| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Original Research
Volume 12, December 2020, pages 129-139
Identification of short-chain pyroglutamyl peptides in Japanese salted fermented soy paste (miso) and their anti-obesity effect
Saki Shirakoa, b, Yumi Kojimaa, Takeo Hasegawac, Toshikazu Yoshikawac, Yasuki Matsumurad, Kaori Ikedae, Nobuya Inagakie, Kenji Satoa, *
aDivision of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwake-cho, Kyoto 606 8502, Japan
bDepartment of Biomedical Sciences, College of Life Sciences, Ritsumeikan University, 1-1-1 Noji-Higashi, Kusatsu, Shiga 525 8577, Japan
cLouis Pasteur Center for Medical Research, 103-5, Tanaka-Monzenmachi, Kyoto 606 8225, Japan
dDivision of Agronomy and Horticultural Science, Graduate School of Agriculture, Kyoto University, Gokasho, Uji, Kyoto 611 0011, Japan
eDepartment of Diabetes, Endocrinology and Nutrition, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawahara-cho, Kyoto 606 8507, Japan
*Corresponding author: Kenji Sato, Division of Applied Biosciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwake-cho, Kyoto 606 8502, Japan. Tel: +81-75-753-6444, E-mail: sato.kenji.7x@kyoto-u.ac.jp
DOI: 10.31665/JFB.2020.12251
Received: December 21, 2020
Revised received & accepted: December 29, 2020
| Abstract | ▴Top |
Miso, a paste of salted fermented soybean, is a seasoning used extensively in traditional Japanese cuisine. Herein, pyroglutamyl peptides present in miso were identified by a liquid chromatography-tandem mass spectrometry (LC-MS/MS), detecting precursor ions, which generated immonium ion of pyroglutamyl residue. By using this method, 13 pyroglutamyl peptides were identified in four types of miso. Administration of the water extract prepared from 0.6 g soybean miso/kg body weight/day significantly suppressed high fat diet-induced weight gain. A similar effect was exerted by the hydrophobic pyroglutamyl peptide fraction, including pyroglutamyl proline (pEP), pEV, pEI, and pEL. Administration of a mixture of synthetic pEP, pEV, pEI, and pEL in a ratio to that in miso or pEL alone also suppressed the weight gain in a dose dependent manner. These results suggest that the short-chain hydrophobic pyroglutamyl peptides present in miso are effective in suppressing high fat diet-induced obesity.
Keywords: Fermented food; Miso; Pyroglutamyl peptide; Pyroglutamyl leucine; Obesity
| 1. Introduction | ▴Top |
Miso, a paste of salted fermented soybean, is used as a seasoning in Japanese cuisine. The Japanese diet, referred to as washoku, is mainly composed of steamed rice, main and side dishes (fish, meat, or vegetables), and miso soup (Suzuki et al., 2018). Miso soup is prepared by the addition of a spoonful (approximately 12 g per serve) of miso into a soup stock made from dried bonito flakes (katsuobushi), dried anchovies (niboshi), or dried kelp (kombu), and so on. Miso is also used as a seasoning in cooked meat including fish. Although, miso consumption has decreased to half of the consumption in 1970s (Okouchi et al., 2019), it remains an important constituent of the Japanese diet (Suzuki et al., 2018).
Miso is made from steamed soybeans, a fungus starter (koji), and salt. Koji is prepared by inoculating steamed rice, barley, or soybean with Aspergillus oryzae which is then cultured at approximately 30 °C for 40 h, which is referred to as rice koji, barley koji, or soybean koji, respectively. For preparing miso, washed soybeans are soaked in water and steamed. Then, the steamed soybeans are mixed with koji and salt, and aged for several weeks (short aging type), months, or years (long aging type). In Japan, different types of miso are produced with various kinds of ingredients and distinct fermentation periods. Depending on the koji ingredients, the final product is called rice miso, barley miso, or soybean miso. Approximately 80% of miso available in Japan is rice miso, while the remaining 20% is barley and other miso. During this process, the protein in soybean and barley or rice is degraded into peptides and amino acids by proteases from A. oryzae, thereby giving an umami taste to the miso. The characteristic flavor and aroma of miso could, in addition, be generated by the products of Maillard reaction and fermentation by air borne salt-resistant lactic acid bacteria and yeasts.
Several studies have demonstrated the health benefits associated with miso consumption. A cross-sectional study has shown that people who frequently consume miso soup tend to have a lower heart rate (Ito et al., 2017). The Nagahama prospective cohort study also demonstrated that women who consume miso soup every day show lower values of HOMA-IR (homeostatic model assessment for insulin resistance) (Ikeda et al., 2018). Kondo et al. (2019) have reported that miso consumption for a duration of 8 weeks reduces nighttime blood pressure in human subjects. Kitano et al. (2014) have shown that Japanese diet in 1975 had high anti-obesity effect on mice than that in 1960, 1990, and 2005. Okouchi et al. (2019) have reported that daily miso consumption, along with exercise, suppressed visceral fat accumulation in mice, thereby suggesting that miso may be partially responsible for the anti-obesity effects of the Japanese diet in 1975. These beneficial effects have been attributed to certain compounds present in miso. However, compounds that can be beneficial at a dose accessible by a daily consumption of miso have not been identified yet, even in animal models.
We have previously demonstrated the presence of pyroglutamyl (5-oxoprolyl) peptides in food protein hydrolysates (Sato et al., 1998, 2013; Ejima et al., 2018) and the Japanese rice wine, sake (Kiyono et al., 2013). Pyroglutamyl peptides are spontaneously generated during processing and storage of peptides with a glutaminyl residue at the amino terminal (Sato et al., 1998; Higaki-Sato et al., 2006). Short-chain pyroglutamyl peptides resist digestion by both endo- and exopeptidases (Chen et al., 2019). Several studies have reported the in vivo and in vitro biological activities of short-chain pyroglutamyl peptides. Administration of pyroglutamyl leucine (pyroGlu-Leu or pEL) have been shown to attenuate D-galactosamine-induced hepatitis in rats (Sato et al., 2013). In addition, administration of pyroGlu-Leu and pyroGlu-Asn-Ile at low doses (0.1–1 mg/kg body weight) attenuates dextran sulfate sodium (DSS)-induced colitis and colitis-induced dysbiosis (disturbance of microbiota) in mice (Wada et al., 2013; Kiyono et al., 2016). Administration of pyroGlu-Leu also attenuates high fat diet-induced dysbiosis by increasing host antimicrobial peptide (Shirako et al., 2019), and has been shown to exert antidepressant-like effects (Yamamoto et al., 2015). Moreover, pyroglutamyl peptides exhibit anti-inflammatory activity against LPS-stimulated macrophages (Hirai et al., 2014) and IL-1β stimulated hepatocytes (Oishi et al., 2015). Our preliminary investigations based on amino acid analysis and solid phase extraction with a strong cation exchanger indicated that pyroglutamyl peptides represent approximately 10% of all peptides in miso. This suggests that these peptides might be, at least partially, responsible for the beneficial effects of miso. However, the presence of multiple constituents in miso has hindered the identification of the pyroglutamyl peptides.
