| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 11, September 2020, pages 13-30
Composition, polyphenol bioavailability, and health benefits of aronia berry: a review
Erica S. Kinga, Bradley W. Bollinga, *
aDepartment of Food Science, University of Wisconsin-Madison, 1605 Linden Dr., Madison, WI 53706, USA
*Corresponding author: Bradley W Bolling, Department of Food Science, University of Wisconsin-Madison, 1605 Linden Dr. Madison, WI 53706, USA. Tel: 608-890-0212; E-mail: bwbolling@wisc.edu
DOI: 10.31665/JFB.2020.11235
Received: July 4, 2020
Revised received & accepted: September 11, 2020
| Abstract | ▴Top |
Aronia berries (Aronia melanocarpa and Aronia mitschurinii) are underutilized functional food, rich in bioactives. Aronia berries have abundant levels of anthocyanins, proanthocyanidins, flavonols, and phenolic acids that may reduce the risk of non-communicable diseases such as diabetes, metabolic syndrome, and neurological disease. Aronia polyphenols are bioavailable, and the majority are transformed into low molecular-weight phenolics. The impact of biotransformation on aronia polyphenols health effects is not fully understood. The objective of this review is to analyze aronia berry composition, including polyphenols nutrients. Additionally, this review summarizes recent preclinical and clinical studies on the polyphenol bioavailability and health benefits upon aronia berry consumption to better understand its potential as a functional food.
Keywords: Aronia berry; Anthocyanin; Chronic disease; Composition; Polyphenol
| 1. Introduction | ▴Top |
Aronia Medik. (chokeberries), are deciduous shrubs and a member of the Rosaceae family (Sidor and Gramza-Michałowska, 2019; Mahoney et al., 2019). Aronia berry color ranges from red, purple, to black, depending on the species (Sidor and Gramza-Michałowska, 2019). The four main Aronia species include A. arbutifolia (L.), A. melanocarpa (Michx.), A. prunifolia (Marshall), and A. mitschurinii (Mahoney et al., 2019; Kulling and Rawel, 2008). A. mitschurinii is the primary species used for commercial aronia berry production in North America (Brand et al., 2017; Mahoney et al., 2019). The ‘Nero’ and ‘Viking’ cultivars of A. mitschurinii are crosses between A. melanocarpa and Sorbus aucuparia L. and grow from 3 to 6 feet tall (Mahoney et al., 2019; Kulling and Rawel, 2008). From May to June, white flowers grow on the shrubs, and by late August and September, the berries are fully matured, with a diameter between 6.1 to 17.8 mm (Kulling and Rawel, 2008).
Aronia berry is consumed as whole berries, but most of the crop is processed to juice, juice concentrates, extracts, fruit powders, jams, or fermented products. Aronia berry is rich in polyphenols, which may contribute to its health benefits. Aronia berries contain a mixture of polyphenolic components and have abundant anthocyanins and proanthocyanidins (Taheri et al., 2013). Although the in vivo bioactivity and bioavailability of polyphenols are not fully understood, the antioxidant and anti-inflammatory actions of polyphenols may decrease the risk of cardiovascular disease, metabolic syndrome, inflammation, and neurodegenerative disease (Jakobek and Seruga, 2012; Bhaswant et al., 2017). The mechanisms by which anthocyanins, proanthocyanidins, and other polyphenols found in aronia berries are an active reseach area.
Aronia berry remains an underutilized functional food. Prior reviews have addressed aronia berry composition (Sidor and Gramza-Michałowska, 2019), polyphenol bioavailability (Denev, Kratchanov, Ciz, Lojek, and Kratchanova, 2012), and health benefits (Sidor and Gramza-Michałowska, 2019). Additional reports on the composition, polyphenol bioavailability, and bioactive mechanisms have been published since these publications. Therefore, this paper aims to provide an updated and expanded review of the current scientific literature on aronia berry composition, the pharmacokinetics of the polyphenols, and its potential for improving health. We expect that characterizing the progress and knowledge gaps in these areas will accelerate research, development, and aronia berry utilization.
| 2. Methods | ▴Top |
Publications were identified through Medline, Elsevier, Google Scholar, and Pubmed databases using keywords such as aronia, antioxidants, anthocyanins, bioavailability, chokeberry, proanthocyanidins, cancer, cardiovascular disease, diabetes, functional foods, and polyphenols. Studies were limited from 2010 until 2020 and from 2015 for preclinical and human intervention studies. Compositional data were compiled for black aronia berry (A. melanocarpa and A. mitschurinii).
| 3. Aronia composition | ▴Top |
The functional components in aronia berry include nutrients, polyphenols, fiber, and sorbitol. Other components such as organic acids, protein, and lipids contribute to fruit quality and stability. The abundance and distribution of these components vary significantly among the studies reviewed in this paper. Variability may arise from aronia genetic variability, environment (location, humidity, temperature, rain, fertilizers, and infections), harvest time, and other factors (Veberic et al., 2015). Furthermore, analysis may further introduce variability from extraction methods (e.g. selection of solvent, berry particle sizes, solid-solvent ratio, time, and temperature) or analytical approach (e.g. HPLC vs. gas chromatography, specific or non-specific methods) (Denev et al., 2018).
