| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 2, June 2018, pages 37-50
Edible brown seaweeds: a review
K.K. Asanka Sanjeewa, You-Jin Jeon*
Laboratory of Marine Bioresource Technology, Department of Marine Life Science, School of Marine Biomedical Sciences, Jeju National University, Jeju 63243, Republic of Korea
*Corresponding author: You-Jin Jeon, Department of Marine Life Sciences, School of Marine Biomedical Sciences, Jeju National University, Jeju 63243, Republic of Korea
DOI: 10.31665/JFB.2018.2139
Received: March 8, 2018
Revised received & accepted: June 11, 2018
| Abstract | ▴Top |
Seaweeds play a vital role as a source of food and ingredients in traditional Korean medicine. Koreans consume seaweed as fresh vegetables, salad, soups, or snacks. There are several edible brown seaweed species are abundant along the shores of the Korean peninsula, such as Ecklonia cava, Hizikia fusiforme, Laminaria japonica, Pelvetia siliquosa, Sargassum fulvellum, and Undaria pinnatifida. With the growing body of scientific evidence, it is clear that these brown seaweeds are not only good substitutes for land vegetables but also a good source of bioactive secondary metabolites. The secondary metabolites identified from edible Korean brown seaweeds (phlorotannins, sulfated polysaccharides, pigments, and sterols) have the potential to be developed as functional food ingredients. However, seaweeds consumption and their industrial level applications as functional materials are limited compared to the land vegetables. Insufficient awareness about health benefits of seaweeds might be the reason for this issue. Thus, in this review health promotion properties associated with edible Korean brown seaweeds are summarized. The present study might therefore increase consumption and industrial use of brown seaweeds.
Keywords: Seaweeds; Bioactive compounds; Functional foods
| 1. Introduction | ▴Top |
Seaweeds are important macroscopic marine organisms with great economic and ecological value. In East-Asia (Korea, China, and Japan) seaweeds serve as healthy food and medicinal ingredients (Jeong et al., 2015). The term “sea vegetable” is used to define seaweeds include approximately 50 different species of edible seaweeds in this region (Park et al., 2016). Besides the nutrient value, a growing body of scientific evidence indicates that the secondary metabolites present in seaweeds have great potential for developing active ingredients in functional products. Phlorotannins, sulfated polysaccharides, ergosterol, and some pigments isolated from seaweeds have been found to possess outstanding bioactive functionalities (antioxidant, anticoagulant, anticancer, anti-microbial, anti-diabetic, and anti-inflammatory activities) compared to the synthetic chemicals under in vivo and in vitro conditions (Jeong et al., 2015; Son et al., 2018).
Korea is one of the major seaweed producers and consumers in the global seaweed market and makes great contributions to the Korean economy. Seaweeds have more than thousands of year’s history in Korea as food and medicinal ingredients, and increases the food security and healthy lifestyle of Koreans (Choi et al., 2012). During winter (December to February), Koreans enjoy seaweed as a vegetable substitute in the winter season (Park et al., 2016). Figure 1 graphically illustrates common seaweed applications including traditional meals, cosmeceutical and functional food ingredients found in Korea. On the basis of their color and appearance, seaweeds are categorized into three groups as brown, green, and red. All three seaweed types have been found to possess interesting bioactive properties and are useful for a range of industrial applications (Wijesinghe and Jeon, 2012a). However, in the present study, we have focused about the potential health promotion properties of edible brown seaweeds consumed in Korea including Ecklonia cava, Hizikia fusiformis, Sargassum fulvellum, Laminaria japonica, Undaria pinnatifida, and Pelvetia siliquosa.
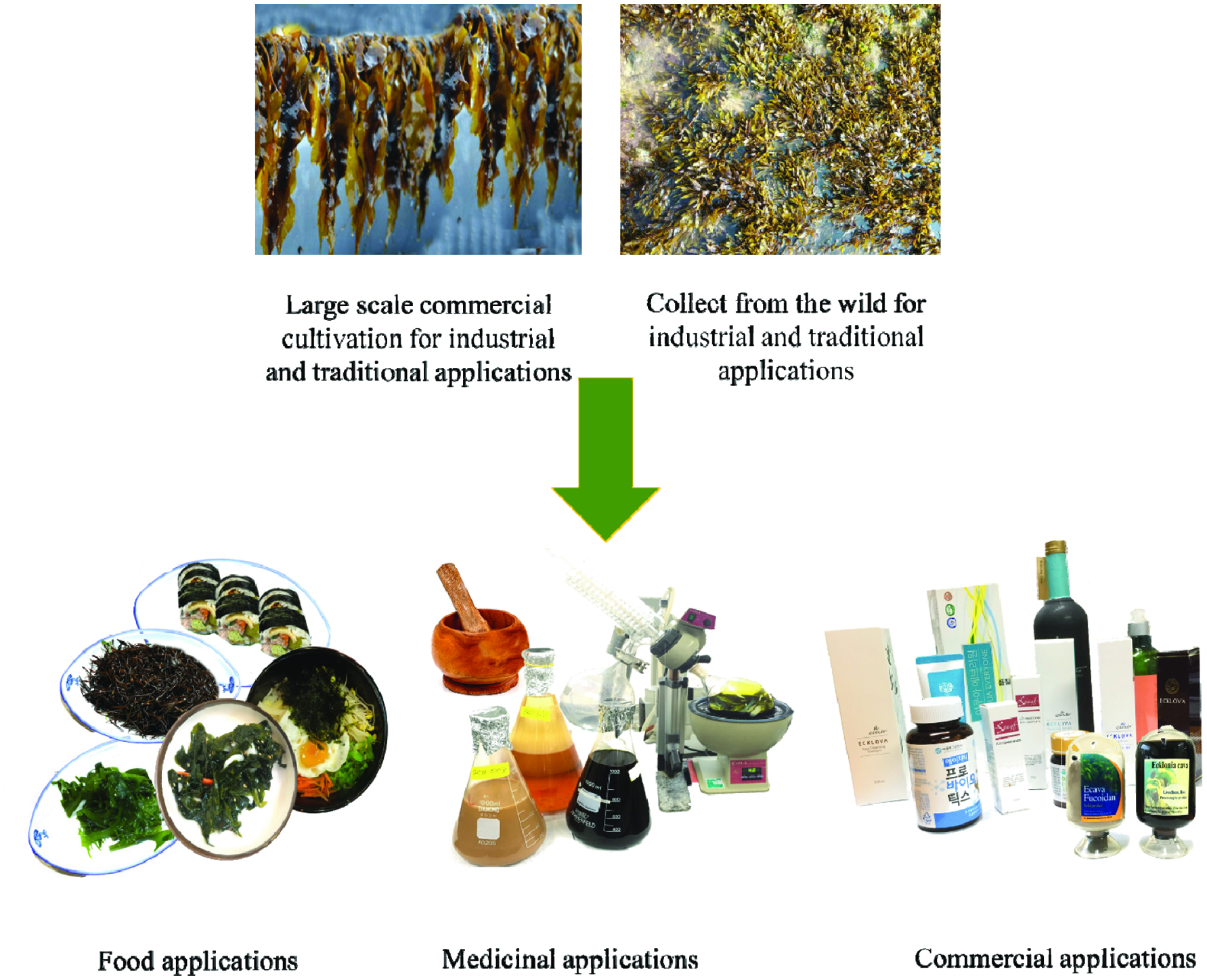 Click for large image | Figure 1. Common applications of seaweeds. |
| 2. Brown seaweeds | ▴Top |
2.1. Ecklonia cava (Kamthe)
Ecklonia cava is a brown alga, abundantly found in the subtidal regions of Jeju Island, Korea. E. cava, is popular as a food ingredient, fertilizer, as an ingredient in folk medicine, and animal feed stock in Korea and Japan (Le et al., 2009). Specifically, E. cava, is commercially cultivated to supply a summer feed for the abalone industry in Korea (Hwang et al., 2012a). E. cava has been identified as a potential source of a wide spectrum of secondary metabolites such as phlorotannins, sulfated polysaccharides, and carotenoids. The compounds isolated from this seaweed have shown interesting biological activities in vital industrial applications including functional foods, pharmaceuticals, cosmeceuticals, and nutraceuticals (Wijesinghe and Jeon., 2012a). Previously, a number of studies have reported that the secondary metabolites isolated from E. cava render bioactive properties such as antioxidant, anti-diabetes, anti-coagulative, anticancer, antimicrobial, anti-inflammatory, and anti-human immunodeficiency virus (HIV).