Therefore, the present study was designed with overall objective of elucidating the structure, abundance, and biological activity of pyroglutamyl peptides in miso. For this purpose, pyroglutamyl peptides were detected by using a liquid chromatography tandem mass spectrometry (LC-MS/MS) as precursor ions generating immonium ion of pyroglutamyl residue. Further, the suppressive effect of pyroglutamyl peptides in miso on body weight gain of rats fed on high fat diets was examined.
| 2. Materials and methods | ▴Top |
2.1. Materials
Rice miso (short aging and long aging types A, B, and C) and barley miso were kind gifts from Marumi Koji Honten (Soja, Okayama, Japan). Rice miso (long aging type D) and soybean miso (long aging type, 2 years) was obtained from the local market.
2.2. Reagents
Acetonitrile (HPLC grade) was purchased from Nacalai Tesque (Kyoto, Japan). Benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate (PyBOP), 1-hydroxybenzotriazole (HOBt), 9-fluorenylmethoxycarbonyl (Fmoc) amino acid derivatives, N-(tert-Butoxycarbonyl)-L-pyroglutamic acid (Boc-pyroGlu), L-proline tert-butyl ester hydrochloride (H-Pro-OtBu·HCl), L-valine tert-butyl ester hydrochloride (H-Val-OtBu·HCl), L-isoleucine tert-butyl ester hydrochloride (H-Ile-OtBu·HCl), and L-leucine tert-butyl ester hydrochloride (H-Leu-OtBu·HCl) were purchased from Watanabe Chemical Industries (Hiroshima, Japan).
2.3. Identification of pyroglutamyl peptides in miso
The pyroglutamyl peptide fraction was obtained as described previously (Ejima et al., 2018). Briefly, a strong cation exchanger (AG50W-×8, hydrogen form, 100–200 mesh, Bio-Rad Laboratories, Hercules, CA, USA) was washed with 50% methanol and packed into a spin column (15 mm × 7 mm i.d., 5.0 μm pore size, Ultrafree-MC, Merck, Darmstadt, Germany). The resin was further washed with 200 μL of 50% methanol and eluted by centrifugation at 815 g (thrice). Then, the resin was equilibrated with 200 μL of 0.1% formic acid containing 10% acetonitrile (twice). Soybean miso was added to 3-volume of ethanol and stirred vigorously for minutes. The resultant suspension was centrifuged at 12,000 g for 10 min at 4 °C. The supernatant (200 μL) was loaded onto the spin column and eluted by centrifugation at 815 g. The eluent was used as the pyroglutamyl peptide fraction for subsequent LC-MS/MS analysis. The abundance of pyroglutamyl peptide in the eluent was determined by amino acid analysis of the HCl hydrolysate obtained from the pyroglutamyl peptide fraction (Bidlingmeyer et al., 1984). The total peptide content was estimated by subtracting the free amino acid content from the total amino acid content of the HCl hydrolysate generated from water extract of miso.
Aliquots of the pyroglutamyl peptide fraction were subjected to LC-MS/MS using an LCMS-8040 setup (Shimadzu, Kyoto, Japan) connected to an Inertsil ODS-3 column (5 μm, 2.1 mm i.d. × 250 mm, GL Sciences, Tokyo, Japan). A binary linear gradient was set up with 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B) at a flow rate of 0.2 mL/min. The gradient program was as follows: 0–30 min, 0–30% B; 30–40 min, 30–100% B; 40–50 min, 100% B; 50–50.1 min, 100–0% B; and 50.1–60 min, 0% B. The column was maintained at a temperature of 40 °C. Total ion intensity was monitored in the positive mode by scanning across mass to charge ratio (m/z) range of 50–150, 150–200, 250–300, and 300–500 (total ion scan). Pyroglutamyl peptides were detected based on specific precursor ions, which generated immonium ion of pyroglutamyl residue (m/z = 84.1) at a collision energy of −35 V in the positive mode in the m/z range of 50–150, 150–200, 250–300, and 300–500 (precursor ion scan). The m/z corresponding to the pyroglutamyl peptide peaks were recorded and utilized for product ion scanning to elucidate the primary structure of the peptides at collision energies of −15, −25, and −35 V. Structure of pyroglutamyl peptides were estimated based on the product ions including immonium and related ions from each amino acid (Falick et al., 1993; Papayannopoulos, 1995).
2.4. Peptide synthesis
Peptides were synthesized by the Fmoc strategy using a PSSM-8 solid-phase peptide synthesizer (Shimadzu) for use as LC-MS/MS standards. The synthetic peptides were purified by reversed phase-high performance liquid chromatography (RP-HPLC) using a COSMOSIL 5C18-MS-II column (10 mm i.d. × 150 mm, Nacalai Tesque). A binary linear gradient was set up using 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B) at a flow rate of 2 mL/min. The gradient program was as follows: 0–20 min, 0–50% B; 20–30 min, 50–100% B; 30–35 min, 100% B; 35–35.1 min, 100–0% B; and 35.1–45 min, 0% B. The column was maintained at a temperature of 40 °C. Peptide elution was monitored based on absorbance at wavelengths of 214 and 254 nm. The purity of the synthesized peptides was confirmed using LC-MS. The abundance of the purified peptides was evaluated by amino acid analysis of the HCl hydrolysate, as described earlier (Bidlingmeyer et al., 1984). Pyroglutamyl proline (pyroGlu-Pro or pEP), pyroglutamyl valine (pyroGlu-Val or pEV), pyroglutamyl isoleucine (pyroGlu-Ile or pEI), and pyroglutamyl leucine (pyroGlu-Leu or pEL) were synthesized in the liquid phase through Boc strategy as described previously (Sato et al., 2013) for use in animal experiments.
2.5. Determination of pyroglutamyl peptide abundance
Aliquots (10 μL) of pyroglutamyl peptide fraction obtained from miso were subjected to LC-MS/MS in the multiple reaction monitoring (MRM) mode using the Inertsil ODS-3 column. The synthetic peptides were used as standards for the optimization of MRM conditions. As before, the binary linear gradient was set up using 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B) at a flow rate of 0.2 mL/min. The gradient program was as follows: 0–20 min, 0–30% B; 20–25 min, 30– 100% B; 25–30 min, 100% B; 30–30.1 min, 100–0% B; and 30.1–40 min, 0% B. The column was maintained at a temperature of 40 °C.
2.6. Preparation of samples for animal experiments
Soybean miso was added to 4 volumes of water and stirred vigorously. The resultant suspension was centrifuged at 3,000 g for 10 min. The supernatant was collected and used as the crude water extract. Pyroglutamyl peptides in the crude extract were fractionated by a series of column chromatography in reversed phase and strong cation exchange mode. Sep-Pak C18 35cc Vac (10 g) cartridges (Waters, Milford, MA, USA) was washed successively with acetonitrile (50 mL) and 0.1% acetic acid (50 mL). The crude water extract (100 mL) was loaded onto the column using a syringe (50 mL), followed by the addition of 50 mL of 0.1% acetic acid. The eluents were combined and used as the non-absorbed (N-A) fraction. The compounds absorbed to the column were then eluted with 0.1% acetic acid containing 10 and 30% acetonitrile (50 mL), termed as 10 and 30% ACN fractions, respectively.