3.1. Carbohydrates
Aronia berry carbohydrates are primarily sugars and fiber (Tables 1 and 2). Fresh aronia berry contains 15 to 20.9 g/100 g fwb of carbohydrates (Sidor and Gramza-Michałowska, 2019). The sugars in aronia berry and juice are mainly fructose and sorbitol with lower amounts of glucose and sucrose, ranging from 6.2 to 20.9 g/100 g fwb or 8.9 to 19.6 g/100 mL. Sorbitol is abundant in aronia berry with 4.36–12.99 g/100 g fwb of the whole berry. As a sugar alcohol, sorbitol contains about 2.6 calories per gram and has diuretic, laxative, and cathartic properties (US Food and Drug Administration, 2020; Featherstone, 2015). A majority of the berry sugars are extracted into juice, whereas its fiber is mainly distributed in the pomace. The pomace contains 57.8 to 71.5 g/100 g dwb of total dietary fiber and insoluble fiber at 43.8 to 61.7 g/100 g dwb (Schmid et al., 2020). The berry pomace fibers include cellulose (34 g/100 g dwb), hemicellulose (32 g/100 g dwb), lignin (22.7 g/100 g dwb), and pectin (7.52 g/100 g dwb). Although these fibers’ structures and solubility vary, increased fiber reduces cardiovascular disease risk, aids in glycemic control, and helps maintain a healthy weight (Schmid et al., 2020; Slavin, 2013).
 Click to view | Table 1. Aronia berry and juice sugar content and profile |
 Click to view | Table 2. Aronia berry pomace fiber content |
3.2. Protein and amino acids
Similar to other berries, the protein and amino acid content of aronia berry is relatively low (Table 3), 700 mg/100 g fwb and 4,900 to 24,000 mg/100 dwb. Most of the protein and amino acids are distributed in the pomace (Sidor and Gramza-Michałowska, 2019). Aronia berry contains both essential and nonessential amino acids. Threonine (0.033 to 0.39 mg/100 g fwb berry) is the most abundant among the amino acids, followed by serine (0.023 to 0.39 mg/100 g fwb berry).
 Click to view | Table 3. Protein content and amino acid profile of aronia berry and pomace |
3.3. Lipids
Aronia berry has a low lipid content, with a total fat of 0.14 % in a fresh berry (Table 4). A significant amount of the lipids are in the seed oil, represented mostly by sterols and phospholipids (Sidor and Gramza-Michałowska, 2019). The remaining lipids are mainly in the pomace, primarily polyunsaturated fatty acids, which is 90.49% of total fatty acids, whereas saturated fatty acid is 9.51%. Among the different types of fatty acids, the linoleic (C18:2) and oleic acids (C18:1) are abundant, at 43.43% and 16.38% of fatty acids, respectively (Sidor and Gramza-Michałowska, 2019). While the lipid content of aronia berry is low, processing strategies to recover lipids from aronia seeds and pomace could be used to develop new sources of seed oils with high proportions of unsaturated fatty acids.
 Click to view | Table 4. Lipid content and profile of aronia berry fruit and pomace. |
3.4. Vitamins and minerals
Aronia berry contains vitamin A, vitamin E, and vitamin C (Table 5). Total carotenoids (including α-, β-, and ζ-carotenes) can be up to 97.8 µg/L of aronia berry juice (Oprea et al., 2014; Sidor and Gramza-Michałowska, 2019). However, vitamin C is the most abundant micronutrient, with 7.25 to 98.75 mg/100 g fwb (Catană et al., 2017). Aronia berries also contain a variety of minerals. The ash content of aronia berry is 0.37 to 0.49 g/100 g fwb, providing major minerals (calcium, magnesium, phosphorus, potassium) and trace minerals (iron, copper, iodine, zinc, and selenium) (MedlinePlus, 2019). Lead and other heavy metals have been reported in aronia berry, but these levels are below thresholds where toxicity is a concern (Juranović Cindrić et al., 2017).