2.2. Hizikia fusiforme (Tot)
Hizikia fusiformis is a huge annual brown seaweed that grows in Korea, China, and Japan. H. fusiformis is abundant in southern coast, west coast, and Jeju Island in Korea. In the traditional Chinese medicine H. fusiformis have been used as an active ingredient for thousands of years (Park et al., 2017). H. fusiformis is a popular edible seaweed in East-Asian countries. Besides Korea, H. fusiformis is also popular in Japan and China as an edible seaweed (Choi et al., 2009). Tot grows from the foot of the eulittoral to the upper part of the sublittoral zone, around the Korean peninsula. Tot is preserved as a salad, fried with vegetables, and often used as an ingredient in bi-bim-bap. Bi-bim-bap is a mixture of seasoned vegetables, rice, and a paste prepared from chili paper. Recently, the market demand for food and other functional products prepared from H. fusiformis has increased due to the excellent nutritional value as well as health promotion properties associated with H. fusiformis (Hu et al., 2013). However, Nakamura et al. (2008) have reported that cooked tot also contains a considerable amount inorganic arsenic. Thus, the authors have proposed that the regular consumption of cocked tot might increase cancer risk due to the presence of inorganic arsenic in tot (Nakamura et al., 2008).
2.3. Laminaria japonica (Da-si-ma)
Laminaria japonica is an important seaweed cultured in Korea and commercially cultivated in the temperate seaside areas of northwest Pacific region. L. japonica is a popular sea vegetable in many Asian and Pacific countries including Korea. L. japonica is used as an ingredient in traditional Korean medicine. A soup prepared from L. japonica is used to increase maternal health recovery of women after giving birth (Post-natal). (Choi et al., 2012; Islam et al., 2013). In addition, L. japonica use as a medicinal ingredient in traditional Japanese and Chinese medicine (anti-thrombosis, gall disease, hard lump, edema, anti-thyroid tumor, tuberculosis and beriberi drugs) to treat various disease conditions (Mei et al., 2017; Wang et al., 2018; Zeng et al., 2017). Sliced L. japonica (5–6 cm long strips) is boiled with hot water to prepare soup and dried L. japonica is used as a cookie with green tea, or pickled in vinegar. In addition, L. japonica is also mixed with various traditional Korean food products like bread, muffins, and cake to enhance flavor and quality of the food products (Choi et al., 2012).
2.4. Pelvetia siliquosa (Tumbugi)
Pelvetia siliquosa is another popular edible brown seaweed in Korea. P. siliquosa belongs to the family Fucaceae and abundantly grows in the rocky areas of the southern seashores of Korea. P. siliquosa is consider an important source of seafood and used as a source of alginic acid. Traditionally, P. siliquosa has been consumed as seasoned sea greens for religious services, as well as in soup or fresh seafood salad (Hwang et al., 2012b). A number of studies have reported that the metabolites present in tumbugi have bioactive properties such as anti-diabatic and antioxidant effects (Lee et al., 2003; Lee et al., 2004).
2.5. Sargassum fulvellum (Mo-Ja-ban)
Sargassum fulvellum is a popular seafood in the Korean food market. S. fulvellum is an abundant seaweed from southern coast to the eastern coast of Korea. People living in Jeju Island of Korea use this seaweed to prepare salad, soup (Mom-Guk) or use it to prepare side dishes (Choi et al., 2007a; Hwang et al., 2007). In addition to its food value, in the traditional medicine S. fulvellum is used as a medicine to treat diseases such as a dropsy, lump, swollen, painful scrotum, and to treat urination problems (Gwon et al., 2013; Kang et al., 2008). As claimed by the previous studies, S. fulvellum is a potential candidate for developing functional material due to the interesting bioactive properties such as antioxidant, anticancer, and anticoagulant (De Zoysa et al., 2008; Kang et al., 2008).
2.6. Undaria pinnatifida (Mi-Yeok)
Korea is one of the largest commercial cultivator of U. pinnatifida (Choi et al., 2007b). This seaweed has a long history in Korea and Japan as an edible product. The coastal areas located in south part of Korea is popular for growth and cultivation of U. pinnatifida. Fucoxanthin is one of the major bioactive compounds present in Mi-Yeok. Fucoxanthin isolated from Mi-Yeok owing a number of bioactive properties such as antioxidative, anti-cancerous, anti-obesity, angiotensin-converting-enzyme inhibitory, and anti-inflammatory (Prabhasankar et al., 2009; Suetsuna and Nakano, 2000). U. pinnatifida is generally served in soup, salad, and side dishes (Kim, 2010). In addition, U. pinnatifida is a popular seafood among Korean mothers. Soup prepared from Mi-Yeok is served to lactating mothers to help to increasing postpartum convalescence (Cho et al., 2007).
| 3. Bioactive compounds reported from edible brown seaweeds | ▴Top |
3.1. Carotenoids
Carotenoids are red, orange, and yellow color pigments present in plant leaves, fruits, and flowers. Plants, bacteria, fungi, and algae are the producers of carotenoids. However, carotenoids are also responsible for color spot present in birds, insects, fish, and crustaceans, because many animals incorporate carotenoids to their bodies from their diet (Stahl and Sies, 2003). Carotenoids in photosynthetic organisms help in photosynthesis, photo-protection, phototropism, and photo-reception, and act as a repellent for their natural enemies (Dembitsky and Maoka, 2007; Matsuno, 2001). The central chain of carotenoids is build up with cyclic end-groups substituted with oxygen-containing functional groups. According to their composition, carotenoids are divided into two groups as carotenes and oxocarotenoids or xanthophylls (Stahl and Sies, 2003). Fucoxanthin (Fig. 2) is a well-known carotenoid pigment present in brown seaweeds. Fucoxanthin accounts for around 10% of the total natural production of seaweed carotenoids (Matsuno, 2001). With interesting bioactive properties associated with fucoxanthin, which is a popular ingredient in industries like functional food, cosmeceutical, and nutraceuticals (Peng et al., 2011; Miyashita and Hosakawa, 2018).
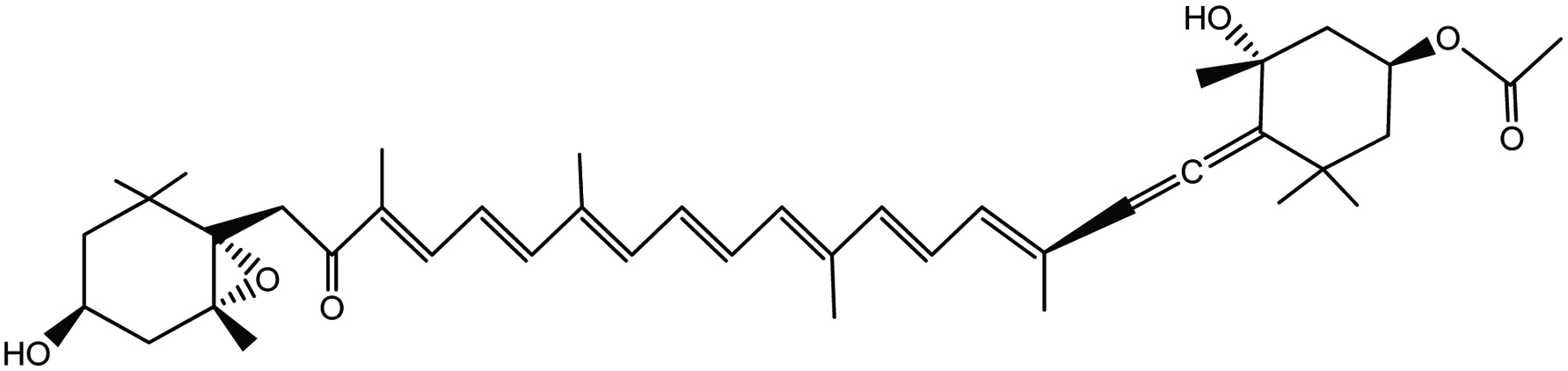 Click for large image | Figure 2. Chemical structure of fucoxanthin. |
3.2. Polyphenols
Polyphenols are water-soluble chemical compounds present in cell vacuole (Harborne, 1998; Manach et al., 2005). Polyphenols are capable of reacting or pairing with one-electron oxidants formed during biological processes. Due to their high electron donating ability, polyphenols are considered as one of the best class of bioactive compounds present in nature (Handique and Baruah, 2002). Polyphenols reported from land plants are polymers of flavonoids and gallic acid and similarly occur in brown seaweeds (phlorotannins) as chains of 1,3,5-trihydroxybenzene formed in the acetate-malonate pathway (Li et al., 2011). Phlorotannins produced by brown seaweeds act as herbivore deterrent, digestive inhibitor, UV screen, and antibacterial agents that protect seaweeds from their natural enemies (Targett and Arnold, 1998). In addition, some studies have confirmed that, phlorotannins render a range of bioactive properties such as antioxidant, anticancer, ant-inflammation, anti-allergic, antibacterial, and matrix metalloproteinase inhibition (Wijesinghe and Jeon, 2012b). Due to these diverse bioactive properties, phlorotannins have been identified as an important seaweed metabolite to use as a functional ingredient in food products, pharmaceuticals, and cosmeceuticals (Li et al., 2011). Figure 3 illustrates the structures of some phlorotannins isolated from brown seaweeds.
