| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 13, March 2021, pages 20-31
Stilbenoids: chemistry, occurrence, bioavailability and health effects—a review
Won Young Oh, Yue Gao, Fereidoon Shahidi*
Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1B 3X9
*Corresponding author: Fereidoon Shahidi, Department of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, Canada A1B 3X9. Tel: +1 709 737 8552 E-mail: fshahidi@mun.ca
DOI: 10.31665/JFB.2021.13256
Received: August 1, 2020
Revised received & accepted: August 31, 2020
| Abstract | ▴Top |
Stilbenoids are synthesized by plants in response to external stimuli such as infection and UV-irradiation, thus known as phytoalexins. Stilbenoids include resveratrol, piceid, piceatannol, pterostilbene, astringin, and viniferin and have been of interest due to their myriad of health benefits, such as antioxidant, anti-inflammatory, anticancer, and anti-diabetic effects. Despite their numerous health effects, the bioavailability of stilbenoids is poor. However, the existing literature on this subject is fragmented and thus collective effort is needed to better understand their role before and after consumption. Therefore, this contribution provides an overview of the synthesis, occurrence, bioavailability and health benefit of stilbenoids.
Keywords: Stilbenoid; Occurrence; Bioavailability; Health effect; Resveratrol
| 1. Introduction | ▴Top |
Stilbenes are naturally occurring secondary metabolites, which have an important disease resistance mechanism and are known as phytoalexins (Langcake and Pryce, 1977; Kuć, 1995; Roupe et al., 2006a; Błaszczyk et al., 2019). Stilbenoids, named by Gorham in 1980, are hydroxylated derivatives of stilbenes (Gorham, 1980; Xiao et al., 2008). Stilbenoids consist of two benzene rings joined by an ethylene bridge and belong to one of the categories of phenolic compounds that share their synthesis pathway with flavonoids. So far, more than 1,000 stilbenoids have been identified (Shen et al, 2013) and these include resveratrol, piceid, piceatannol, pterostilbene, astringin, and viniferin. Grapes are the primary food source of stilbenoids (Adrian et al., 2000; Rimando et al., 2004; Viñas et al., 2011; Vrhovsek et al., 2012).
Stilbenoids have been of interest due to their antioxidant (Oh and Shahidi, 2017; Oh et al., 2019; Treml et al., 2019), anti-inflammatory (Leláková et al, 2019; Hošek et al, 2019), anticancer (Cuzick et al., 2015) cardioprotective (Zamora-Ros et al., 2012), anti-Alzheimer’s (Moussa et al., 2017), and anti-diabetic (Banaszewska et al., 2016) activities. In addition, resveratrol, for example, has a higher lipophilicity compared to other phenolic compounds, which may lead to its better cell permeability (Neves et al., 2013). Indeed, resveratrol shows better absorption compared to other phenolics (Walle et al., 2004). However, its bioavailability is relatively poor due to its extensive metabolism, thus no correlation exists between in vitro and in vivo results (Walle et al., 2004; Roupe et al., 2006b; Baur and Sinclair, 2006). Therefore, it is also important to understand their absorption and metabolism. This review provides details about the synthesis, occurrence, bioavailability and health benefit of stilbenoids.
| 2. Chemistry and biosynthesis | ▴Top |
Stilbenes are C6-C2-C6 compounds consisting of two bezene rings linked by an ethylene bridge (Figure 1). The double bond located between the two benzene rings can either assume E (trans) or Z (cis) configuration. Trans-configuration is the predominant form and it has better antioxidant and anticancer activities (Roupe et al., 2006a). Stilbenoid backbone contains one or more hydroxyl and can also have other substituents such as methyl, methoxy, prenyl, and/or sugar groups and possibly with different degrees of polymerization (dimer, trimer, etc.) (Shen et al., 2013). Stilbenoids include resveratrol, piceid, pterostilbene, piceatannol, astringin, pinosylvin, and rhapontigenin (Figure 2).