 Click to view | Table 5. Micronutrient, nitrate/nitrite, and mineral content of aronia berry fruit |
3.5. Organic acids
The content of organic acids in aronia leads to its sour flavor (Famiani et al., 2015). The titratable acidity is in range with other berries at 0.85 to 1.22% (Bolling et al., 2015). At least eight organic acids are present in aronia berry, including quinic, malic, ascorbic, shikimic, citric, oxalic, succinic, and isocitric acids (Table 6). Fumaric and tartaric acids were also reported in juice. Quinic acid is the most abundant organic acid in aronia berry (293 to 591 mg/100 g fwb), followed by malic acid (308 to 350 mg/100 g fwb), but others have reported that malic acid is the primary organic acid (Denev et al., 2018; Kulling and Rawel, 2008).
 Click to view | Table 6. Organic acid content of aronia berry and juice |
3.6. Polyphenols
Aronia berry has a significantly higher polyphenol and antioxidant content than most fruits and vegetables (Nour et al., 2015; Jakobek and Seruga, 2012; Pérez-Jiménez, 2010). Aronia berry total polyphenols range from 1.0 to 3.6 g/100 g fwb (Denev et al., 2018; Nour et al., 2015). The primary polyphenols in aronia berries include anthocyanins, proanthocyanidins, flavonols, and phenolic acids. These polyphenols contribute to the health benefits as well as the astringent and bitter flavor associated with aronia berry.
3.6.1. Anthocyanins
Within black aronia berry, anthocyanins are the most abundant polyphenol and pigment (Wathon et al., 2019; Sidor and Gramza-Michałowska, 2019). The structure of anthocyanins determines the pigmentation of the fruit by the type of aglycon base or flavylium ring, sugar, and acylation (Sidor and Gramza-Michałowska, 2019). Anthocyanins also reduce photooxidation and limit CO2 assimilation in plant tissue. Thus, most of the anthocyanins are located on the skin’s external layer in the pomace (Veberic et al., 2015). There are six major anthocyanin aglycons in berries: cyanidin, delphinidin, petunidin, peonidin, pelargonidin, and malvidin, which typically contain a sugar moiety (Bueno et al., 2012). In aronia berry, cyanidin glycosides account for 90 to 98.7% of the total anthocyanin content (Veberic et al., 2015; Denev et al., 2018). The major cyanidin glycosides are include its 3-galactoside (126 to 990 mg/100 g fwb), 3-glucoside (1.7 to 21.5 mg/100 g fwb), 3-arabinoside (52 to 399 mg/100 g ), 3-xyloside (2.7 to 81.2 mg/100 g fwb), 3,5-hexoside (epi)catechin (14.3 mg/100 g dwb), 3-pentoxide-(epi)catechin (7.26 mg/100 g dwb), 3-hexoside-(epi)cat-(epi)cat (13.6 mg/100 g dwb) (Table 7). On a fresh weight basis, aronia berries are among the richest dietary sources of anthocyanins (Denev et al., 2018; Pérez-Jiménez et al., 2010).
 Click to view | Table 7. Anthocyanin content and profile of aronia berry, juice, and pomace |
3.6.2. Proanthocyanidins
Aronia contains proanthocyanidins with predominately (−)-epicatechin units (32.2 to 99.6 mg/100 g) with a trace amount of (+)-catechin (Table 8, Jurikova et al., 2017). Oligomeric and polymeric (−)-epicatechins make up monomers, dimers, tetramers, hexamers, octamers, and decamers, but most proanthocyanidins in aronia berries have polymerization greater than 10 (Taheri et al., 2013). Aronia berry proanthocyanidins are distributed 70% in the flesh, 25% in the skin, and 5% in the seeds (Mayer-Miebach et al., 2012). Proanthocyanidin consumption may have a wide range of human health benefits, including reducing oxidative stress, improving blood circulation, and reducing cancer symptoms (Rauf et al., 2019). Additionally, when used as food ingredients, proanthocyanidins create foamability, oxidative stability, and heat stability and increase astringency (Shi et al., 2003).
 Click to view | Table 8. Tannin content and profile of aronia berry and pomace |
3.6.3. Flavonols
Flavonols are less abundant in aronia berry relative to proanthocyanidins and anthocyanins. The major flavonol in aronia is quercetin (Table 9), and it can increase by 5% during juice pasteurization (Jurikova et al., 2017). Quercetin distributed as its 3-rutinoside (3.9 to 61.7 mg/100 g fwb), 3-glucoside (4.03 to 29.2 mg/100 g fwb), 3-galactoside (6.6 to 30.2 mg/100 g fwb), 3-robinobioside (1.03 to 11.3 mg/100 g fwb), 3-vicianoside (2.36–5.38 mg/100 g fwb) (Table 9). Other aronia berry flavonols include myricetin, isorhamnetin, and kaempferol.