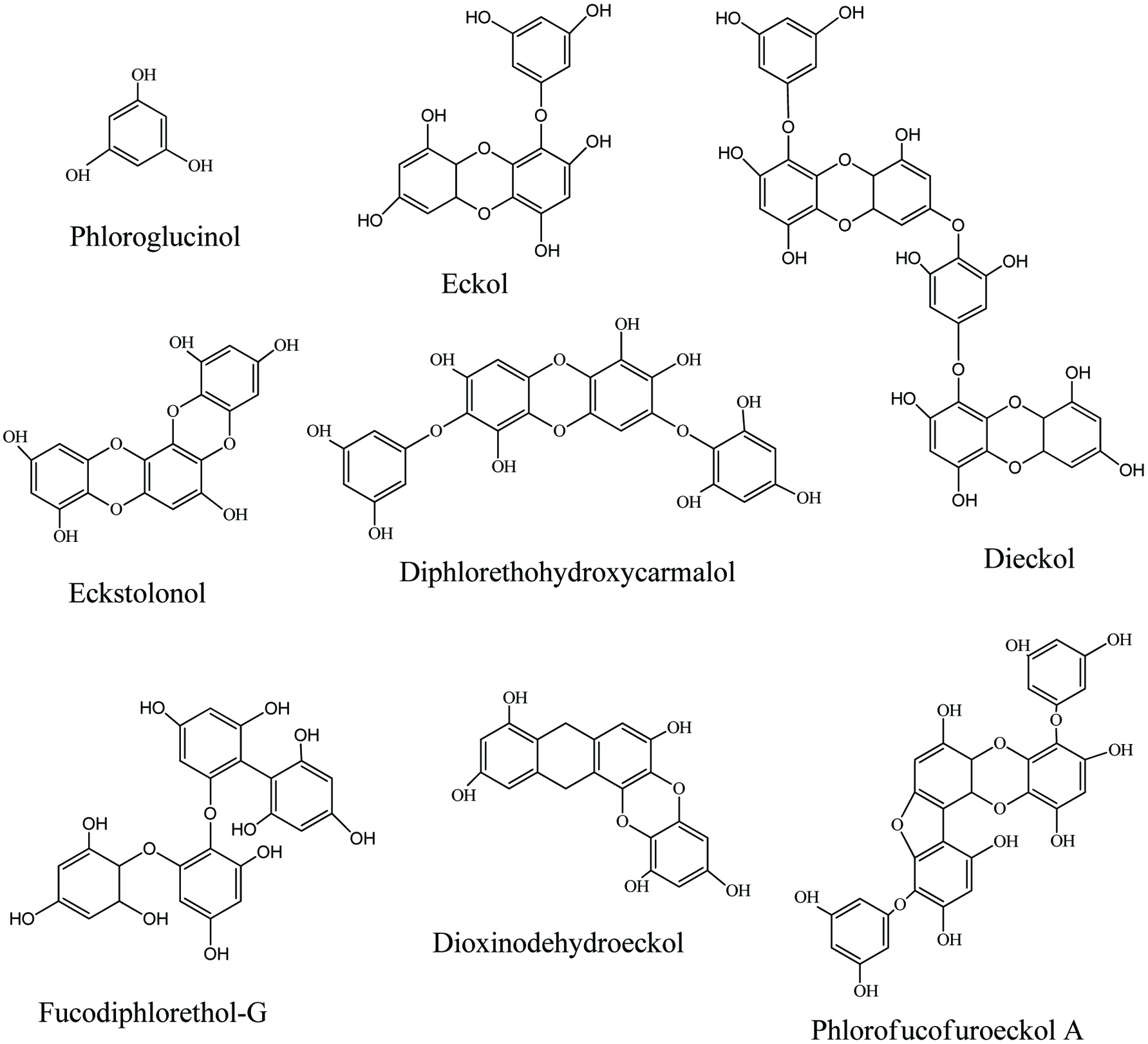 Click for large image | Figure 3. Bioactive phlorotannins isolated from brown seaweeds. |
3.3. Polysaccharides
3.3.1. Alginic acid
Alginic acids are high-molecular-weight polysaccharides reported from the brown seaweed cell wall. Alginic acids are alkali-soluble polysaccharide where the other cell wall constitutes such as fucoidan and laminarin are water-soluble. In water, alginic acid forms a mechanically stable porous hydrogel. Moreover, seaweeds contain approximately 10–40% of alginic acid from on a dry weight basic. Growing depth and seaweed growth season determine the amount of alginic acid in the seaweed biomass (Marriott et al., 2016; Rioux et al., 2007). Alginic acid comprises two kinds of hexuronic acids including α-1,4-linked L-guluronic acid and β-1,4-linked D-mannuronic acid residues arranged in homopolymeric blocks separated by regions of alternating sequences of the two monomers (Fig. 4) (Marriott et al., 2016). In the food industry, alginates are used as thickening agents or metal ion chelators (Gupta and Abu-Ghannam, 2011). In addition, alginates have also been used as an immune-stimulant in aquaculture to increase disease resistance and non-specific immune responses in aquatic species such as abalone, shrimp, and grouper (Lee et al., 2017).