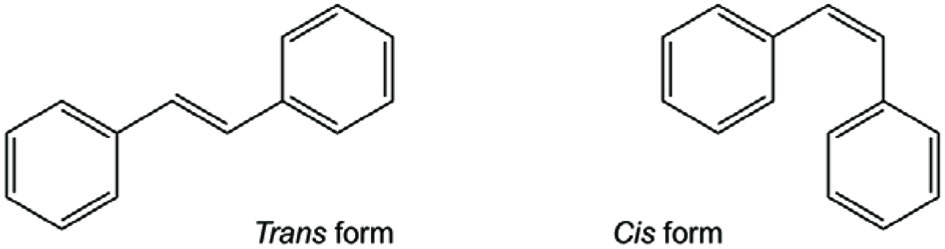 Click for large image | Figure 1. Basic skeleton of stilbenes. |
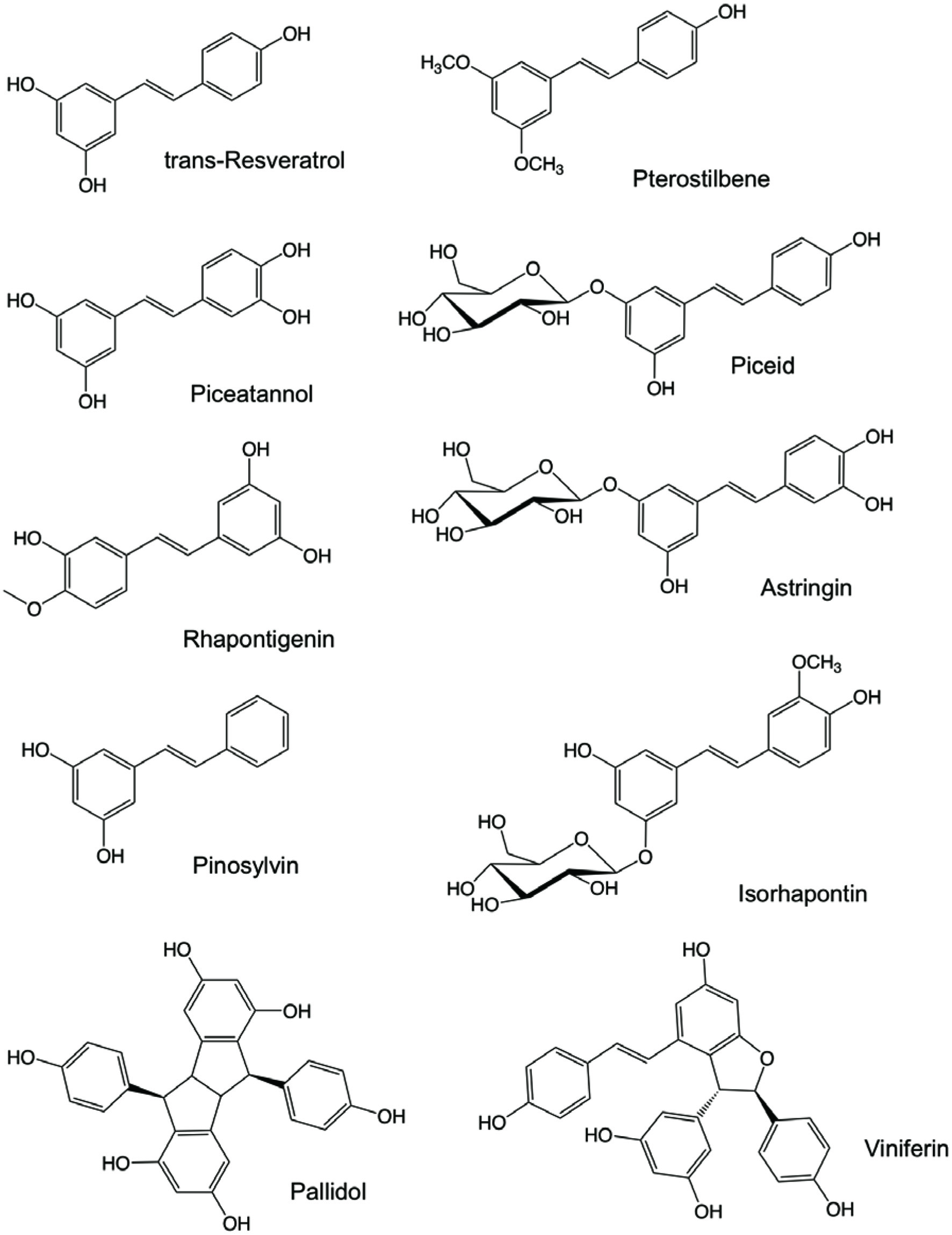 Click for large image | Figure 2. Chemical structures of selected stilbenoids. |
Stilbenoids are derived from phenylpropanoids pathway as shown in Figure 3. The synthesis of stilbenoids starts from phenylalanine by phenylalanine ammonia lyase (PAL) forming carbon-carbon double bond (Shahidi and Naczk, 1995). In some cases, tyrosine can replace phenylalanine in this pathway and the enzyme in this process is tyrosine ammonia lyase (TAL). The product, trans-cinnamic acid, is further processed by monooxygenase (cytochrome P450), yielding p-coumaric acid. CoA ligase turns p-coumaric acid into p-coumaryl CoA and this interacts with 3 molecules of malonyl CoA (Roupe et al., 2006a). The tetraketide formed undergoes aldol reaction by stilbene synthase and forms resveratrol, the best known stilbenoid (Dewick, 2002; Shen et al., 2013).