 Click to view | Table 9. Flavonol content reported in aronia berry fruit, juice, and pomace |
3.6.4. Phenolic acids
The phenolic acid profile of aronia berries is mainly neochlorogenic and chlorogenic acids with lower levels of other phenolic acids (Table 10). These other phenolic acids include vanillic, ferulic, syringic, and gallic acids.
 Click to view | Table 10. Phenolic acid content reported in aronia berry fruit, juice and pomace |
3.6.5. Non-extractable polyphenols
In the studies reviewed, most of the experiments have used analytical methods to assess extractable polyphenols (EPs).The solvents used to obtain EPs are typically aqueous-organic, commonly containing water, methanol, or acetone (Han et al., 2019). In contrast, non-extractable polyphenols (NEPs), also known as insoluble-bound phenolics, require enzymatic, acidic, or alkaline hydrolysis to be liberated to the extraction medium (de Camargo et al., 2016). These methods dissociate non-extractable polyphenols from cellulose, hemicellulose, polysaccharides, and polypeptides. Advanced techniques, such as ultrasound-assisted extraction and microwave-assisted extraction, are not able to successfully extract NEPs. The most effective method to release the NEPs is hydrolysis (Dzah et al., 2020). However, hydrolysis conditions may degrade polyphenols. After juicing, aronia pomace is a good source of NEPs. Enzyme-assisted extraction and high-pressure extraction methods have also been applied to recover NEPs from aronia berry pomace (Grunovaitė et al., 2016; Kitrytė et al., 2017); however, alkaline hydrolysis is expected to be the most efficient method to recover NEPs (de Camargo et al., 2016). The profile of aronia berry NEPs recovered by enzyme- assisted extraction and high-pressure extraction is similar to its free polyphenol profile (Grunovaitė et al., 2016; Kitrytė et al., 2017).
3.7. Astringent compounds
Although aronia berries show potential for numerous health benefits, consumption of whole aronia berry is limited by its astringency and bitterness. Adding sugar or ethyl butyrate to aronia juice may reduce its bitterness, but does not change astringency and consumers’ preference (Duffy et al., 2016). Amygdalin is an aromatic cyanogenic glucoside compound that is responsible for a bitter-almond smell and contributes to aronia berry astringency. There are no recent studies on amygdalin content in aronia berries, but prior studies have reported 52.3 mg/100 g fwb in pomace (Kulling and Rawel, 2008). Proanthocyanidins also contribute to astringency, due to its interactions with salivary proteins (Soares et al., 2018). Some hydroxycinnamic acids, including vanillic and syringic acid, may also contribute to astringency (Sáenz-Navajas et al., 2010). Additional research to characterize the relative astringency of these components in aronia berries can help inform product development and masking strategies in food production.
| 4. Polyphenol bioavailability | ▴Top |
Aronia berries contain higher antioxidant capacity than most foods (Pérez-Jiménez et al., 2010), but these tests neglect polyphenol metabolism and bioavailability. Thus, to understand the antioxidant and human health-promoting mechanisms of aronia polyphenol consumption, it is necessary to consider the metabolism and bioavailability of its polyphenols. While reports on polyphenol bioavailability have increased, there is still limited knowledge about the dynamics of polyphenol metabolism (Neilson et al., 2017; Shahidi et al., 2019). Bioavailability depends on their physicochemical stability, complex formation, food interaction, gastrointestinal absorption, and hepatic and gut metabolism (Luca et al., 2019). Recent work has described how the gut microbiome contributes to aronia polyphenol metabolism and bioavailability (Istas et al, 2019). Significant inter-individual variability of polyphenol metabolism and bioavailability may explain the variable outcomes in human intervention studies and are summarized in Table 11. The following subsections focus on describing the metabolism and bioavailability of aronia berry polyphenols.
 Click to view | Table 11. Summary of aronia berry juice and extract polyphenol bioavailability to plasma |
4.1. Anthocyanins
Anthocyanin structure and stability is pH-dependent. Upon consumption, the oral cavity pH is 5.6–7.9, which enhances anthocyanin hydrolysis. Given the short time period at this pH, only a fraction are hydrolyzed in the oral cavity (Braga et al., 2018). In the stomach, the acidic pH of 1.5–3.5 increases the proportion of anthocyanins as flavylium cations (Braga et al., 2018). At the small intestine, the pH of 6.7 to 7.4 favors the anthocyanin chalcone and quinoidal base formation, promoting hydrolysis to low molecular weight phenolics (Braga et al., 2018). A fraction of anthocyanins are directly absorbed in the stomach and small intestine. However, anthocyanins have low absorption in the small intestine, and the majority of them will be catabolized by gut microbiota (Denev et al., 2012). Upon absorption, anthocyanins and their metabolites undergo phase I and phase II metabolism in enterocytes and the liver (Denev et al., 2012). Phase I metabolites include phloroglucinaldehyde, 3,4-dihydroxybenzaldehyde, and hydroxybenzoic acid. Phase II metabolites are glucuronidated and methylated cyanidin. In the colon, gut microbiota hydrolyzes the anthocyanins into phenolic acids such as hippuric acid, phenylpropanoid acid, and ferulic acid (Wiczkowski et al., 2010; Denev et al., 2012). A human supplementation study utilizing 13C-cyanidin-3-glucoside reported 5.4% urinary excretion of anthocyanins and its metabolites (Czank et al., 2013). It would be expected that cyanidin-3-galactoside metabolism would be similar (Figure 1).