The pyroglutamyl peptide fraction was obtained by passing the N-A, 10% ACN, and 30% ACN fractions through the strong cation exchange (AG50W-×8) resin. Then, the AG50W-×8 was washed with 60% acetonitrile and packed into an XK column (16 mm i.d. × 200 mm, GE Healthcare Chicago, IL, USA). The column was equilibrated with water, and each fraction was loaded onto the column using a Perista Pump (ATTO, Tokyo, Japan). Pyroglutamyl peptide fractions from the N-A eluent were stored separately, whereas those from the 10 and 30% ACN were pooled and termed as ACN. All pyroglutamyl fractions were freeze dried and used for animal experiments
2.7. Animal experiments
All animals used in this study were treated and cared for in accordance with the guidelines of the National Institutes of Health (NIH) for the use of experimental animals. All experimental procedures were approved by the Animal Care Committee of Louis Pasteur Center for Medical Research (no. 20162 for experiments 1 and 2, and no. 20172 for experiments 3 and 4). Five-week-old male Wistar/ST rats (120–140 g) were purchased from Japan SLC (Shizuoka, Japan). All rats were housed in individual cages maintained at 24 ± 1 °C with a 12 h light/dark cycle. The rats were allowed free access to a normal diet (solid type of certified diet MF; Oriental Yeast, Tokyo, Japan) and drinking water for 1 week. After 1 week acclimatization period, the rats were maintained on normal or high fat diets, with peptide samples being administered via drinking water for 5 weeks. Body weight, total food intake, and total water intake were measured every 2 days. Body weight gain (%) was calculated as (final body weight – initial body weight)/initial body weight×100.
2.7.1. Experiment 1 (administration of water extract of miso to rat fed on high fat diet)
After the acclimatization period (1 week), the rats were divided into 5 groups (n = 4 for each group). One group was fed on normal diet (N). The other 4 groups were fed on a solid type of high fat diet (HF; 45% kcal/total kcal; D12451, Research Diets, New Brunswick, NJ, USA). The 4 HF groups were administered with vehicle, crude water extract of miso (CE), pyroglutamyl peptide fraction prepared from the N-A fraction (N-A), or that from the pooled ACN fractions (ACN) via drinking water. CE, N-A, and ACN, prepared from 0.6 g of miso/kg (body weight of rat) were administered to each rat per day. The abundance of pyroglutamyl peptides in each fraction was determined by the method described in section 2.5, and the dosage of each peptide is shown in Figure 1a. The rats were allowed free access to either the normal or high fat diet, as well as drinking water. Drinking water containing CE, N-A, or ACN was prepared every week to administer above-mentioned dose of pyroglutamyl peptides on the basis of their water consumption of the previous week. After 5 weeks, all rats were sacrificed by puncturing the inferior vena cava under isoflurane-induced anesthesia. Plasma was obtained from the peripheral blood and stored at −80 °C until analysis.
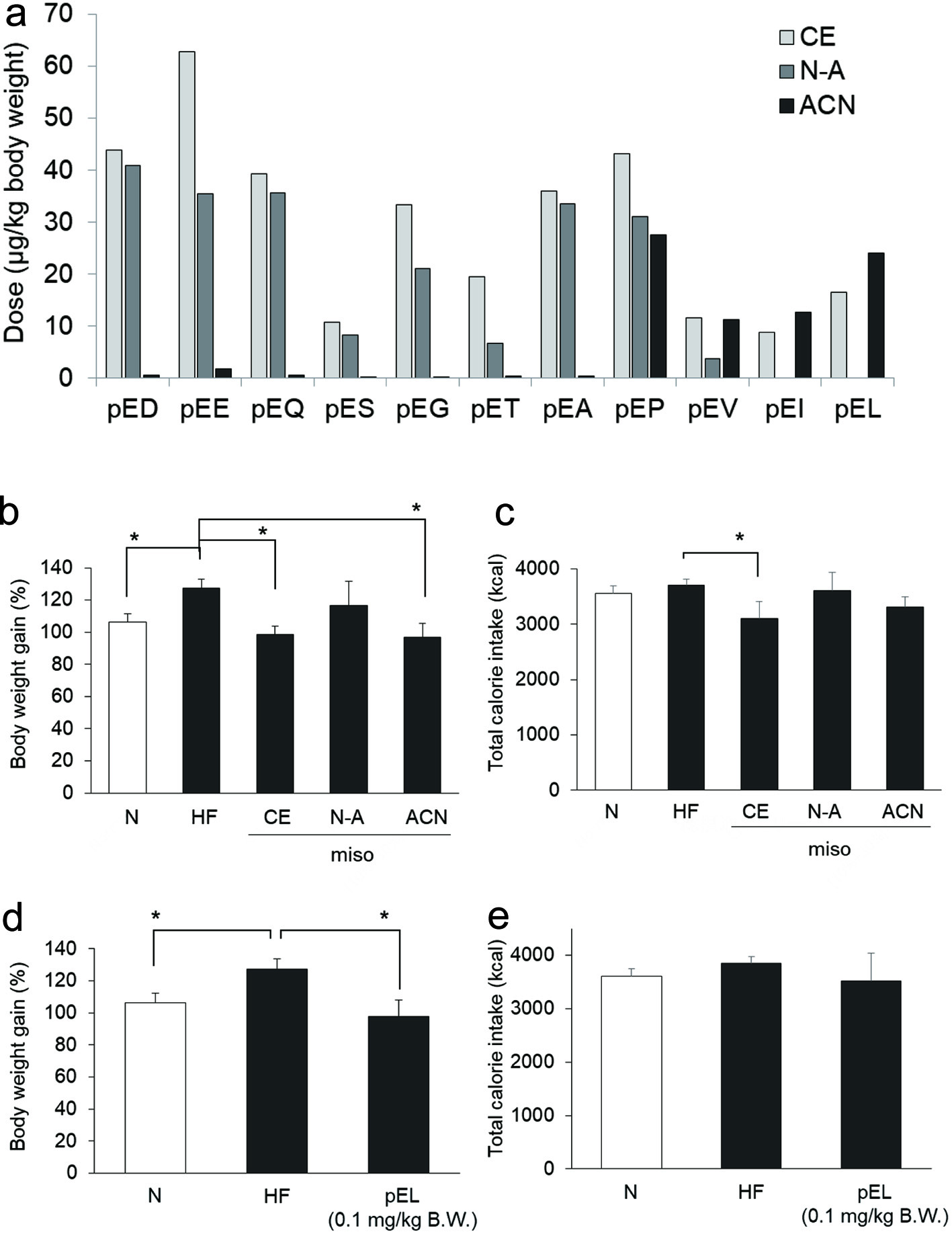 Click for large image | Figure 1. Effects of water extracts of miso and synthetic pyroGlu-Leu on weight gain in 45% high fat diet-fed rats. The dose of pyroglutamyl peptides in crude water extract of miso (CE), non-absorbed fraction (N-A), and pooled mixture of 10% and 30% ACN fractions (ACN) (a). Rats were fed on normal (open column) and 45% high fat diet (closed column). Effect of miso extracts on body weight gain (b) and total calorie intake (c) in 45% high fat (HF) diet-fed rats. Effect of synthetic pyroGlu-Leu (0.1 mg/kg body weight) on body weight gain (d) and total calorie intake (e) in the rats. One-letter codes are used for all amino acid residues in panel (a). For b–e, data are shown as mean ± SD (n = 4). * represents significant difference with p < 0.05, as estimated by Dunnett’s test vs HF. |
2.7.2. Experiment 2 (administration of synthetic pyroGlu-Leu to rat fed on high fat diet)
After the acclimatization period (1 week), 2 groups were fed on normal diet (N) and the remaining 2 groups were fed on the high fat diet. One N and one HF group received pyroGlu-Leu (at a dosage of 0.1 mg/kg body weight), while the other 2 were treated with the vehicle. These experimental groups were referred to as N, N+pEL, HF, and HF+pEL, respectively. The rats were allowed free access to either the normal or high fat diet. Synthetic pyroGlu-Leu was orally administered via drinking water. Drinking water containing pyroGlu-Leu (0.36–0.83 mg/L) was prepared every week to administer pyroGlu-Leu at the above-mentioned dosage on the basis of their water consumption of the previous week. After 5 weeks, all rats were sacrificed by the same manner as described above.