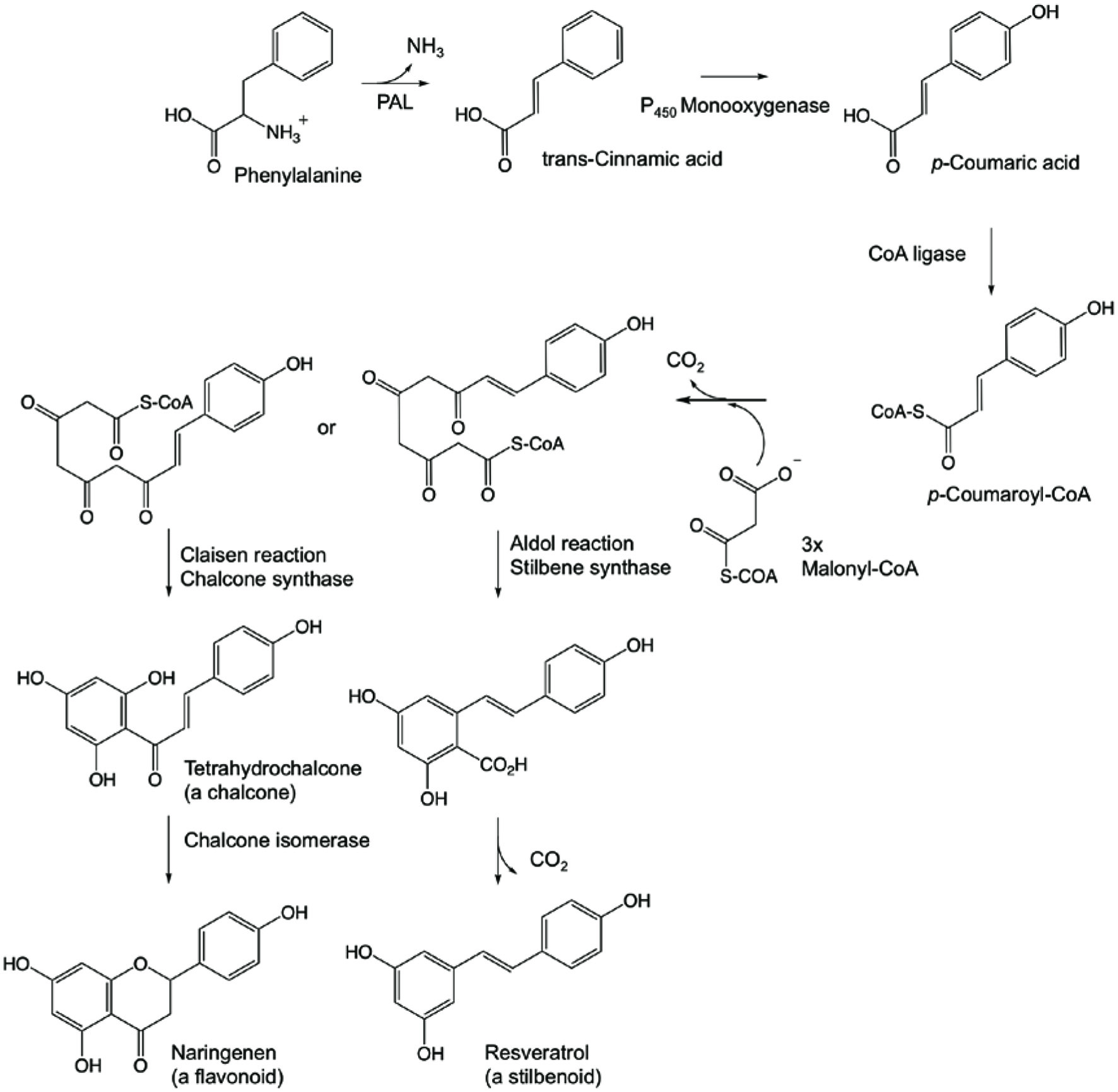 Click for large image | Figure 3. Synthetic pathway of stilbenoid and flavonoid from phenylalanine (Adapted from Shahidi and Naczk, 1995; Roupe et al., 2006a; Vermerris and Nicholson 2008; Dewick, 2002; Shen et al., 2013). PAL denotes phenylalanine ammonia lyase. |
| 3. Occurrence | ▴Top |
Stilbenes are produced in response to stress, injury, infection, or UV-irradiation (Kuć, 1995; Soleas et al., 1997). In addition, the concentration of stilbene is affected by many factors such as cultivar, genotype, soil type, climate, light exposure, pest management, agronomy, ripeness, storage condition, and postharvest treatment (Roupe et al., 2006a; Błaszczyk et al., 2019).