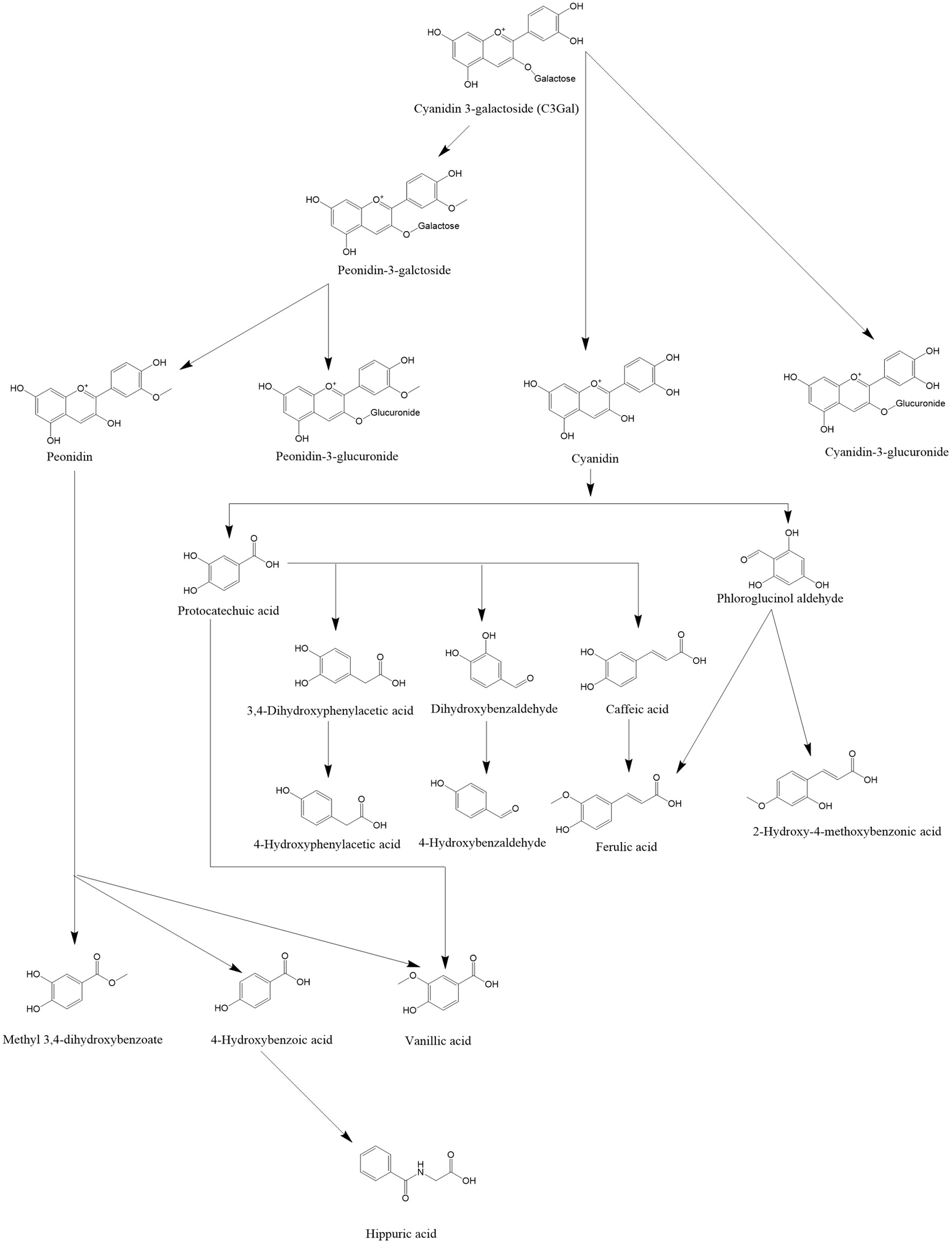 Click for large image | Figure 1. Overview of cyanidin-3-galactoside metabolism, adapted from de Ferrars et al. (2014). Cyanidin-3-galactoside undergoes methylation by the host to form peonidin-3-galactoside, or is hydrolyzed and glucoronidated. Anthyocyanins are subsequently hydrolyzed in host tissue or by the gut microbiota into phenolic acids. Phenolic catabolites can undergo enterohepatic circulation and are subjected to further host metabolism prior to excretion. |
4.2. Proanthocyanidins
Proanthocyanidins have limited bioavailability because of their polymeric structures. Larger polymers are unable to translocate across the phospholipid bilayer of intestinal cells membrane, be transported by carrier proteins, and entirely dissolve into the aqueous phase of the small intestine (Luca et al., 2019). Moreover, human enzymes are not able to hydrolyze proanthocyanidins. Therefore, only monomers or dimers can be absorbed in the small intestine (Williamson and Clifford, 2017). The majority of proanthocyanidins reach the colon unchanged. A proportion of B-type proanthocyanidins are hydrolyzed by gut microbiota to valerolactones and phenolic acids (Appeldoorn et al., 2009). Thus, phenolic catabolites’ rate and profile are highly dependent on the gut microbial composition (Luca et al., 2019). The remaining proanthocyanidins are excreted and in the feces (Neilson et al., 2016).