2.7.3. Experiment 3 (administration of a synthetic mixture of hydrophobic pyroglutamyl peptides to rat fed on very high fat diet)
The major pyroglutamyl peptides in ACN (pyroGlu-Pro, pyroGlu-Val, pyroGlu-Ile, and pyroGlu-Leu) were synthesized and mixed in the same proportion as that in the ACN. After the acclimatization period (1 week), the rats were divided into 4 groups (n = 4 for each group). One group was fed on normal diet (N). The other 3 groups were fed on another solid type of very high fat diet (VHF; 60% kcal/total kcal; D12492, Research Diets). The very high fat diet groups were administered with the vehicle or the mixture of synthetic pyroglutamyl peptides in doses similar to their abundance in ACN (Figure 1a) and in 10 times higher doses. These groups were referred to as VHF, ×1 ACN-pep, and ×10 ACN-pep, respectively. The synthetic pyroglutamyl peptide mixture was orally administered via drinking water. After 5 weeks, all rats were sacrificed in the same manner, as described above. The weight of epididymal adipose tissue and liver were measured.
2.7.4. Experiment 4 (administration of synthetic pyroGlu-Leu to rat fed on very high fat diet)
After the acclimatization period (1 week), 2 groups were fed on normal diet (N) and the other 2 groups were fed on the very high fat diet (n = 4 for each group) and treated as described in section 2.7.2. Synthetic pyroGlu-Leu was orally administered at a dosage of 1 mg/kg body weight via drinking water by method described above. After 5 weeks, all rats were sacrificed in the same manner as described above. The weight of epididymal adipose tissue and liver were measured in the sacrificed rats.
2.8. Blood analysis
Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were determined by outsourcing to Oriental Yeast. Plasma leptin and ghrelin levels were determined by ELISA kits (Rat Leptin Assay Kit, Immuno-Biological Laboratories, Fujioka, Japan, and Rat Ghrelin ELISA Kit, MyBioSource, San Diego, CA, USA) according to the manufacture’s instruction.
2.9. Statistical analysis
The results of the experiments were presented as mean ± standard deviation (SD). In animal experiments 1 and 3, significant differences compared to the high fat diets group were evaluated by the Dunnett’s test. On the other hand, significant differences by administration of pyoGlu-Leu in each diet group in animal experiments 2 and 4 were evaluated by the t-test. Differences associated with p < 0.05 were considered significant. Differences associated with 0.05 < p < 0.1 were considered to show tendency. Statistical analysis was performed using GraphPad Prism version 6.04 (GraphPad Software, San Diego, CA, USA).
| 3. Results | ▴Top |
3.1. Identification and determination of pyroglutamyl peptides abundance in miso
Pyroglutamyl peptides in soybean miso were analyzed by LC-MS and LC-MS/MS in the precursor ion scan mode, targeting the immonium ion generated from the pyroglutamyl residue (m/z = 84.1). Mass chromatograms were obtained by scanning across different m/z ranges (50–150, 150–200, 200–250, 250–300, and 300–500) as shown in Figure 2a–e. The mass chromatograms from the LC-MS/MS (lower) were different from those from the LC-MS (upper), with some peaks being observed only in the lower chromatograms. Precursor ions corresponding to the peaks in the LC-MS/MS chromatograms (Figure 2, lower) were further analyzed by LC-MS/MS in the product ion scan mode to elucidate the peptide primary structure. The observed product ions including the immonium ones are summarized in Table 1. Based on the precursor and product ion scans, 13 pyroglutamyl di- and tri-peptides (pyroGlu-Gly, pyroGlu-Ser, pyroGlu-Thr, pyroGlu-Asp, pyroGlu-Ala, pyroGlu-Pro, pyroGlu-Val, pyroGlu-Ile/Leu, pyroGlu-Glu, pyroGlu-Gln, pyroGlu-Gly-Ser, and pyroGlu-Phe) were identified (peaks a–m in Figure 2, lower). Free pyroglutamic acid (peak x) and its methyl (peak y) and ethyl (peak z) esters were also observed. Adduct formation with acetonitrile and proton were observed (marked with *) for pyroglutamyl esters and pyroGlu-Ile/Leu. In addition, adduct ions generated from 2 molecules of pyroglutamic acid, its esters, and pyroGlu-Pro with single-proton were also observed (marked with **). Most of the peaks observed by LC-MS/MS in the precursor ion scan mode, targeting immonium ions generated from the pyroglutamyl residue, could be assigned to pyroglutamyl peptides and their derivatives.