Table 1 shows representative food sources of stilbenoids. Among stilbenoids, resveratrol has been distributed more widely than others. The most abundant dietary source of resveratrol is grape, thus it can also be found in grape products such as wine and grape juice (Rimando et al., 2004; Vitrac et al., 2005; Romero-Pérez et al., 1999). Stilbenoids also found in peanut and peanut products. According to Sobolev and Cole (1999), roasted peanuts, peanut butter, and boiled peanuts contained trans-resveratrol at a level of 0.018–0.080, 0.148–0.504, 0.048 (liquid)-7.092 (kernel) μg/g, respectively. Ibern-Gómez et al. (2000) also reported similar results in peanut butter products (0.265–0.753 μg/g). In addition, they observed trans-piceid that ranged from 0.067 to 0.225 μg/g in peanut butter products. Hurst et al. (2008) studied the stilbenoids (trans-resveratrol and trans-piceid) contents of cocoa-containing and chocolate products that were correlated with the quantity of non-fat cocoa solid in the products. Rogab et al. (2006) found stilbenoids such as cis-resveratrol, trans-resveratrol, cis-piceid, and trans-piceid in tomato varieties. These compounds were also found in commercial seedless red table grape, with total stilbenoids content of at least 10-fold higher than that in tomato (Table 1; Rogab et al., 2006). Xie and Bolling (2014) reported that California almond (Prunus dulcis) contained piceid, trans-resveratrol, and pterostilbene, however, the level of trans-resveratrol, and pterostilbene were less than the detection limit. Jerkovic et al. (2005) observed trans-piceid (4.15–8.89 mg/kg), cis-piceid (2.30–6.01 mg/kg), and trans-resveratrol (0.22–1.00 mg/kg) in 9 hop varieties (Sterling, Wilhamette, Cascade, Nugget, Vanguard, Simcoe, Warrior, Tomahawk, and Amarillo).
 Click to view | Table 1. Source of stilbenoids in food. |
| 4. Health benefit | ▴Top |
Stilbenoids have received much attention in recent years due to their general health benefits as antioxidants and anti-inflammatory agents. In the past ten years, medical researchers have invested much effort into exploring the benefits of stilbenoids on conditions ranging from dry skin to Alzheimer’s disease.
4.1. Antioxidant effect
Stilbenoids and related compounds are produced by plants for antifungal and antibacterial purposes. Nineteen stilbenoid derivatives were tested in a cellular model system, some of which exhibited antioxidant properties while others were pro-oxidative (Treml et al., 2019). Among these, resveratrol was found to have direct scavenging effect on reactive oxygen species (ROS) (Akinwumi et al., 2018). However, this scavenging effect was relatively low compared to other antioxidants such as ascorbate and cysteine (Bradamante et al., 2004; Li et al., 2012).
On a cellular level as a gene regulator, stilbenoids follow multiple mechanisms of action. Stilbenoids-rich Vitis vinifera (grape) roots extract, which contained 7 identifiable naturally occurring stilbenoid derivatives, exhibited antioxidant properties via different mechanisms. First, they were able to stop hydrogen peroxide-induced DNA damage, possibly by up regulation of genes that lead to the activation of antioxidant enzymes which target hydrogen peroxide, such as superoxide dismutase (SOD), catalase and glutathione peroxidases. Second, they activate nuclear factor erythroid 2-related factor-2 (Nrf2) gene and its target genes encoding heme oxygenase-1 (HO-1) and γ-glutamylcysteine synthetase (γ-GCS). The up-regulation of these effector molecules directly decreases ROS. Grape root extract also increased in vitro paraoxonase 1 production, which is a high-density lipoprotein (HDL) associated liver protein that prevents low-density lipoprotein (LDL) oxidation (Esatbeyoglu et al., 2016).
Resveratrol is the principle stilbenoid derivative in red wine and is often purified and studied on its own. Its antioxidant effects are assessed in different ways. Resveratrol can bind to around 20 proteins with specific affinity (Britton et al., 2015), leading to the up-regulation of antioxidants and down-regulation of ROS. Among its targets, the activation of NAD+-dependent histone/protein deacetylase sirtuin 1 (SIRT1), the Nrf2 gene, and estrogen receptors are particularly important. Activation of SIRT1 leads to SIRT1-dependent up-regulation of antioxidant enzymes in human cells and in lab animals in vivo. Activation of the Nrf2 gene leads to Nrf2-dependent up-regulation of antioxidant enzymes, both in cells and in vivo. Binding of resveratrol to estrogen receptors in the cytoplasm leads to the activation of a signaling cascade that regulates the expression of many genes. The end result is both an increase in ROS detoxification and a reduction in ROS production from vascular NADPH oxidases, mitochondria, and uncoupled endothelial nitric oxide synthase (eNOS) (Xia et al., 2017).
4.2. Anti-inflammatory effect
Health benefits of stilbenoids are attributed to their antioxidant properties and potent anti-inflammatory effects. In vitro testing of 25 non-prenylated stilbenoids revealed that 5 of them had significant inhibitory effect against cyclooxigenase-1 (COX1) and cycclooxigenase 2 (COX2), which are enzymes that induce inflammation. Among these, trans-resveratrol showed a higher inhibitory effect against both COX1 and COX2 than ibuprofen, although it was more selective for COX1 than COX2. Pinostilbene showed the greatest selectivity for COX2, even greater than ibuprofen. This is desirable because long-term use of nonsteroidal anti-inflammatory drugs (NSAIDS), which are more selective for COX1, can cause gastrointestinal damage. When considering inhibitory effect against three inflammation enzymes, namely COX1, COX2, and 5-lipoxygenase (5-LOX), piceatannol and pinostilbene showed activities comparable to zileuton and ibuprofen, respectively. Most of the 25 tested stilbenoids showed inhibitory effect against lipopolysaccharide (LPS) stimulated activation of both nuclear factor (NF)-κB and activator protein (AP)-1 transcription factors. Among these, 5 showed comparable effect to prednisone. Reduced transcription of NF-κB and AP-1 gene attenuated the expression of tumor necrosis factor (TNF)-α, which is a powerful pro-inflammatory cytokine. Stilbenoids inhibit NF-ĸB/AP-1 activity though upstream inhibition of the phosphorylation of mitogen-activated protein kinase (MAPKs) (Leláková et al, 2019).