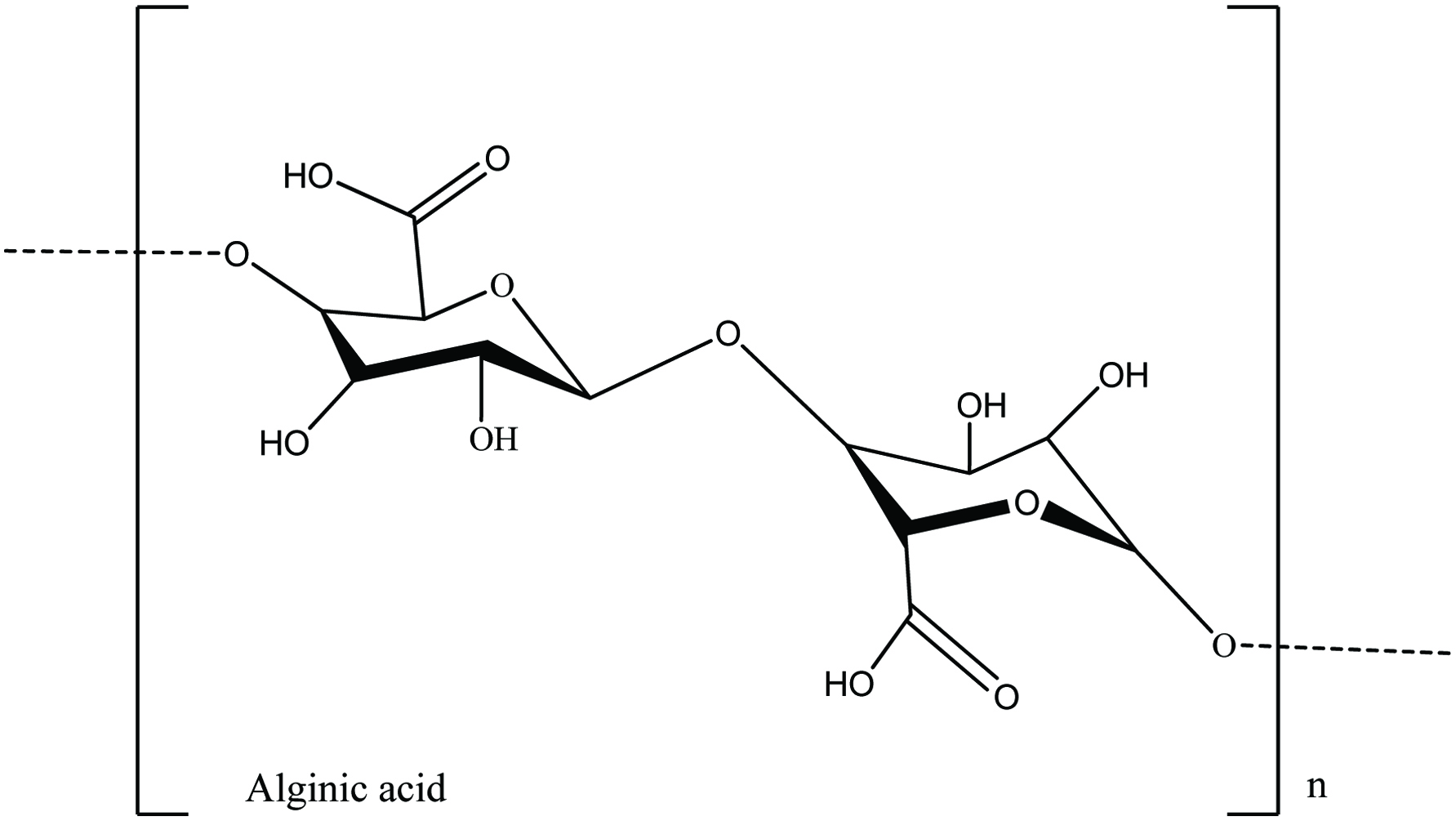 Click for large image | Figure 4. The chemical structure of alginic acids present in brown seaweeds. |
3.3.2. Fucoidans
Fucoidans are a class of sulfated polysaccharide extracts from brown seaweeds. During the past few decades, fucoidan has gained considerable attention in the biomedical research studies due to its diverse bioactive properties like anti-coagulant, antioxidant, anticancer, anti-inflammatory, and immune-modulatory effects (Sanjeewa et al., 2017). Classically, backbone of fucoidan is made of α(1 → 3)-L-fucopyranose residues or alternating α(1 → 3) and α(1 → 4)-linked L-fucopyranosyls or both forms. Moreover, fucoidans contain monosaccharides such as fucose (mainly), mannose, galactose, glucose, arabinose, uronic acids, and xylose (Sanjeewa et al., 2017). Due to the significant differences in fucoidan structure (fucose linkage, sulfate position, and sugar composition), they are very diverse (Fig. 5) and their structures depend on the species, season, location, and growth stage of the seaweed (Fletcher et al., 2017).
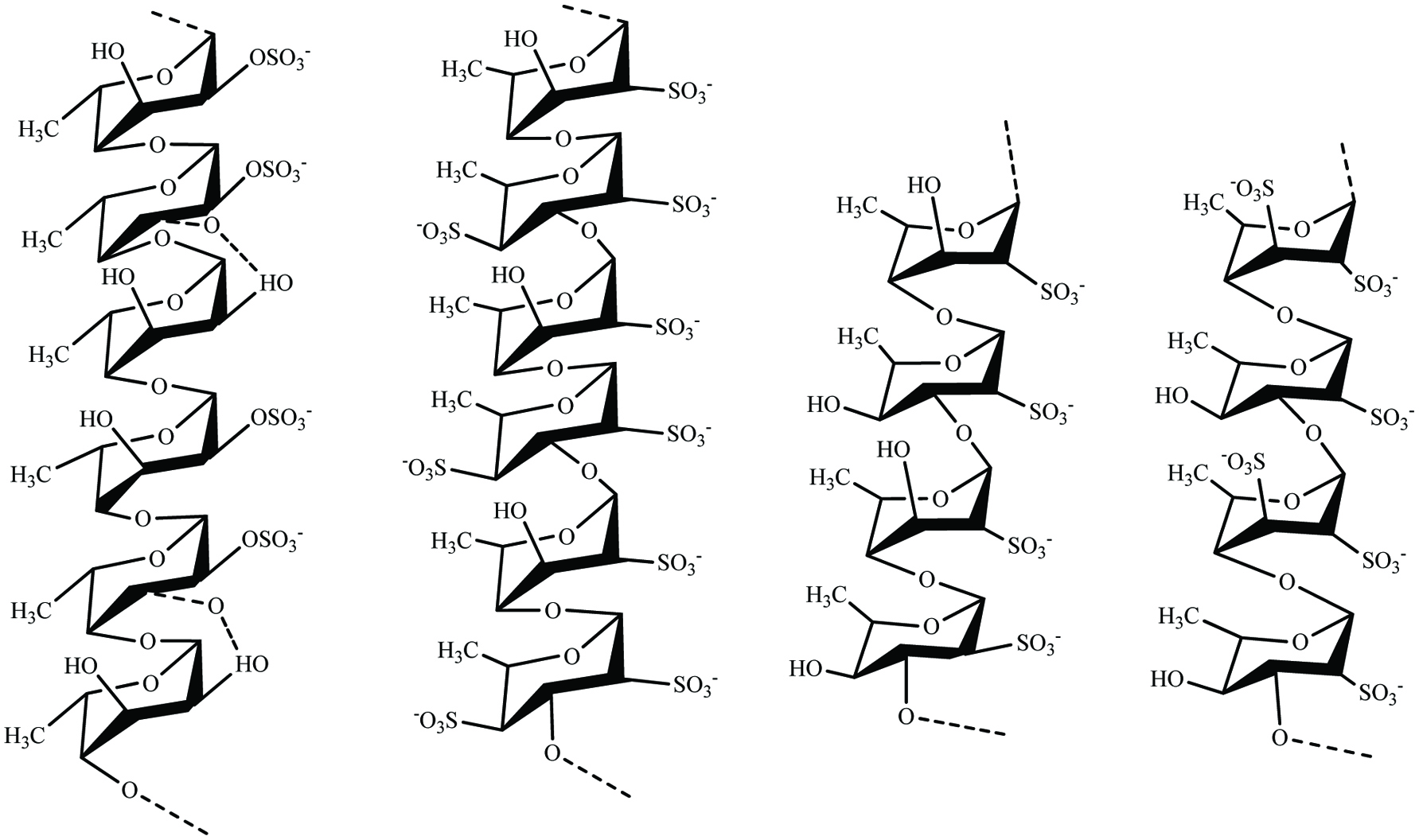 Click for large image | Figure 5. Structural motifs of fucoidans present in brown seaweeds. |
3.3.3. Laminaran
Laminaran is a major storage polysaccharide present in brown seaweeds (Fig. 6). Laminaran represents 35% of seaweed on a dry weight basis. The percentage of laminaran depends on seaweed species, harvesting time, and extraction method (Yin et al., 2014). Laminaran consists of glucose monomers joined together mainly by (1,3)-β-D-glucan backbone with β (1,6) glycosidic bonds. Laminarans may be separated into two groups as G and M chains, where M chains end with a mannitol as the terminal reducing end and glucose is attached to the end of the G chains. It has been reported that laminaran possesses interesting bioactive properties such as antioxidant, antitumor, anti-inflammatory, and anticoagulant activities (Sanjeewa et al., 2017).