4.3. Quercetin
The metabolism and bioavailability of quercetin depends on its glycosylation (Kaşıkcı and Bağdatlıoğlu, 2016). Quercetin glucosides and galactosides are absorbed in the small intestine, whereas rutinosides are not. Rutin (quercetin-3-rutinoside) is deglycosylated by microbiota and then absorbed through passive diffusion (Luca et al., 2019). Quercetin glucosides and galactosides are hydrolyzed in the gut and liver, and aglycones mainly undergo Phase II metabolism. If the flavonols are not absorbed in the small intestine, they enter the gut, where the microbiota hydrolyzes the quercetin (Luca et al., 2019). The bacteria hydrolyze quercetin to low molecular polar metabolites (Santhakumar et al., 2018). Some of these compounds are 4-dihydroxyphenylacetic acid (DOPAC), 3-hydroxyphenyl acetic acid (3-OPAC), 3,4-dihydroxybenzoic acid (PCA), and vanillic acid (Ameida et al., 2018). The rate of formation of microbial metabolites depends in-part on nutrients presence (Rodriguez-Castaño et al., 2019). These low molecular compounds are absorbed in the large intestine and then metabolized in the liver (Atala et al., 2017). Quercetin metabolites are excreted through feces and urine, mainly as benzoic and hippuric acid (Luca et al., 2019).
4.4. Phenolic acids
The predominant phenolic acids in aronia, chlorogenic and neochlorogenic acids, require de-esterification of quinic acid prior to absorption (Denev et al., 2012). The bioavailability of these compounds are low because esterase is limited in the small intestine. In the colon, bacteria release caffeic acid from chlorogenic acid (Heleno et al., 2015). Subsequently, absorbed phenolic acids undergo phase II metabolism in tissues prior to excretion.
4.5. Aronia polyphenol bioavailability
The maximum plasma concentration of dietary polyphenols in humans is in the nanomolar or low micromolar range (Istas et al., 2019). The highest level of polyphenols reported in plasma after aronia consumption has been 1.4 and 592 nmol/L, and appearing in the plasma between 0.5 and 4 h after consumption (Pojer et al., 2013). This wide range of absorption is partly because of differences in fruit composition, glycosylation, and anthocyanidin type. For example, a review on anthocyanins bioavailability concluded that galactosides were more bioavailable than arabinosides (Pojer et al., 2013). Additionally, the report compared aronia berry and elderberry extract bioavailability, containing 721 mg and 720 mg anthocyanidins, respectively (Pojer et al., 2013; Cao et al., 2001; Kay et al., 2005). After participants consumed both extracts, plasma anthocyanin concentrations were similar at 96.08 nM and 97.20 nM, respectively (Cao et al., 2001; Kay et al., 2005). In a double-blind, placebo-controlled study compared polyphenol metabolites in plasma after the consumption of whole aronia fruit or aronia extract (Istas et al., 2019). The total plasma polyphenol concentration was 30 ± 156 µM and 14 ± 106 µM after 12 weeks of consuming extract and whole berries, respectively (Table 11). In addition, 20 metabolites were found in the volunteers consuming aronia extract, while only five metabolites were found after whole fruit consumption.