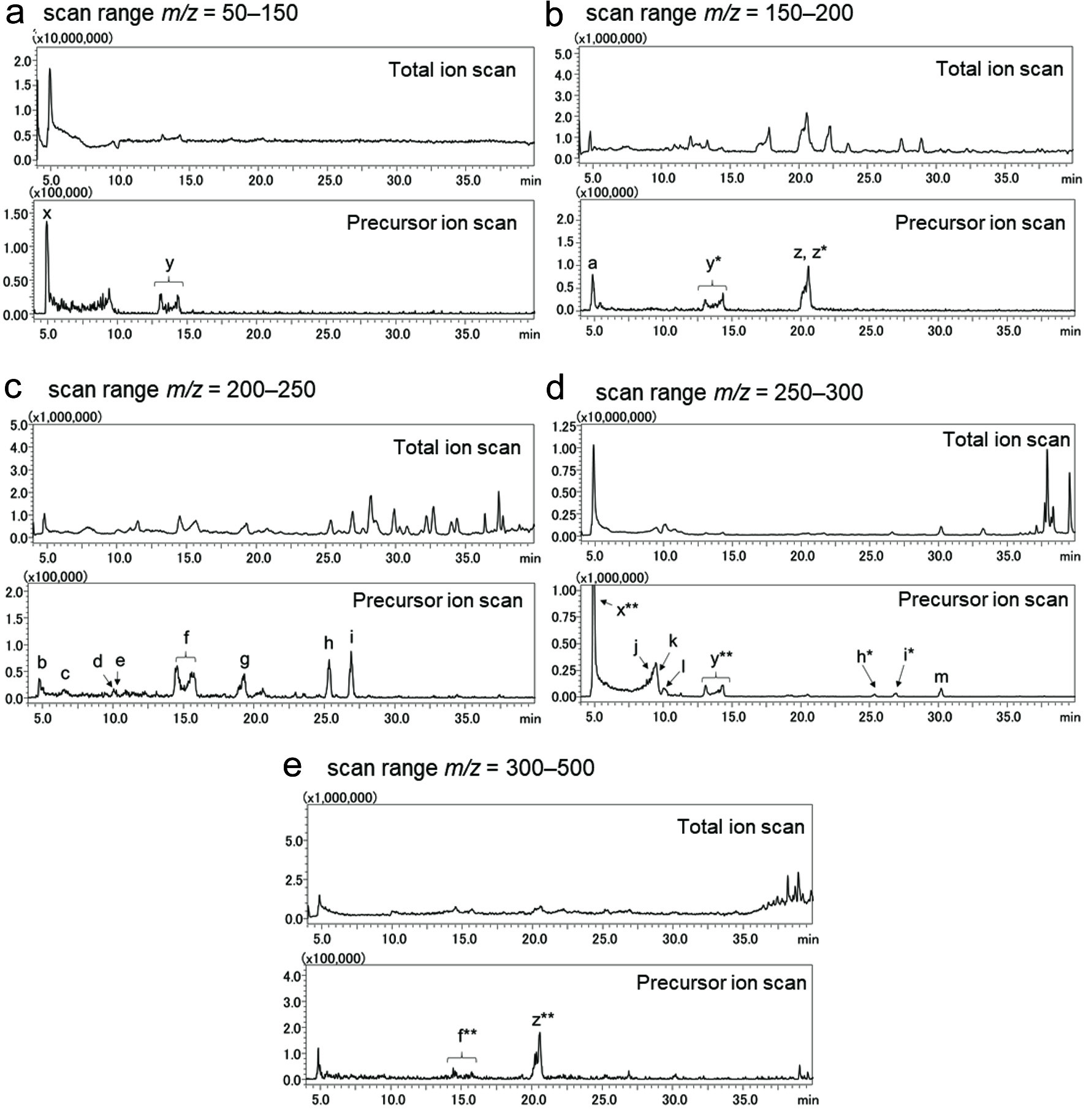 Click for large image | Figure 2. Mass spectrometry chromatograms of pyroglutamyl peptide fraction of soybean miso acquired in the total (upper) and precursor (lower) ion scan modes. Total ion intensity was monitored in the positive mode across the mass to charge ratio (m/z) range of 50–150 (a), 150–200 (b), 200–250 (c), 250–300 (d), and 300–500 (e) (total ion scan). In the precursor ion mode, precursor ions, which generated immonium ion of pyroglutamyl residue (m/z = 84.1), were detected in the positive mode across the same range as the total ion scans. Peaks marked with alphabets (peak a–m and x–y) were further subjected to MS/MS analysis (product ion scan). |
 Click to view | Table 1. Sequences of pyroglutamyl peptides and derivatives in miso |
The presence of pyroglutamyl peptides, including pyroGlu-Ile and pyroGlu-Leu, were confirmed by comparison with retention times of synthetic peptides estimated by LC-MS/MS in the MRM mode. The amounts of pyroglutamyl di-peptides in representative commercially available miso are shown in Figure 3. Approximately 0.05–0.5 μmol/g of pyroglutamyl peptides were present in these miso samples. Short aging type of rice miso showed lower amounts of pyroglutamyl peptides compared to long aging types. Abundances of some hydrophilic pyroglutamyl peptides (pyroGlu-Asp, pyroGlu-Glu, pyroGlu-Gln, and pyroGlu-Gly) and pyroGlu-Pro were higher than those of the hydrophobic peptides (pyroGlu-Val, pyroGlu-Ile, and pyroGlu-Leu).
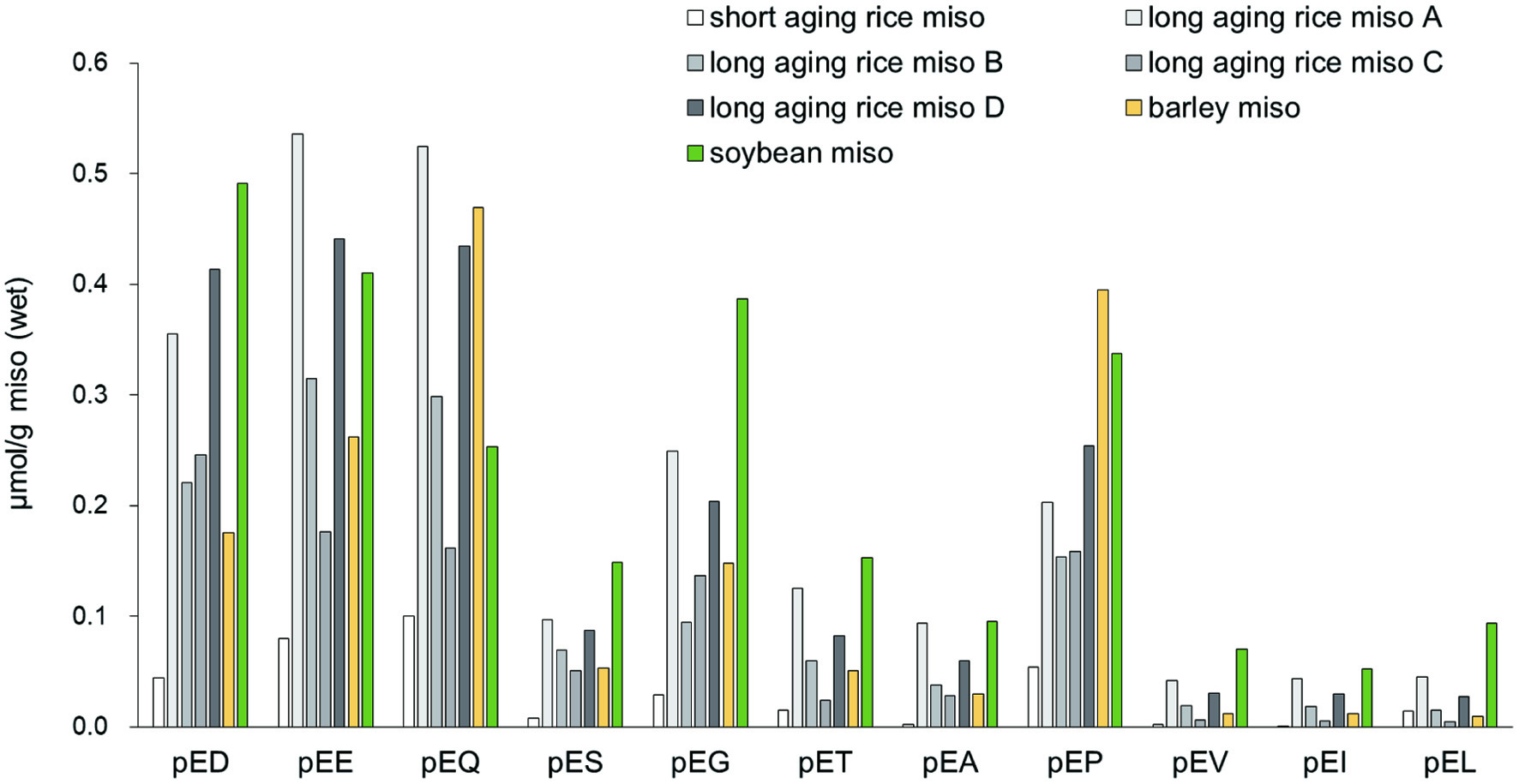 Click for large image | Figure 3. Amount of pyroglutamyl di-peptides in 4 types of miso. Short aging and long aging types of rice miso (A, B, C, and D), barley miso, and soybean miso were analyzed by LC-MS/MS in the MRM mode. Data are shown as the average (n = 3–6). The one-letter codes are used for all amino acid residues. pE represents pyroglutamyl (pE) residue. |
3.2. Effect of water extracts of miso and pyroGlu-Leu on 45% high fat diet-induced obesity
As shown in Figure 1b and c, feeding with 45% high fat diet significantly increased body weight gain in rats compared to those fed on a normal diet without increasing the calorie intake. Administration of crude water extracts of soybean miso (CE) significantly suppressed the high fat diet-induced body weight gain, along with a concomitant significant reduction in calorie intake. ACN administration was associated with significant suppression of high fat diet-induced body weight gain; however, no significant differences were observed in total calorie intake. As shown in Figure 1a, ACN was predominantly composed of pyroGlu-Pro, pyroGlu-Val, pyroGlu-Ile, and pyroGlu-Leu, while pyroGlu-Pro was also found in N-A. N-A administration had no discernible effect on high fat diet-induced body weight gain in the rats.