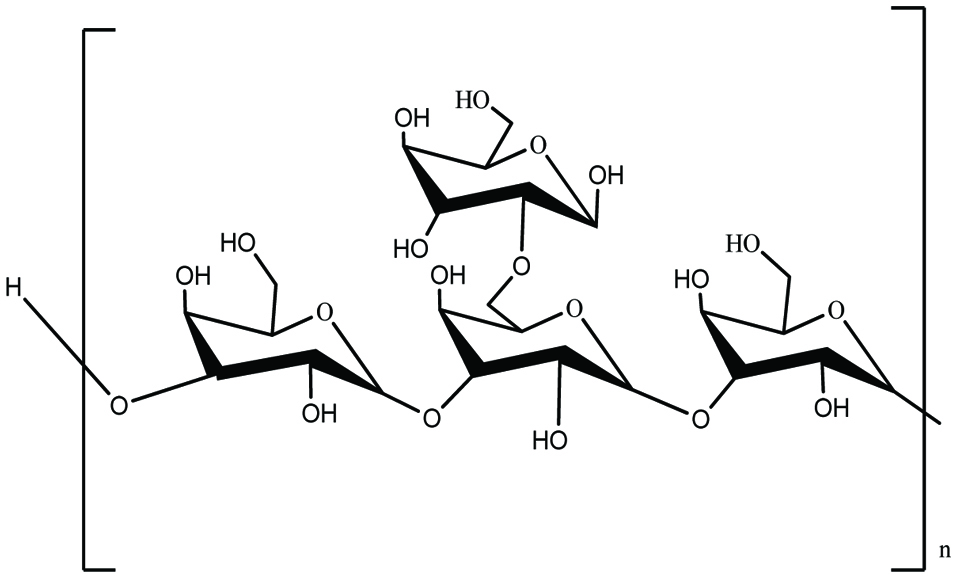 Click for large image | Figure 6. Chemical structure of laminarin presents in brown seaweeds. |
3.4. Sterols
Sterols are major components in the plasma membranes of all eukaryotes (20–30%) but are absent in prokaryotes. Cholesterol is the major sterol in animals, fungi, and yeast capable to produce ergosterol. Other than that phytosterols and fucosterol are major sterols present in higher plants and seaweeds, respectively (Mouritsen et al., 2017; Sanjeewa et al., 2016). Sterols in seaweeds serve as a substantial source of bioactive ingredients. A number of studies have reported that sterols such as ergosterol and fucosterol (Fig. 7) have the potential to be incorporated as an active ingredient in functional food products (Kim and Ta, 2011).
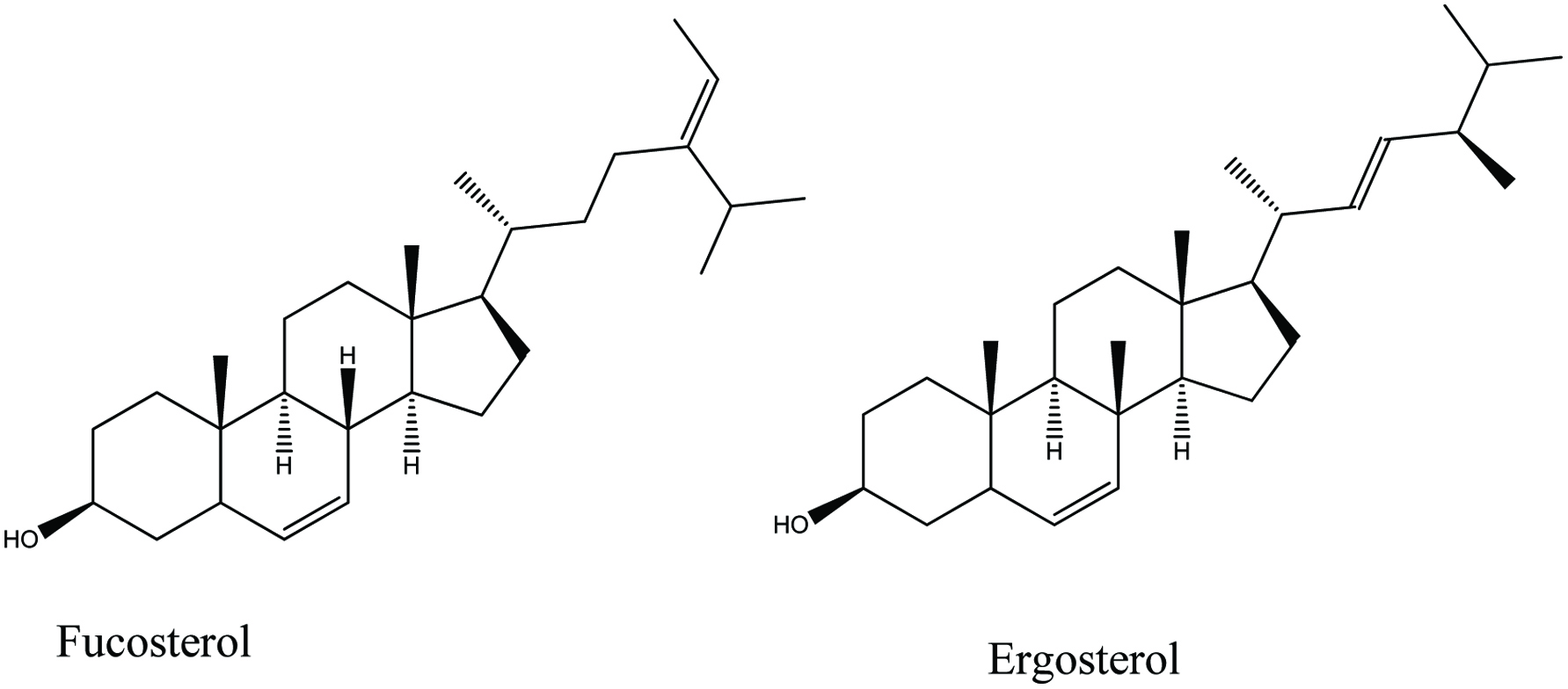 Click for large image | Figure 7. Chemical structure of two major sterol present in seaweeds. |
| 4. Bioactivities of brown seaweed polysaccharides | ▴Top |
4.1. Antioxidant ability
According to the previous reports, edible seaweeds grow along the shores of the Korean peninsula, H. fusiformis, L. japonica, and E. cava bio-synthesize secondary metabolites with strong antioxidant properties. Some antioxidant properties of edible brown seaweeds growing along the shores of the Korean peninsula are discussed here.