Another study evaluated the pharmacokinetics of anthocyanins and selected metabolites in adults’ plasma (n = 6) that consumed 500 mg of aronia extract (Xie et al., 2016). Hippuric acid had the highest plasma concentration (0.87 to 3.5 μg/mL), followed by 3-(4-hydroxyphenyl)propionic acid (0.033 to 0.48 μg/mL), peonidin-3-O-galactoside (0.029 to 0.266 μg/mL), cyanidin-3-O-glucoside (0.014 to 0.180 μg/mL), and lastly protocatechuic acid (0.004 to 0.007 μg/mL) (Xie et al., 2016). A separate study found eight metabolites in the blood and urine consuming of 0.8mg of anthocyanins/ kg of body weight from aronia juice (Wiczkowski et al., 2010). The concentration of anthocyanins in the plasma was maximal at 1.3 hours after consumption, reaching 20.4 to 51.8 nmol/L. However, all of these studies had significant inter-individual variability in polyphenol pharmacokinetics. Larger studies are needed to characterize the importance of these variations to human health.
| 5. Health benefits of aronia berry consumption | ▴Top |
Non-communicable diseases (cancer, diabetes, cardiovascular diseases, and depression) have an enormous social and economic toll worldwide (Centers for Disease Control and Prevention, 2020). Thus, multiple studies have focused on effective treatments to lower the risks of non-communicable diseases. Given the high polyphenol content of aronia berries, preliminary evidence indicates its preventive and therapeutic effects on non-communicable diseases. Here, we summarize recent preclinical (Table 12) and human intervention studies (Table 13) with aronia berry, juice, or extracts.
 Click to view | Table 12. Recent preclinical studies on aronia berry health benefits |
 Click to view | Table 13. Recent human intervention studies with aronia berry interventions |
5.1. Cancer prevention
Recent studies have demonstrated anti-carcinogenic mechanisms of aronia polyphenols in vitro. Aronia extract prevents the growth, migration, and invasion of SK-Hep1 human liver cancer cells (Thi and Hwang, 2018). Liver cancer cell growth, adhesion, and migration were reduced by the aronia extract. Furthermore, aronia extract inhibited the expression of proteases involved in metastasis (MMP-2/9, MT-1 MMP). Another study isolated catechol from fermented aronia juice and characterized its inhibition of cancer stem cells (Choi et al., 2018). Catechol inhibited the formation of cancer stem cells and reduced the production of IL-6, which enhances cancer cells’ survival. Additionally, catechol inhibited Stat3, a key transcription factor necessary for cancer stem cell formation. Thus, aronia components can inhibit multiple cancer mechanisms in vitro. Further studies that account for aronia polyphenol and bioavailability are needed to determine these studies’ relevance to human health.
5.2. Diabetes
Preclinical and human studies have reported that aronia consumption may reduce insulin resistance. In rodents, aronia juice concentrate consumption increased plasma levels of adiponectin, the most abundant peptide secreted by key monomers that have an inter-relationship between insulin resistance and inflammation (Baum et al., 2016). Aronia juice consumption also decreased intestinal glucosidase activity and increased DPP IV activity in diabetic mice (Yamane et al., 2016). An open-label trial of aronia juice for adults with type 2 diabetes reported improvement of glycemic control, with decreased fasting blood glucose and glycated hemoglobin (Milutinović et al., 2019). Thus, aronia consumption appears to be a promising treatment for diabetes. Further well-controlled human intervention studies on aronia berry are needed to increase the evidence base for its anti-diabetic activity.
5.3. Cardiovascular disease
Cardiovascular disease is a leading cause of death in the US (Centers for Disease Control and Prevention, 2020). Poor diets, low physical activity, excessive drinking, and smoking may increase cardiovascular disease risk. Increased blood pressure, adiposity, total and LDL cholesterol, and elevated inflammation and oxidative stress increase cardiovascular disease risk. Preclinical experiments have demonstrated aronia berry extract increases vasodilatory nitric oxide in cultured endothelial cells, and L-NAME induced hypertensive rats (Varela et al., 2015; Cebova et al., 2017). In rats, this increase is associated with increased nitric oxide synthase activity, reduced inflammation, and hypertension (Cebova et al., 2017). Consumption of aronia berry powder, extract, and juice also inhibits weight gain, lipid dysmetabolism, and inflammation in diet-induced obesity in mice (Bhaswant et al., 2017; Jeong and Kim, 2019; Yamane, Kozuka, Yamamoto, et al., 2016).