As shown in Figure 1d and e, administration of pyroGlu-Leu at a 0.1 mg/kg body weight dosage significantly suppressed high fat diet-induced body weight gain in rats fed on 45% high fat diet, without significant reduction in total calorie intake. These data indicate that the hydrophobic pyroglutamyl peptides are, at least partially, responsible for the suppression of high fat diet-induced body weight gain induced by miso consumption. There were no significant differences in plasma AST and ALT level between the groups (data not shown).
3.3. Effect of synthetic hydrophobic pyroglutamyl peptides on weight gain in rats fed on 60% very high fat diet
As shown in Figure 4a, rats maintained on the 60% VHF diet showed a significant increase in body weight gain compared to those fed on normal diet (N) without increasing calorie intake. Increase in weight gain associated with the 60% very high fat diet was higher than that associated with the 45% high fat diet. Mixtures of synthetic pyroGlu-Pro, pyroGlu-Val, pyroGlu-Ile, and pyroGlu-Leu (×1 ACN-pep and ×10 ACN-pep) were administered to the 60% high fat diet-fed rats for 5 weeks. As shown in Figure 4a and b, administration of high dose of synthetic peptide mixtures (×10 ACN-pep) tended to reduce high fat diet-induced body weight gain and total calorie intake (p = 0.07 and 0.05 for body weight gain and total calorie intake, respectively). As shown in Figure 4c and d, administration of pyroGlu-Leu at a 1 mg/kg body weight dosage has a similar suppressive effect on very high fat diet-induced body weight gain, along with a significant reduction in total calorie intake. However, synthetic pyroGlu-Leu administration did not affect body weight gain and total calorie intake in rats fed on a normal diet. Administration of a high dose (×10 ACN-pep) of the mixture of 4 pyroglutamyl peptides tended to decrease liver weight in rats fed on a high fat diet, but did not significantly affect the epididymal adipose tissue weight (Figure 5a and b). Administration of pyroGlu-Leu (1 mg/kg) significantly decreased liver weight in rats fed on a very high fat diet, but not in rats fed on a normal diet (Figure 5c). PyroGlu-Leu also tended to decrease epididymal adipose tissue of the rat fed high fat diet (Figure 5d).
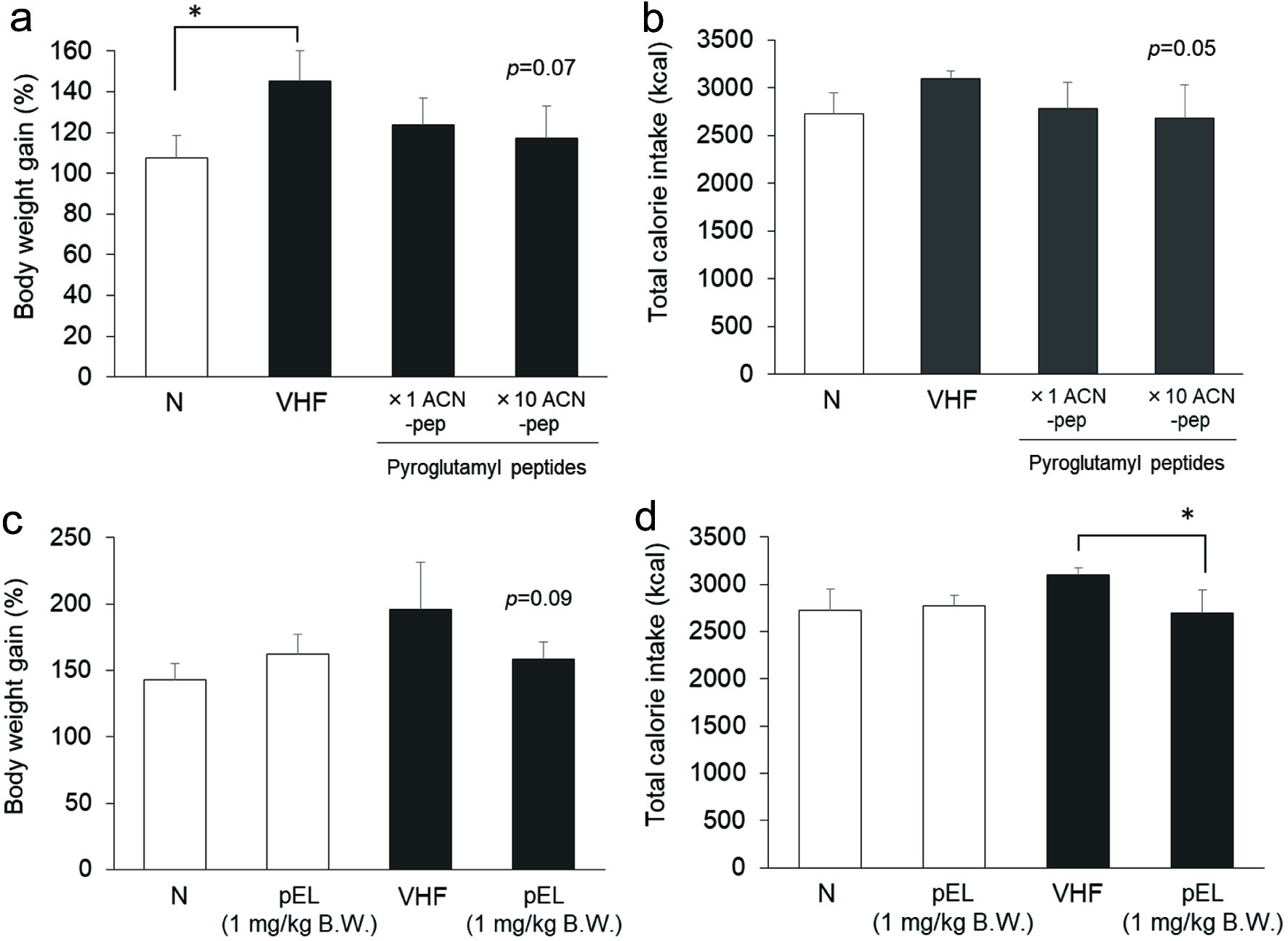 Click for large image | Figure 4. Effect of synthetic pyroglutamyl peptides on weight gain in 60% very high fat diet-fed rats. Rats were fed on normal (open column) and 60% very high fat diet (closed column). Effect of a mixture of hydrophobic synthetic pyroglutamyl peptides (pEP, pEV, pEI, and pEL) at doses corresponding to those in ACN (×1 ACN-pep) and 10 times higher (×10 ACN-pep) on body weight gain (a) and total calorie intake (b) in rats fed on 60% very HF diet (VHF). Effect of synthetic pyroGlu-Leu (1 mg/kg body weight) on body weight gain (c) and total calorie intake (d). Data are shown as mean ± SD (n = 4). For the mixture of hydrophobic pyroglutamyl peptides, * represents significant difference with p < 0.05, as estimated by Dunnett’s test vs VHF. For pyroGlu-Leu, * represents significant difference with p < 0.05, as estimated by t-test between each diet group. |