Siriwardhana et al. (2003) studied the reactive oxygen species (ROS) scavenging and lipid peroxidation inhibition activities of water and organic extracts separated from H. fusiformis respectively. According to the results, water extract of H. fusiformis had the highest DPPH radical scavenging activity among the other tested extracts (methanol and ethanol). Additionally, the authors reported, that the radical scavenging activities of water extract were stronger than those of the commercial antioxidant (BHA, BHT, and α-tocopherol) under tested conditions (Siriwardhana et al., 2003). Tong et al. (2012) evaluated the antioxidant properties of the extracts separated from three seaweeds including H. fusiforme, Capsosiphon fulvescens, and U. pinnatifida collected from Wando, Korea. Among the tested seaweed extracts, H. fusiforme had a better DPPH radical scavenging activity (90.12%, at a 0.32 mg/mL concentration), followed by U. pinnatifida. Additionally, the reducing power of extract of U. pinnatifida and H. fusiforme had better effects compared to Capsosiphon fulvescens (Tong et al., 2014). Lee and Kang (2015) evaluated DPPH radical scavenging effect of 70% ethanolic extract separated from H. fusiformis. According to the authors, H. fusiformis extract significantly scavenged DPPH radicals with IC50 of 9.64 ± 0.78 μg/mL (Lee and Kang, 2015). Wu et al. (2016) reported a novel lectin (65 kDa) isolated from H. fusiformis which has the potential to incorporate it as an active ingredient in functional food products. At 1.6 mg/mL concentration, the isolated lectin inhibited DPPH and hydroxyl radicals by 77.23 and 33.65%, respectively (Wu et al., 2016). However, compared to the DPPH inhibitory effect of 70% ethanolic extract (reported by Lee and Kang 2015), the inhibitory effect of lectin was very low. Kim (2010) evaluated the total phenolic contents and antioxidant activities of U. pinnatifida and Capsosiphon fulvescens under different drying conditions. The authors reported that radical scavenging activity and phlorotannins contents were comparatively high in the extracts separated from vacuum dry method compared to the hot air dry method. Thus, the antioxidant properties of seaweeds extracts depended on the processing method employed (Kim, 2010). Zha et al. (2016) evaluated the antioxidant properties of different molecular weight polysaccharides separated from L. japonica using superoxide radical scavenging properties of polysaccharides. Their results confirmed, that the low-molecular-weight polysaccharide fraction had a better superoxide radical scavenging effect than the high-molecular-weight fraction (Zha et al., 2016). Cui et al. (2016) reported that a polysaccharide [mole ratio of galactose, mannose and fucose (26.1:1.3:1) and 1,3- and 1-linked galactose] separated from L. japonica had remarkably high oxygen radical absorbance capacity (1247.22 μmol trolox equants g−1) and ABTS (−70% at 4mg/mL) scavenging activities (Cui et al., 2016). In another similar study, antioxidant effect of polysaccharide separated from L. japonica was documented by Yao et al. (2017). In this study, the authors compared the antioxidant capacity of polysaccharide extracted from different extraction methods such as water extraction and acidic extraction (citric acid, sulfuric acid, hydrochloric acid, and phosphoric acid). According to the results, polysaccharides from citric acid aided extraction method had good ABTS radical scavenging activity (IC50 = 1.06 mg/mL) and oxygen radical absorbance capacity (341.87 μmol trolox equants g−1) under the test conditions (Yao et al., 2017). In addition, antioxidant properties of pigment-free fucoidan separated from L. japonica was also reported (Zhao et al., 2018). The results revealed L. japonica fucoidan had the potential for developing as antioxidant materials. Under the test conditions, the isolated fucoidans had a better DPPH radical scavenging (IC50 = 4.64 mg/mL) and hydroxyl radical scavenging activity. A sulfated polysaccharide fraction (529 kDa) from S. fulvellum possessed a strong hydrogen peroxide scavenging activity compared to the commercial antioxidant such as BHA and α-tocopherol. Additionally, the separated polysaccharides inhibited the superoxide radicals, nitric oxide radicals, and DPPH in a dose-dependent manner (Choi et al., 2007a).
Other than the radical scavenging effects, some studies reported that extracts and pure compounds separated from edible brown seaweed render strong antioxidant properties under in vitro and in vivo conditions. Here we briefly introduce a few curious studies reporting in vitro and in vivo antioxidant properties of edible Korean brown seaweeds. Cha et al. (2012) studied the protective effect of E. cava phlorotannins on UV-induced ROS production in in vivo zebrafish model. The results confirmed that, pretreatment of eckol, eckstolonol, phloroglucinol, and triphlorethol-A (50 μM) protect zebrafish against UV-induced ROS generation, NO production, and cell death. In addition, the authors also studied the Inhibitory effect of phlorotannins on UV-B-induced hyperpigmentation in zebrafish embryos. Further they have reported that phlorotannins inhibited the UV-B induced melanin production in zebrafish embryos (Cha et al., 2012). Later, Wu et al. (2013) proclaimed that the water-soluble polysaccharides isolated from H. fusiformis had a protective effect on CCL4 induced liver damage in BALB/c mouse. In relation to the results, administration of separated polysaccharide at a rate of 200 mg/kg/day significantly reduced the CCl4 induced liver damage compared to the control group (Wu et al., 2013). Mohibbullah et al. (2015) compared the neuroprotective effect of 23 ethanolic extracts separated from seaweed against hypoxia/reoxygenation-induced oxidative stress in cultured hippocampal neurons. According to the authors, Gracilariopsis chorda (red alga) had the highest neuro-protection at 15 μg/mL, followed by U. pinnatifida. (Mohibbullah et al., 2015). Kim et al. (2015) also evaluated the protective effect of H. fusiforme extract on radiation-induced damage in splenocytes (C57BL/6 mouse). In this study the authors demonstrated pre-incubation of splenocytes with H. fusiforme (3.1–25 μg/mL) extract prior to gamma-ray irradiation (1.5 Gy) which had the potential to increase cell viability and proliferation by reducing elevated ROS levels in gamma irradiated splenocytes compared to the control group (Kim et al., 2015). In addition, fucoidan separated from E. cava was also found to possess protective effect against hydrogen peroxide-induced cytotoxicity in PC-12 and MC-IXC neuronal cells (Park et al., 2018). The isolated fucoidan restored mitochondrial damage by increasing the mitochondrial membrane potential (Δψm) and ATP levels as well as regulating mitochondrial-mediated proteins (p-AMPK and BAX). The aforementioned antioxidant properties are summarized in Table 1.
 Click to view | Table 1. Antioxidant properties reported from edible Korean brown seaweeds |
4.2. Anticancer activity
Induction of apoptosis in cancer cells is a major strategy for eliminating cancer cells. Tumor necrosis factor-related apoptosis-inducing (TNFAI) ligand is a transmembrane protein which capable of binding to its membrane-bound death receptors. Coupling of TNFAI and its receptor transmit an apoptotic signal via their intracellular death domains. Which can induce apoptosis in cancer cells (Kim et al., 2009). Kim et al. (2009) evaluated the anticancer effects of ethanolic extract separated from H. fusiforme on AGS human gastric adenocarcinoma cells. The results revealed that H. fusiforme extract (0–25 μg/mL) had the potential to induce apoptosis in cancer cells via up-regulating tumor necrosis factor-α (TNF-α) expression in cancer cells (Kim et al., 2009). Later, Kim and Choi (2010) reported ethyl alcohol fraction separated from H. fusiforme has a potential to inhibit matrix metalloproteinase (MMP) activity and regulates tight junction related protein expression in Hep3B human hepatocarcinoma cells. In this study, the authors demonstrated the regulation of MMP activity related genes (MMP-1, MMP-2, TIMP-1, and TIMP-2) and tumor invasiveness and metastasis-related genes (claudins, E-cadherin, IGF-1R, and TSP-1) are responsible for induce apoptosis in cancer cells (Kim and Choi, 2010). In addition, 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone isolated from 80% ethanolic extract of H. fusiforme was also found to possess promising anticancer properties against human AGS carcinoma cells and DR4 in cancer cells (Kim et al., 2012). The isolated compound dose-dependently increased (1–10 μg/mL) the death receptor-associated apoptosis mediators including Fas, Fas L, FADD, TRADD. In addition, Son et al. (2018) proclaimed that ethanol extract from H. fusiforme had the potential to reduce azoxymethane-induced colonic aberrant crypt foci (ACF) formation in F344 male rats (5 weeks, 150 g). ACF is a term used to describe the pre-neoplastic lesions in the colon which progress into colorectal cancers (Son et al., 2018). Kang et al. (2011) noted that ethyl alcohol extract of H. fusiforme had the potential to induce apoptosis in human leukemia U937 cells. Their results indicated that the down-regulated expression of IAP family members such as IAP-1, IAP-2, and XIAP, as well as inhibition of Bcl-2 proteins were responsible for the apoptotic cell death of U937 cells (Kang et al., 2011).