Prenylated stilbenoids show similar effects in all of the above-mentioned mechanisms (Hošek et al, 2019). Theoretically, because of the additional hydrophobic group, prenylated stilbenoids have higher bioavailability and higher distribution rate in the human body than non-prenylated stilbenoids (Araya-Cloutier et al, 2018).
In an experiment that tested 13 stilbenoids and derivatives, natural non-glycosylated stilbenoids were found to down regulate the PI3K/Akt pathway, which is a pathway involved in the regulation of inflammation. The three most potent compounds, piceatannol, pinosylvin, and pterostilbene, were tested in mice with carrageenan-induced paw inflammation. All three stilbenoids suppressed inflammatory edema and down regulated inflammatory mediators interleukin (IL) 6 and monocyte chemoattractant protein (MCP)-1 and their effects were comparable to commercial PI3K inhibitor LY294002 (Eräsalo et al., 2018).
4.3. Cardiovascular protection
Due to its antioxidant and anti-inflammatory effects, stilbenoids are tested for their health benefits in a wide range of diseases and conditions. It seems that with the amount of resveratrol level achieved in typical western diet, resveratrol level in the blood is not correlated with protection from all-cause mortality in community dwelling older adult (Semba et al., 2014). For resveratrol to exert any health benefit, supplementation is needed on top of regular diet.
One disease/condition of particular interest is cardiovascular disease (CVD). In a study that tested the effect of resveratrol in the primary prevention of CVD, 75 patients were divided into three groups of placebo, resveratrol-rich grape supplement, and conventional grape supplements without resveratrol. These patients were at risk of CVD, but did not yet have cardiovascular event. After a year of consumption, compared to the placebo and supplement without resveratrol, resveratrol-rich grape supplement significantly improved CVD biomarkers in the test group. Improvement in biomarkers such as high-sensitivity C-reactive protein, TNF-α, plasminogen activator inhibitor type 1, and IL-6/IL-10 as well as anti-inflammatory IL-10, signified improved inflammatory and fibrinolytic status in the test group, which is directly correlated to CVD risk. No adverse effect was observed in all patients (Tomé-Carneiro et al., 2012). An interesting study conducted in Spain interviewed 100 participants for their lifestyle, then took blood and urine samples for analysis. In this study, total urinary resveratrol metabolites (TRMs) was used as a biomarker for wine and dietary resveratrol consumption. From analysis of the blood and urine samples, a clear correlation was established that the test subjects with higher TRMs also had lower fasting glucose level, slower heart rate, and better blood lipid profile (lower triacylglycerol and higher high density lipoprotein (HDL)). All these parameters are closely related to CVD, and it can be inferred that resveratrol can decrease the risk of CVD (Zamora-Ros et al., 2012). Another study showed that a 4-week intake of grape pomace extract significantly increased serum resveratrol, and decreased trimethylamine N-oxide (TMAO), another risk factor of CVD (Annunziata et al., 2019).
In overweight patients, effects of resveratrol in improving biomarkers of CVD, as well as anti-inflammatory effects were less pronounced. In patients with schizophrenia, who were generally overweight or obese and had several metabolic disorders, a 4-week supplement of resveratrol (200 mg/day) did not bring changes to body weight, waist circumference, glucose, and total cholesterol. However, it is worth noting that the blood lipid level (triacylglycerol, HDL) worsened for the control group during the study, but did not change for the test group (Zortea et al., 2016). In another study involving overweight and slightly obese subjects, a 4-week resveratrol supplement (150 mg/day) did not result in differences in any of the fasting serum or plasma metabolic risk markers such as Apo A-I, Apo B100, HDL, LDL, triacylglycerol, glucose, and insulin. No difference in plasma markers of inflammation or endothelial function was observed (van der Made et al., 2015). In a smaller scale, but well designed, study participants with central obesity were fed high fat meals, which were thought to induce postprandial inflammation. Resveratrol/curcumin supplements were taken before consuming the high fat meal. After the meal, serum samples were taken and analyzed. The supplementation did not make any significant difference in circulating inflammatory markers (C-reactive protein, IL-6, IL-8, MCP-1), adhesion molecules, or whole blood NFκB1 and peroxisome proliferator activated receptor alpha (PPARA) gene expression (Vors et al., 2018). However, this study only tested acute administration of resveratrol/curcumin. Chronic resveratrol intake can potentially give a different result. In a study concerning patients with non-alcoholic fatty liver disease, a 12-week supplementation of resveratrol (500 mg/day) did not result in improvement of waist circumference, insulin resistance biomarkers, lipid profile or blood pressure. However, it improved alanine aminotransferase (ALT) and hepatic steatosis, which are indicators of liver health (Faghihzadeh et al., 2015). In patients with dyslipidemia, a 2-month supplementation of resveratrol (100 mg/day) significantly improved serum concentration of total cholesterol and trigacylglycerol, without significant improvements in HDL or LDL (Simental-Mendía et al., 2019). This study lasted longer than the other previously mentioned studies. Perhaps in overweight or obese patients, it takes a longer duration of supplementation for resveratrol (or other stilbenoids) to show effectiveness on improving CVD biomarkers.