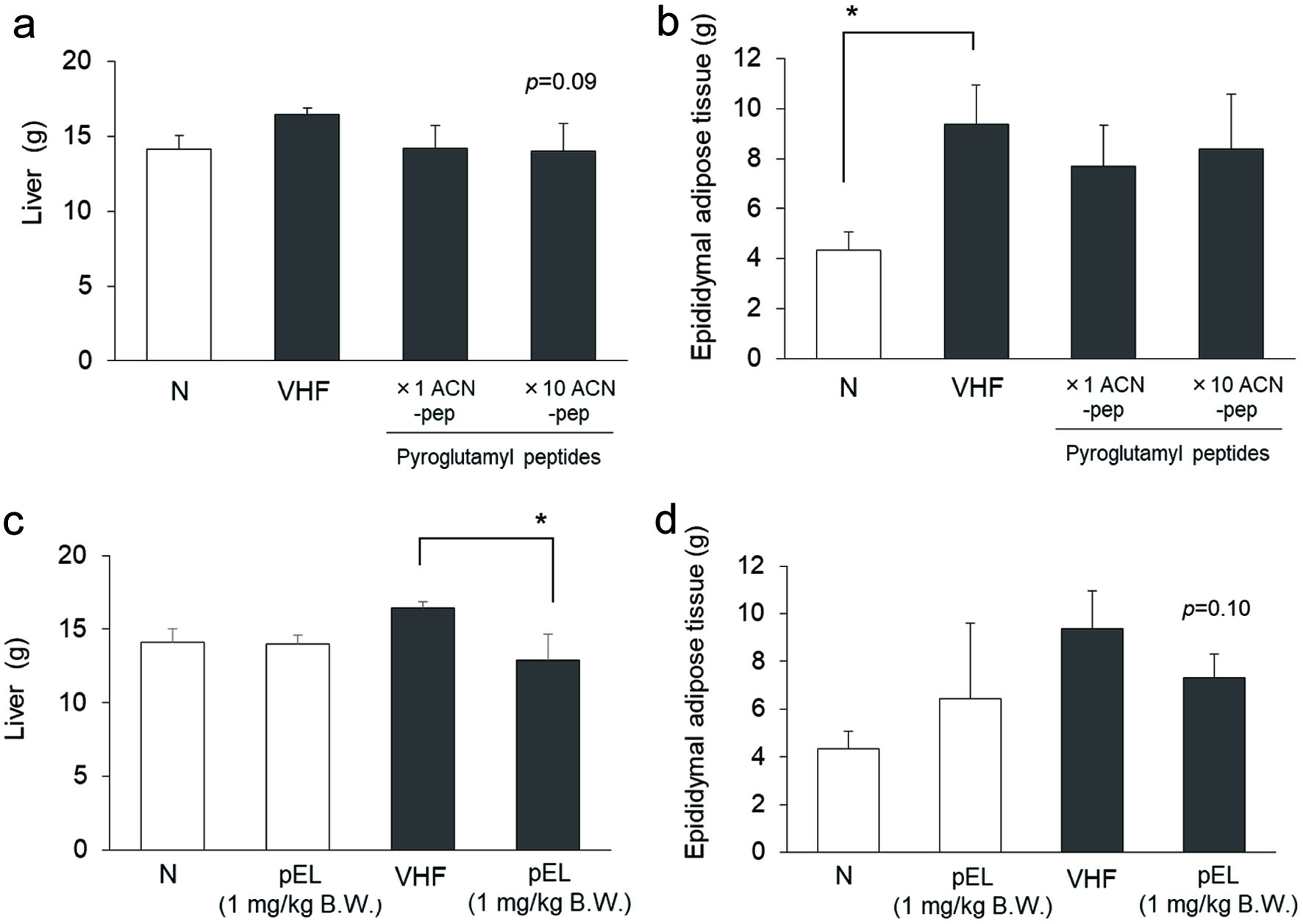 Click for large image | Figure 5. The effect of pyroglutamyl peptides on weight of epididymal adipose tissue and liver in 60% very high fat diet-fed rats. Rats were fed on normal (open column) and 60% very high fat diet (closed column). Refer the legends for Figure 4 for abbreviation. The effect of mixture of hydrophobic pyroglutamyl peptides (pEP, pEV, pEI, and pEL) on weight of liver (a) and epididymal adipose tissue (b). The effect of synthetic pyroGlu-Leu (1 mg/kg body weight) on weight of liver (c) and epididymal adipose tissue (d). Data are shown as mean ± SD (n = 4). For mixture of hydrophobic pyroglutamyl peptides, * represents significant difference with p < 0.05, as estimated by Dunnett’s test vs VHF. For pyroGlu-Leu, * represents significant differences with p < 0.05, as estimated by t-test between each diet group. |
In experiments 3 and 4, no significant differences in plasma AST and ALT levels were observed across the groups, indicating that negligible liver damage was induced in the course of these experiments. The plasma leptin level was significantly higher in the 60% high fat diet group compared to the normal diet one. While administration of synthetic peptide mixtures at high dose (×10 ACN-pep) and pyroGlu-Leu (1 mg/kg) to the very high fat diet group tended to decrease total calorie intake, administration of these peptides did not influence plasma leptin and ghrelin levels (data not shown).
| 4. Discussion | ▴Top |
We have previously demonstrated the presence of pyroglutamyl peptides in food protein hydrolysates (Sato et al., 1998, 2013; Ejima et al., 2018) and the Japanese rice wine, sake (Kiyono et al., 2013). Pyroglutamyl peptides with known structure such as pyroGlu-Leu have also been detected in other fermented foods including miso using LC-MS/MS in the MRM mode (Sato and Kiyono, 2017). However, the unambiguous detection of pyroglutamyl peptides with unknown structures in a complex food matrix such as miso is complicated due to presence of multiple substances in the latter. In this preliminary experiment, compounds present in miso were pre-fractionated using size exclusion chromatography (SEC), followed by separation and detection by LC-MS analysis. However, most of the peaks in the LC-MS chromatograms of SEC fractions could not be assigned to pyroglutamyl peptides (data not shown). To circumvent this problem, LC-MS/MS in the precursor ion scan mode, targeting immonium ion of pyroglutamyl residue (m/z = 84.1), was performed in narrow scan ranges. As shown in Figure 2, all peaks, obtained by precursor ion scans were well resolved and could be assigned to pyroglutamyl peptides and their derivatives (Table 1). Consequently, 13 pyroglutamyl peptides and 11 related ions were identified without the SEC-mediated pre-fractionation step. The precursor ion scan method targeting immonium ion of pyroglutamyl residue could, therefore, be useful for the detection of pyroglutamyl peptides with unknown structures in a complex food matrix.