Ermakova et al. (2011) attempted to studied the anticancer properties of fucoidans isolated from three seaweed species grown in the Korean costs including S. hornery, E. cava, and Costaria costata. According to their results, fucoidans (100 μg/mL) inhibited the colony formation of skin melanoma cell (SK-MEL-28) and the colon cancer cells (DLD-1). Cervical carcinoma is a major cancer in women globally and threatens the health of females who are in the reproductive age (Zhai et al., 2014). Crude polysaccharides from L. Japonica were evaluated against two cervical carcinoma cells (HeLa and U14) to explore the potential of L. Japonica polysaccharides to develop as anticancer drugs (Zhai et al., 2014). According to the results, L. Japonica polysaccharide induced apoptosis in HeLa and U14 cells with IC50 values of 85.74 and 76.91 μg/mL, respectively. The nasopharyngeal carcinoma cells inhibitory effect of a polysaccharide separated from L. Japonica was recently documented by Zeng et al. (2017). In this study, the authors demonstrated that the administration of 12.5–50 mg/kg polysaccharide had significant inhibitory effect on tumor growth (nasopharyngeal cancer cell transplanted BALB/C nude mouse). In another recent study, fucoxanthin isolated from L. Japonica was evaluated for its anticancer effect on lung cancer cells including A549, H460, SPC-A1, and H1299. In this study, the up-regulated expression of p53, p21waf1/cip1, PUMA, and FAS and the down-regulated expression of Bcl-2 were found to be responsible for inducing apoptosis in tested cancer cells (Mei et al., 2017). In addition to fucoxanthin, a water-soluble polysaccharide separated from L. Japonica was found to possess anticancer properties in mouse (female kunming mouse) bearing H22 liver cancer cells as well as in vitro in H22 hepatoma cells. Moreover, they found that administration of polysaccharides induced the expression levels of interleukin-2 (IL-2), TNF-α and inhibited the expression of vascular endothelial growth factor (Zhu et al., 2016).
Synytsya et al. (2010) evaluated the anticancer properties of fucoidan isolated from U. pinnatifida using four cancer cell lines, including cervical cancer cells (HeLa), prostate cancer cells (PC-3), carcinomic human alveolar basal epithelial cells (A549), and hepatocellular carcinoma cells (HepG2). According to the results, fucoidans had considerable anticancer effect against all tested cancer cells at concentrations of 0–0.8 mg/mL. However, the authors reported that fucoidans from U. pinnatifida had slightly lower activity than the commercial fucoidan against PC-3 and A549 cancer cells (Synytsya et al., 2010). Other than the aforementioned studies, Cho et al. (1997) studied anti-mutagenic and anticancer properties of 9 seaweeds collected from Korean peninsula including ceylon moss, chlorella, sea lettuce, sea mustard, sea tangle, sporophyll of sea mustard, and purple laver. The authors assessed the anticancer effect of 20% methanolic extracts collected from the aforementioned seaweeds on Salmonella typhimurium TA100 cells and human colon cancer (HT-29) cells. The 20% methanol extracts had strong anti-mutagenic activity against aflatoxin B1 and N-methyl -N′-nitro-N-nitrosoguanidine in S. typhimurium TA100. Moreover, sea mustard extracts, and sea tangle had cytotoxic effects on HT-29 human colon carcinoma cells at the 0.2 mg/mL (Cho et al., 1997). Table 2. represent some anticancer properties reported in edible Korean seaweeds.
 Click to view | Table 2. Anticancer properties reported from edible Korean brown seaweeds |
4.3. Anti-inflammatory activity
Senevirathna et al. (2012) studied the nitric oxide (NO) inhibitory effect of L. japonica enzymatic digests on lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. According to the results, Celluclast- and AMG enzyme-assisted digests had more than 60% NO inhibition at concentrations between 50 and 500 μg/mL compared to the LPS-stimulated RAW 264.7 cells (Sevevirathne et al., 2012). Jung et al. (2013) attempted to evaluate the in vitro anti-inflammatory activities of fucosterol and seven phlorotannins (phloroglucinol, eckol, dieckol, phlorofucofuroeckol-A, dioxinodehydroeckol, 7-phloroeckol) collected from edible brown seaweed E. bicyclis. The results revealed that the seven phlorotannins isolated from E. bicyclis effectively inhibited the LPS-Induced NO production via down-regulating inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) protein expression in LPS-stimulated RAW 264.7 cells. Additionally, fucosterol inhibited tert-butylhydroperoxide-induced ROS production and down-regulated the expression of iNOS and COX-2 protein expressions (Jung et al., 2013). Gwon et al. (2013) investigated the anti-inflammatory effect of the hexane fraction of S. fulvellum in LPS-induced RAW 264.7 cells and phorbol 12-myristate 13-acetate (PMA)-induced mouse-ear edema. The results revealed that S. fulvellum extracts had the potential to inhibit the LPS-induced inflammatory responses in macrophages by reducing NF-κB transcriptional activity and NF-κB translocation into the nucleus by inhibiting phosphorylation of inhibitor κB-α. Additionally, down-regulated expression of Akt and mitogen-activated protein kinases (MAPKs) in the LPS-stimulated RAW 264.7 cells was also observed in this study. In addition, S. fulvellum extracts suppressed PMA-induced mouse-ear edema.
Cho et al. (2007) studied the anti-inflammatory effect of dichloromethane, ethanol, and boiling water extracts of the brown seaweeds U. pinnatifida and L. japonica using a BALB/c mouse model. The ethanol and dichloromethane (0.4 mg/ear) extracts of U. pinnatifida inhibited inflammatory symptoms induced by phorbol 12-myristate 13-acetate (PMA) in mouse ear edema by 95.3 and 65.5%, respectively (Cho et al., 2007). Furthermore, Khan et al. (2009) studied the protective effect of U. pinnatifida methanol extracts on PMA induced ear edema in BALB/c mouse model. According to the results, the methanol extract of the seaweed had considerable protective effect in ear edema induced by PMA, with an IC50 of 10.3 mg/mL. Nevertheless, compared to the study carried out by Cho et al. (2006) the ethanol extract of U. pinnatifida had more than 25 times activity against PMA induced ear edema in BALB/c mouse compared to the methanol extract of U. pinnatifida. In contrast to the organic solvent extraction methods, supercritical fluid extraction (SFE) method is a novel green extraction technique which has the potential to increase the bioactive compounds recovery, reduce the environmental pollution, and enhance the extraction rates (da Silva et al., 2016). Kang et al. (2016) attempted to evaluate the anti-inflammatory effects of U. pinnatifida using SFE in a BALB/c mouse (8–10 weeks old, 20–25 g body weight) model. In this study, the authors reported that the essential oil from U. pinnatifida had a protective effect against BALB/c mouse ear inflammation induced by PMA, with IC50 values of 87, 134, and 158 µg per ear for edema, erythema, and blood flow, respectively (Kang et al., 2016). Fucoidan isolated from U. pinnatifida also possessed anti-inflammatory properties against IL-1β-induced COX-2 expression in rabbit articular chondrocytes in a dose-dependent (0–100 μg) and time-dependent (0–48 h) manner (Phull et al., 2017).
Microglial cells (BV-2) are specialized macrophage-like immune cells present in the central nervous system, which are involved in the initiation of innate immune responses. Specifically, the activated cells are responsible for the production of pro-inflammatory mediators such as NO, prostaglandin E2 (PGE2), and TNF-α (Kang et al., 2013). Jung et al. (2007) reported that the methanolic extract obtained from H. fusiforme had a suppressive effect on TNF-α production in LPS-stimulated BV-2 cells. According to the results, pre-incubation of H. fusiforme extract together with BV-2 cells prior to LPS stimulation inhibited the LPS-induced TNF-α production by inhibiting the nuclear factor-κB (NF-κB) translocation (Jung et al., 2007). Kang et al. (2013) evaluated the anti-inflammatory effects of 5-hydroxy-3,6,7,8,3′4′-hexamethoxyflavone isolated from H. fusiforme. In this study the authors demonstrated that the isolated compound had the potential to suppress NF-κB associated proteins in LPS-induced BV-2 cells. Lee and Kang (2015) attempted to evaluate the anti-inflammatory effects of 70% ethanol extract collected from H. fusiformis using BV-2 microglial cells. Accordingly, pretreatment of H. fusiformis extract (0–100 μg/mL) inhibited the activation of BV-2 cells through down-regulation of inflammation-related gene expression (iNOS) and inhibiting pro-inflammatory cytokine production including TNF-α and IL-6.
