidant activity of the cereal-legume mixtures depended on the type of legume and level of incorporation. For instance, the wheat/soybean mixture blend that contained 50% soybean flour exhibited highest DRSA and FRAP whereas, the flour with 30% soybean in the wheat/soybean blend showed stronger potency in terms of MCA. The 50/50 millet/peanut mixture had the highest SRSA (56.7%), while the 70/30 wheat/soybean flour blend exhibited the highest (95.0%) HRSA. The results suggest that the cereal-legume composite flours have enhanced potentials as nutritional and bioactive ingredients for the formulation of health-promoting food products.
Acknowledgments
The authors acknowledge support from the Natural Sciences and Engineering Council of Canada (NSERC), funding reference number RGPIN 2018-06019.
Conflict of interest
Author declares no conflict of interest.
| References | ▴Top |