Okouchi et al. (2019) reported that miso supplementation to normal diet (5% fat) at amounts similar to its proportion in Japanese diet in 1975 significantly decreased epididymal adipose tissue weight and adipocyte sizes of mice compared to control, while there was no significant change in body weight gain. The present study demonstrates that administration of a crude water extract prepared from soybean miso (0.6 g miso/kg body weight of rat) suppressed body weight gain in rats fed on 45% high fat diet. The dosage of 0.6 g miso/kg body weight corresponds to consumption of 3 cups of miso soup/day (3 × 12 g miso/cup/60 kg of body weight). These results suggest that daily intake of miso could have a beneficial effect on human health. The hydrophobic compounds without primary and secondary amines in the crude water extract of miso were responsible for the observed suppression of weight gain (Figure 1b), and contained pyroGlu-Pro, pyroGlu-Val, pyroGlu-Ile, and pyroGlu-Leu; their dose were 10–30 μg/kg body weight/day. Administration of synthetic pyroGlu-Leu at a 100 μg/kg body weight dose, approximately corresponding to the sum of the 4 pyroglutamyl peptides, also suppressed body weight gain in 45% high fat diet-fed rats. Very high fat (60%) diet significantly increased body weight gain compared to the 45% high fat diet. A mixture of the 4 synthetic hydrophobic short-chain pyroglutamyl peptides (×10 ACN-pep) tended to inhibit weight gain in rats fed on the 60% very high fat diet (Figure 4a) at a 10 times higher dose for the 45% high fat diet-fed rats (doses of ACN in Figure 1b and ×1 ACN-pep in Figure 4a). In addition, administration with the mixture of synthetic peptides (×10 ACN-pep) tended to reduce calorie intake, while no significant effects were observed at the same dose for the 45% high fat diet-fed rats (doses of ACN in Figure 1b and ×1 ACN-pep in Figure 4a). PyroGlu-Leu also tended to suppress the increase of body weight at a 10 times higher dose (1 mg/kg body weight) with a significant reduction in calorie intake. On the contrary, pyroGlu-Leu (1 mg/kg body weight) had no significant effect on body weight gain and calorie intake of rats fed on normal diet (12.8% fat). In addition, administration of synthetic pyroglutamyl peptides and miso fraction did not increase plasma AST and ALT levels (data not shown). Moreover, there were no significant differences in water intake (data not shown). Therefore, the reduction in high fat diet-induced body weight gain was not due to toxic effects of synthetic peptides and any related impurities, if present. The short-chain hydrophobic pyroglutamyl peptides are capable of suppressing high fat diet-induced body weight gain with a reduction in calorie intake in a dose-dependent manner, while there is no effect on the body weights of rats fed on normal diet. According to the Ministry of Health, Labour and Welfare in Japan, the average consumption of fat in Japan contributes to approximately 28.3% of the total in calorie intake. Our results suggest that the short-chain hydrophobic pyroglutamyl peptides ingested by daily consumption of miso could prevent obesity in Japanese people induced by a fat-rich diet. A well-designed human clinical trial needs to be performed to prove this hypothesis.
It is difficult to elucidate the mechanism underlying the suppression of body weight gain in rats fed on high fat diet by the chronic administration of a very low dose of peptides. First, the effects of pyroglutamyl peptides on appetite-related hormones were evaluated. Leptin and ghrelin are known to control food intake (Klok et al., 2007). Leptin is a hormone mainly produced by the adipose tissue (Klok et al., 2007), which decreases appetite and body weight (Farooqi et al., 2001; Licinio et al., 2004). On the other hand, ghrelin is a hormone mainly produced by the stomach (Klok et al., 2007), which stimulates food intake and weight gain in rats (Nakazato et al., 2001), and increases appetite in humans (Wren et al., 2000). However, administration of hydrophobic pyroglutamyl peptides did not exert any significant effect on the plasma levels of these hormones at sacrifice (data not shown). However, chronological changes of these hormones in each group were not examined. Further studies on the effects of these short-chain hydrophobic pyroglutamyl peptides on chronological changes in the appetite-related hormones should be examined. Alternatively, the short chain hydrophobic pyroglutamyl peptides might act on vagal nerve and central brain system. Goswami et al. (2018) have reported that injection of short-chain fatty acid (SCFA) suppresses food intake via stimulating vagal afferent signaling, which provides novel target to treat obesity and metabolic syndrome. Furthermore, Kozuka et al. (2012) reported that brown rice reduces the preference for dietary fat in mice. They also revealed that γ-oryzanol in brown rice is responsible for this effect via suppression of high fat diet-induced hypothalamic endoplasmic reticulum stress and brain reward system stimulated by animal fat (Kozuka et al., 2012; Kozuka et al., 2017). Further studies on the effects of these short-chain hydrophobic pyroglutamyl peptides on consumption of high fat diet without going through appetite hormone are now investigated.
A high fat diet has been shown to increase the abundance of Firmicutes and decrease the abundance of Bacteroidetes in gut microbiota in animal models (Zhang et al., 2012; Shirako et al., 2019). Firmicutes is known to increase host energy intake by converting indigestible substances in the gut to substances accessible to the host digestive system (Bäckhed et al., 2005; Turnbaugh et al., 2006). In addition, obese people have lower abundance of Bacteroidetes and higher abundance of Firmicutes in their gut compared to lean people (Ley et al., 2006). Therefore, gut microbiota can modulate obesity. We have earlier demonstrated that the administration of 1 mg/kg body weight of pyroGlu-Leu attenuates high fat (60%) diet-induced dysbiosis in rats by increasing host antimicrobial peptide (Shirako et al., 2019). These facts suggest that administration of short-chain hydrophobic pyroglutamyl peptides present in miso could represent a potential dietary approach to curb obesity via the modulation of gut microbiota. Effects of miso consumption on human gut microbiota need to be examined for this.
| 5. Conclusion | ▴Top |
In this study, a newly developed LC-MS/MS method, based on targeting immonium ion of puroglutamyl residue in the precursor ion scan mode, revealed the presence of 13 types of pyroglutamyl peptides in miso, one of the traditional fermented food popular in Japan. In addition, administration of the hydrophobic pyroglutamyl peptide fraction of miso, including pyroGlu-Pro, pyroGlu-Val, pyroGlu-Ile, and pyroGlu-Leu, suppressed weight gain in 45% high fat diet-fed rats. The administration of pyroGlu-Leu at an approximate sum of the dosage of short-chain hydrophobic pyroglutamyl peptides (0.1 and 1 mg/kg body weight) also showed a similar suppressive effect in rats fed with high (45%) and very high fat (60%) diets. These findings suggest that the intake of hydrophobic pyroglutamyl peptides obtained by daily consumption of miso could be potentially useful to counter obesity.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing. This study was supported by the Integrated Research for Agriculture and Interdisciplinary Fields, Ministry of Agriculture, Fisheries and Forests, Japan (grant number 14532022).
| References | ▴Top |