4.4. Immunomodulation properties
Other than anti-inflammatory properties, some brown seaweed metabolites were found to possess immunomodulatory properties under in vitro and in vivo conditions. Park et al. (2017) attempted to study the immunomodulatory properties of extract separated from H. fusiforme using RAW 264.7 macrophage and using seven weeks old male C57BL mouse model (Park et al., 2017). According to the results, pre-incubation of H. fusiforme extract with RAW 264.7 cells increased the production of NO, PGE2, and TNF-α activity in murine macrophage cells at concentrations of 0–200 μg/mL without altering cell viability. Specifically, the authors found that the active compound in the extract was fucosterol which was responsible for the immunomodulatory properties of Khan the extract. Jeong et al. (2015) attempted to evaluate the immunomodulatory properties of polysaccharides separated from H. fusiforme using murine macrophage and splenocytes. According to the results, the polysaccharides exposed cells dose-dependently induced the production of NO, iNOS, and inflammation related cytokine production from macrophage cells (Jeong et al., 2015). In another study, Feng et al. (2015) reported the immunomodulatory effects of a homogeneous polysaccharide (molecular mass of 2.24 × 106 Da) separated from L. japonica. The isolated polysaccharide induced the activation of macrophage cells by up-regulating inflammation associated signal pathways such as NF-κB and MAPK (Fang et al., 2015). In addition, Zha et al. (2015) also reported a polysaccharide isolated from L. japonica (molecular mass of 2.89 × 106 Da) has a potential to act as immunostimulaory agent. Specifically, in this study the authors observed that the isolated polysaccharide had the potential to upregulate proteins associated with NF-κB and MAPKs signaling pathways in RAW 264.7 cells (Zha et al., 2015). Table 3. illustrates some anti-inflammatory and immune-modulatory properties reported from edible Korean brown seaweeds with their references.
 Click to view | Table 3. Anti-inflammatory and immune-modulatory properties reported from edible Korean brown seaweeds |
4.5. Antidiabetic and Anti-obesity activities
Kim and Lee (2012) evaluated the anti-obesity properties of fucoidan isolated from U. pinnatifida. The authors evaluated the obesity-specific therapeutic property of fucoidan in 3T3-L1 adipocytes. According to the results, exposure of adipocytes to fucoidan (1–100 μg/mL) inhibited the activation of key markers in adipocyte differentiation including CCAAR/enhancer-binding protein α, PPARγ, and adipocyte protein-2 proteins as well as activation of TNFα, MCP-1, and PAI-1 in 3T3-L1 cells (Kim and Lee, 2012). Kim et al. (2014) reported in vivo anti-obesity effect of fucoidan purchased from a commercial outlet (Haewon Biotech, Seoul, Korea). The authors studied the anti-obesity activity using a high fat diet (HFD)-induced obesity in C57BL/6 mouse model. Their results revealed that, HFD + 1% fucoidan and HFD + 2% fucoidan suppressed mRANA expression of aP2, PPARγ, and ACC in epididymal fat tissues compared to the only HFD administrated group (Kim et al., 2014). Oh et al. (2016) studied the anti-inflammatory and anti-diabetic effects of four brown seaweeds (U. Pinnatifida, L. Japonica, S. fulvellum, and H. fusiforme) in a high-fat diet-induced obese mouse. The authors reported that mouse fed L. Japonica with HFD had improved insulin resistance and reduced circulating pro-inflammatory cytokines despite being obese. In addition, supplementation of HFD with U. Pinnatifida, S. fulvellum, and H. fusiforme reduced inflammation in the absence of decreased adipose depot mass and improvement of insulin resistance male C57BL/6N mouse (Oh et al., 2016). Polysaccharide isolated from L. japonica was also found to possess antidiabetic properties in mouse with alloxan-induced diabetes (Jia et al., 2014). According to the authors, administration of L. japonica (200 mg/kg for 28 days) prevented body-weight loss, fasting blood glucose levels, and increased serum insulin levels in diabetic mouse. In addition, the tested polysaccharide decreased the total cholesterol, triglycerides, and low-density lipoprotein-cholesterol levels, and increased high-density lipoprotein-cholesterol levels in diabetic mouse.
4.6. Anti-microbial properties
Thompson and Dragar (2004) attempted to evaluate antiviral properties of a galactofucan isolated from U. pinnatifida against herpes simplex virus (HSV). In this study, the authors evaluated, antiviral activity of 32 clinical strains of HSV which included 14 strains of HSV-1 and 18 strains of HSV-2. Enterovirus 71 is a major etiological agent of hand, foot, and mouth disease as well as being is responsible for severe neurological symptoms developed in young children (Yue et al., 2017). Recently, Yue et al. (2017) assessed inhibitory effects of a polysaccharide purified from L. japonica against Enterovirus 71 (E71). In this study, the authors reported that the incubation of RD cells with isolated polysaccharides had the potential to reduce infection of E71 to human RD cells in a dose- and time-dependent manner. Cao et al. (2016) reported that polysaccharide separated from L. japonica has inhibitory effect on respiratory syncytial virus. The results revealed the polysaccharide extracted from L. japonica had a dose-dependent inhibitory effect on respiratory syncytial virus replication (Cao et al., 2016). In addition to antiviral activity, Liu et al. (2017) reported that depolymerized fucoidans separated from L. japonica had the potential to suppress the growth of Staphylococcus aureus and Escherichia coli at concentrations between 5 and 10 mg/mL (Liu et al., 2017). Additionally, methanolic extract separated from H. fusiformis were found to possess antibacterial properties against several pathogenic bacterial strains including Escherichia coli, S. aureus, Bacillus subtilis, Enterobacter aerogenes, and Shewanella sp (Ming-Jiang et al., 2016).
Antimicrobial properties of ethanolic extract separated from S. fulvellum were previously assessed by Yoon et al. (2010). The authors reported that, S. fulvellum extract inhibited the growth of several bacterial pathogens including, B. subtilis, Clostridium perfringens, Lactobacillus plantarum, S. aureus, Listeria monocytogenes, Saccharomyces cerevisae, and Candida tropicalis at a concentration of 4 mg/mL (agar diffusion assay) (Yoon et al., 2010).
4.7. Anti-coagulant properties
Heparin is a well-known commercial anticoagulant drug available in the market. However, oral administration of heparin and low-molecular-weight heparin is limited because the enzymes secreted by bacteria in the gut increase the degradation and desulfation before those anticoagulants enter to the blood circulation (Zhao et al., 2016). Zhao et al. (2016) evaluated the antithrombotic activity of orally administered low-molecular-weight fucoidan isolated from L. Japonica. According to the authors, the low-molecular-weight fucoidan (7.6 kDa) had better absorption and antithrombotic activity in male wistar rats than the medium-molecular-weight fucoidan (35 kDa). Specifically, oral administration of the low-molecular-weight fucoidan at 400–800 mg/kg prolonged the activated partial thromboplastin time (APTT) and thrombin time (TT) in male wistar rats. Anticoagulant properties of a sulfated polysaccharide isolated from E. cava and commercial fucoidan was earlier assessed by Wijesinghe et al. (2011). The results revealed the polysaccharide isolated from E. cava had strong anticoagulant activity compared to that of a commercial fucoidan in APTT, TT, and prothrombin time (PT) clotting assays in vitro (Wijesinghe et al., 2011).
| 5. Conclusions | ▴Top |
Edible brown seaweeds are abundant in the coastal areas of the Korean peninsula and its Islands such as Jeju. This review reports on the available edible Korean brown seaweeds, dealing with health promotion properties and compounds being isolated from Korean edible brown seaweeds. Most of the studies reported that, the isolated compounds and crude extracts isolated from these seaweeds exhibited promising bioactive properties such as antioxidant, anticancer, anti-inflammatory, immunomodulatory, anti-diabetic, anti-microbial, and anti-coagulant effects. Some comparative studies reported the secondary metabolites present in these edible seaweeds which are more active than the commercial drugs. However, most of the studies limited to cultured cells and/or with laboratory animals like zebrafish or mouse models. Lack of clinical trials and detail investigations with those extracts reduced the industrial level applications of this untapped resource. Therefore, continued research with those identified compounds is required to develop functional products such as cosmeceuticals, nutraceuticals, functional foods, and commercial drugs. In addition, dissemination of scientific results to general population is an important requirement to increase seaweed consumption all around the world. Future research work dealing with edible Korean seaweed bioactivities should be focus to explain the mode of action of isolated compounds under in vivo conditions. These approaches will be a useful approach to develop industrially useful products.
| References | ▴Top |