4.4. Alzheimer’s disease
Stilbenoids can cross the blood brain barrier (BBB) due to their hydrophobic nature. Due to anti-inflammatory effect on organs outside the central nervous system (CNS), stilbenoids may have a similar effect once they cross the BBB. There is also a hypothesis that since mild stressors of caloric restriction (CR) can postpone and prevent diseases of aging in animal models, and because they do so through the activation of sirtuins, then resveratrol as a potent SIRT1 activator, can mimic the physiological effect of CR, thereby postponing the diseases of aging (Pasinetti et al., 2015). The above-mentioned hypothesis led to the testing of resveratrol in Alzheimer’s disease (AD).
A 52 weeks long study which supplemented AD patients with 2 grams of synthetic resveratrol daily showed interesting results regarding both the brain conditions and general health. Compared to the placebo group, the test group patients experienced significant weight loss. Nanomolar amounts of native resveratrol were observed in the cerebrospinal fluid (CSF), suggesting the crossing of BBB and binding to molecular targets. Compared to the placebo, resveratrol stabilized the progressive decline in CSF Aβ40 and plasma Aβ40 levels upon advancement of dementia. In individuals with baseline level of CSF Aβ42 less than 600 ng/mL, a confirmation criterion of AD, resveratrol also stabilized CSF Aβ42 level. In the one-year study period, resveratrol attenuated decline in the Alzheimer’s Disease Cooperative Study-Activity of Daily Living (ADCS-ADL) score. Because aging is a primary cause of cancer, and resveratrol has anti-aging effect, fewer cases of cancer were found in the resveratrol-treated group (1 vs 7 cancer in six participants in the placebo group). Notably, a high dose of resveratrol increased brain volume loss, yet tau protein level remained unaffected (brain volume loss is not due to neuronal loss). This volume loss could be due to the anti-inflammatory effect of resveratrol, which decreases CNS edema in AD brain. Consistent with this hypothesis, pro- and anti-inflammatory biomarkers were significantly better in the CSF and plasma of resveratrol-treated participants (Moussa et al., 2017). Similarly, another study which lasted 52 weeks used 500 mg resveratrol/day, similar but less pronounced results were observed. This suggests that the health benefits of resveratrol are manifested in a dose dependent manner. In this study, decline in CSF Aβ40 and plasma Aβ40 levels, which is a measure of dementia progression, was less in the resveratrol group. There was also a significant weight loss and increased brain volume loss in the treatment group (Turner et al., 2015).
A lower dose of resveratrol, mixed with other nutritional supplements, showed interesting effects on the brain function of healthy elderly individuals. In a 6-month study, healthy elderly participants were supplemented with a combination of 3,000 mg omega-3 PUFAs [1,500 mg docosahexaenoic acid (DHA) and 1,500 mg eicosapentaenoic acid (EPA)], 10 μg vitamin D3, 150 mg resveratrol and 8 g whey protein isolate. No improvement was observed in their cognitive function test scores. However, the treatment group performed better in the Stroop Color and Word test at the 3- and 6-months follow-up (Moran et al., 2018). Unfortunately, it was impossible to determine how much of that enhanced performance was due to resveratrol. It would be interesting to see if a combination of nutrients, in high doses, could postpone cognitive deterioration in AD patients.
Raloxifene, a stilbene derivative, works as a selective estrogen receptor modulator. It has beneficial effects in patients with ovarian cancer, but does not seem to have any beneficial effect in women with AD. A 52 weeks supplementation of 120 mg of raloxifene daily showed no difference in dementia rating, activities of daily living, behavior, or a global cognition composite score in the treatment group compared to the control group (Henderson et al., 2015).