Aronia berry consumption improves biomarkers associated with cardiovascular disease risk in human intervention studies, but these changes depend on the participant populations. Aronia extract (116 mg) and powder (12 mg) consumption improved flow-mediated dilation, a marker of vascular function, in healthy men (Istas et al., 2019). Consumption of 300 mL/day of aronia juice and 3 g/day of aronia powder reduced systolic/diastolic blood pressure in adults with mildly elevated hypertension, but did not modulate serum lipids (Loo et al., 2016). Aronia extract reduced total and LDL cholesterol in healthy former smokers, and these changes were associated with increased urinary excretion of peonidin-3-galactoside, cyanidin-3-galactoside, and 3-(4-hydroxyphenyl)propionic acid (Xie et al., 2017). Open-label trials of aronia juice for adults with type 2 diabetes and aronia extract for adolescents with metabolic syndrome have also been promising for modulating serum lipids and reducing oxidative stress and inflammation biomarkers. A systematic review and meta-analysis of literature concluded that among human interventions, aronia consumption leads to increases in HDL and diastolic blood pressure (Rahmani et al., 2019). Thus, further trials are needed to strengthen the evidence base for the specific populations that benefit from aronia berry consumption.
5.4. Modulation of gut microbiota
The microbiota contributes to modulation of the immune system and is now recognized to contribute to the progression of chronic disease (Festi et al., 2014). Furthermore, the gut microbiota contributes to the development of the metabolic syndrome and affects energy, lipid, and insulin metabolism. Furthermore, diets high in prebiotics that increase commensal microbes may positively change the microbiota composition and reduce inflammation related to metabolic syndrome. In aronia, sorbitol, fiber, and polyphenols may modulate microbial populations. Aronia polyphenols have direct anti-microbial activity against some food pathogens; however, the in vivo modulation of these microbes are not clear (Table 12). In mice, gut microbiota modulation precedes its anti-inflammatory and immunomodulatory effects (Pei et al., 2019). In healthy adults, polyphenols’ consumption equivalent to 75 g of aronia berry or berry powder equivalent to 10 g of aronia berry for 12 weeks did not affect microbial diversity (Istas et al., 2019). However, both groups had significant increases in Anaerostipes and Bacteroides, which are thought to be beneficial commensals. In a simulated human microbiota experiment, aronia juice increased firmicutes, proteobacteria and Akkermansia (Wu et al., 2018). Further studies are needed to characterize the specific effects of whole aronia berry and juice on gut microbiota and its relationship with inflammation outcomes and other health effects.
5.5. Neuroprotection
Experiments in rodents support the neuroprotective effects of aronia consumption (Table 12). When applied to cultured hippocampal and microglial cells, aronia reduces oxidative stress and inflammation (Lee et al., 2018; H. Y. Lee et al., 2017). In aged rats, aronia juice consumption increased hippocampal nerve fibers (Daskalova et al., 2019). Aronia supplementation may improve memory impairment and motor skills in rats (Daskalova et al., 2019; Lee et al., 2018).
Furthermore, aronia juice consumption reduced anxiety-like behaviors in adult rats (Tomić et al., 2016). While more research is necessary, these findings suggest aronia juice or extracts could benefit cognitive function and improve neural health.
5.6. Colitis
Colitis leads to dysmetabolism, loss of body weight, and microbiota changes (Ungaro et al., 2019; Pei et al., 2018). Anthocyanins inhibit intestinal inflammation, promote intestinal barrier function, and maybe protective against colitis (Valdez and Bolling, 2019). Aronia berry powder is protective against adoptive transfer and chemical-induced colitis in rodents. In adoptive transfer colitis, aronia consumption reduces colonic CD4+ cells and increases colonic Tregs and anti-inflammatory Th17 (Pei et al., 2018). These changes are linked to reduced oxidative stress and modulation of colonic antioxidant function (Pei et al., 2019). In the trinitrobenzene sulfonic acid (TNBS) colitis model, aronia juice consumption inhibits inflammation (Valcheva-Kuzmanova et al., 2018). The aronia berry juice decreased the lesion extension, adhesion score, and the colon’s wall thickening score in colitic Wistar rats. Although these studies are promising, human intervention studies are necessary to determine the efficacy of aronia berry or its co-products to inhibit inflammatory bowel diseases.
| 6. Conclusions | ▴Top |
Aronia berries are one of the richest plant sources of anthocyanins and other bioactive polyphenols. However, these are presently underutilized in the diet because of its sourness and astringency. Aronia extract, juices, and pomace may be useful as functional ingredients, given their polyphenol content. Developing a better understanding of the contribution of bioactives to astringency could help create aronia products with improved palatability. Developing a more complete nutrient profile of aronia berry and juice will help improve nutrient databanks. Establishing the relationship of aronia plant genotype to berry quality will improve the production of berries with increased bioactive content. Emerging evidence describes the beneficial effects of aronia berry for prevention of diabetes, hypertension, cardiovascular disease, cancer, and colitis. However, future studies on the health benefits of aronia berry should utilize well-characterized aronia material, including description of the genotype, polyphenols, sorbitol, fiber, and micronutrient content.
Acknowledgments
The authors were supported by the USDA Specialty Crop Multi-State Program (#18-13-352).
| References | ▴Top |