4.5. Breast cancer
Tamoxifen, a stilbene derivative, functions as an estrogen receptor modulator in vivo, and has long-term preventative effect against breast cancer. In a study that tested the long-term effect of tamoxifen, participants who were at high risk of breast cancer received either tamoxifen (20 mg daily) or placebo for 5 years. After this 5-year treatment, participants were followed for 10 years. During this 10-year follow-up period, there was significantly less breast cancer developed in the treatment group. However, the reduction was mainly in invasive estrogen receptor-positive breast cancer and ductal carcinoma in situ, not so much in invasive estrogen receptor-negative breast cancer (Cuzick et al., 2015). In women who are diagnosed with estrogen receptor (ER)-positive early breast cancer, treatment with tamoxifen for 5 years as an adjuvant substantially reduces the breast cancer mortality rate throughout the first 15 years after diagnosis. If tamoxifen treatment duration was increased to 10 years rather than stopping at 5 years, the mortality rate and recurrence rate were reduced even further, particularly after year 10. The protective effect of tamoxifen was only seen in ER-positive cases, and not in ER-negative type of breast cancer (Davies et al., 2013). This study suggests that low-dose tamoxifen would not affect quality of life (Serrano et al., 2018). However, since tamoxifen is extensively metabolized by liver enzyme CYP2D6, it may cause altered availability of other drugs, and its own availability maybe different in patients who have substantially different CYP2D6 gene profile. Using tamoxifen metabolite trans-endoxifen, instead of tamoxifen, can bypass the CYP2D6 metabolism. In endocrine-refractory metastatic breast cancer patients, trans-endoxifen provided substantial drug exposure that was unaffected by CYP2D6 metabolism. It also showed acceptable toxicity, and promising antitumor activity (Goetz et al., 2017). Tamoxifen is an effective adjuvant therapy, but it may not be the optimal adjuvant therapy. In postmenopausal women with hormone-receptor-positive early breast cancer who are receiving ovarian suppression, exemestane as an adjuvant seemed to be more effective than tamoxifen (Pagani et al., 2014).
4.6. Type II diabetes mellitus
Stilbenoids can improve metabolic syndrome, which leads to protective effect against CVD. Their effect on insulin sensitivity is not only a benefit to metabolic syndrome, but also a protective factor against type II diabetes. A study involving patients with polycystic ovary syndrome found that compared to the control, supplementation with 1,500 mg resveratrol daily for 3 month decreased serum fasting insulin level by 31.8%, and increased Insulin Sensitivity Index by 66.3% (Banaszewska et al., 2016). Apart from resveratrol, piceatannol from passion fruit extract is also a stilbenoid of interest. Supplementation of piceatannol (20 mg daily) for 8 weeks significantly decreased fasting serum insulin level, improved insulin sensitivity, and also improved blood pressure and heart rate in overweight men. These effects seemed to be less pronounced in overweight female participants, possibly due to the difference in body composition between overweight male and female participants (Kitada et al., 2017). Piceatannol has also been shown to have protective effect against diabetes in mice models. Although it did not prevent high calorie induced body weight gain or visceral fat gain, it reduced fasting glucose level in obese mice (Maruki-Uchida et al., 2018). Another study in mice model showed that piceatannol decreased body weight and prevented obesity in a dose dependent manner compared to the control. Piceatannol also improved lipid profile (total cholesterol, LDL, HDL), decreased fasting glucose level, liver weight, spleen weight, as well as perigonadal and retroperitoneal fat. Fatty acid synthesis of liver was reduced, therefore decreasing adipose accumulation in the liver. Piceatannol also significantly altered mice gut microbiota composition compared to the control (Tung et al., 2016).
4.7. Dry skin
In vitro studies have shown that passion fruit extract rich in piceatannol can down regulate ROS generation in UVB irradiated human keratinocytes (Maruki-Uchida et al., 2018). It also promotes collagen synthesis and inhibits melanin synthesis in human melanoma and fibroblast cells (Matsui et al., 2010). In women with dry skin, oral supplementation of 5 mg piceatannol daily for 8 weeks significantly increased skin moisture content at week 4 and week 8 compared to the placebo. Transepidermal water loss was also reduced over time. In the end-trial questionnaire, the treatment group also reported a significant reduction in perspiration and fatigue (Maruki-Uchida et al., 2018).
| 5. Bioavailability | ▴Top |
5.1. Resveratrol
Despite the numerous health benefits of resveratrol, concerns have been expressed due to its bioavailability. Resveratrol was investigated for its absorption, bioavailability, and metabolism in humans; 14C-resveratrol was administered orally and intravenously (Walle et al., 2004). Although the highest level of resveratrol and its metabolites in plasma was around 491 ng/mL, unchanged resveratrol was present at less than 5 ng/mL. A rapid decrease of intravenous administration of labeled resveratrol (0.2 mg) was observed due to the distribution, after that it continued to fall during 72-h study period. The recoveries of resveratrol administered orally in the urine and feces were 70.5 and 12.7%, respectively, whereas 64.1 and 10.4% were recovered in the urine and feces, respectively, in the case of intravenous administration. The authors also found five major metabolites using LC/MS and these were two isomers of resveratrol monoglucuronide, dihydroresveratrol monoglucuronide, resveratrol monosulfate, and dihydroresveratrol sulfate. However, only trace amounts of unchanged resveratrol were identified. They concluded that although the rapid metabolism led to low bioavailability of resveratrol, the metabolites and accumulated resveratrol in epithelial cells and aerodigestive tract might have health benefits (Walle et al., 2004). In addition, resveratrol has extremely low water solubility (less than 0.05 mg/mL), which may be another reason for its low bioavailability (Bertacche et al., 2006; Das et al., 2008). There have been many attempts to increase the bioavailability of resveratrol such as increasing its dose (Boocock et al., 2007; Sergides et al., 2016), using cyclodextrin (Das et al., 2008; Silva et al., 2014), solid lipid nanoparticles (Neves et al., 2013), encapsulation (Peñalva et al., 2018), and synthesis of resveratrol derivatives (Sale et al., 2004; Lin and Ho, 2009; Walle, 2011).
5.2. Piceatannol
Piceatannol is a naturally occurring stilbenoid, but is also a metabolite of resveratrol, found in Athymin mice (Niles et al., 2006). The metabolites of piceatannol are piceatannol-diglucuronide, piceatannol monoglucuronide, O-methyl piceatannol monoglucuronide, O-methyl piceatannol monosulfate, isorhapontigenin, and rhapontigenin in rats after oral administration (Setoguchi et al., 2014). Setoguchi et al. (2014) reported that monoglucuronide metabolite was predominant among the above-mentioned metabolites. They also found that the maximum concentration (Cmax) of piceatannol was 3.3 μmol/L at 90 μmol/kg, 7.5 μmol/L at 180 μmol/kg, and 8.1 μmol/L at 360 μmol/kg dose and it reached Cmax 15 min after the administration. In addition, the area under the plasma concentration curve (AUC) of piceatannol and its metabolites was increased in a dose-dependent manner. Roupe et al. (2006b) studied the pharmacokinetic characteristics of rhapontigenin, piceatannol and pinosylvin in Sprague-Dawley rats after intravenously administering a single dose of 10 mg/kg. All of test compounds were glucuronidated and AUC of piceatannol, pinosylvin, and rhapontigenin were 8.48 ± 2.48, 5.23 ± 1.20, 8.39 ± 0.10 μg h/mL, respectively. The total plasma clearance of piceatannol, pinosylvin, and rhapontigenin was 2.130 ± 0.920, 1.840 ± 0.435, and 1.180 ± 0.035 L/h/kg, respectively. More information on the bioavailability of piceatannol and its role in metabolic disease is available elsewhere (Kershaw and Kim, 2017). Lin et al. (2010) studied a pre-clinical pharmacokinetics of piceatannol analog (3,5,3′,4′-tetramethoxystilbene) in Sprague-Dawley rats using 2-hydroxypropyl-β-cyclodextrin as a dosing vehicle. They reported that the half-life, clearance, and oral bioavailability of piceatannol analog were 313 ± 20 min, 33.1 ± 3.9 mL/min/kg, and 50.7 ± 15.0%, respectively.
5.3. Pterostilbene
Pharmacokinetic study of pterostilbene, a 3,5-dimethyl derivative of resveratrol, was carried out by Lin et al. (2009) in rats. The pterostilbene was administrated intravenously at a dose of 5.0 mg/kg. They reported that the half-life, clearance, and oral bioavailability of pterostilbene were 96.6 ± 23.7 min and 37.0 ± 2.5 mL/min/kg, 12.5 ± 4.7%, respectively, which was better pharmacokinetic characteristics than resveratrol. Kapetanovic et al. (2011) also conducted pharmacokinetics of resveratrol and pterostilbene in rats which were administrated orally via gavage for 14 consecutive days (50 or 150 mg/kg/day for resveratrol; 56 or 168 mg/kg/day for pterostilbene). They also prepared two more groups which were administrated once intravenously (10 mg/kg resveratrol; 11.2 mg/kg pterostilbene). The bioavailability of resveratrol and pterostilbene were 20 and 80%, respectively. In addition, the plasma level of pterostilbene and its sulfated metabolites was much higher than that of resveratrol and its sulfated metabolites. On the other hand, the plasma level of resveratrol glucuronide was greater than that of pterostilbene glucuronide, however, the discrepancy of glucuronidated metabolites was smaller compared to the sulfated ones. The authors concluded that pterostilbene had a better pharmacokinetics than resveratrol.
| 6. Conclusion | ▴Top |
Stilbenoids show various beneficial effects such as antioxidant, anti-inflammatory, cardioprotective, neuroprotective, anticancer, and anti-diabetes effects. Among these, antioxidant and anti-inflammatory activity of stilbenoids most probably lead to other health promotion and disease prevention effects. However, the results for stilbenoids, especially resveratrol may not necessarily correlate between in vitro and in vivo due to their extensive metabolism. Therefore, in-depth bioavailability study of stilbenoids is needed. Moreover, there have been many attempts to improve poor bioavailability of resveratrol via substitution (methylation, esterification, etc), using different techniques (cyclodextrin, solid lipid nanoparticles, encapsulation). More studies are needed to evaluate the effect of these techniques in vivo. Except for resveratrol, limited information on health effects and bioavailability of stilbenoids is available. Further research is necessary to assess their bioactivity and pharmacokinetic properties.
| References | ▴Top |