| Journal of Food Bioactives, ISSN 2637-8752 print, 2637-8779 online |
| Journal website www.isnff-jfb.com |
Review
Volume 10, June 2020, pages 32-46
Chemistry and biochemistry of dietary carotenoids: bioaccessibility, bioavailability and bioactivities
Cheng Yanga, b, Lianfu Zhanga, b, Rong Tsaoc, *
aState Key Laboratory of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu, 214122, China
bSchool of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu, 214122, China
cGuelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada
*Corresponding author: Rong Tsao, Guelph Research and Development Centre, Agriculture and Agri-Food Canada, 93 Stone Road West, Guelph, Ontario N1G 5C9, Canada. Tel: +1 226 217 8180. Fax: +1 226 217 8183. E-mail: Rong.Cao@Canada.ca
DOI: 10.31665/JFB.2020.10225
Received: April 10, 2020
Revised received & accepted: June 26, 2020
| Abstract | ▴Top |
Carotenoids are one of the major food bioactives that provide humans with essential vitamins and other critically important nutrients that contribute to the maintenance of human health. This review provides a summary of the most current literature data and information on most recent advances in dietary carotenoids and human health research. Specifically, it addresses the occurrence and distribution of carotenoids in different dietary sources, the effect of food processing and impact of gastrointestinal digestion on the bioaccessibility, bioavailability and bioactivities of dietary carotenoids. Emphasis is placed on the antioxidant and anti-inflammatory effects of carotenoids and their optical/geometric isomers and ester forms, and the molecular mechanisms behind these actions. A particular focus is also on the modulatory ability of carotenoids on biomarkers related to low-grade inflammation, immune response and gut health.
Keywords: Carotenoids; Bioaccessibility; Bioavailability; Antioxidant activity; Anti-inflammatory effect; Low-grade inflammation
| 1. Introduction | ▴Top |
Carotenoids are bioactive compounds that belong to the class of isoprenoids with specific colors such as yellow, orange and red. More than 700 carotenoids have been found thus far, although only ca. 10–15 carotenoids are present in our diets and found in measurable concentrations in human blood and tissues (Pietro et al., 2016). In this review, the main focus will be on research of the most consumed carotenoids, i.e. α/β-carotene, lycopene, lutein, zeaxanthin and astaxanthin in foods and food products.
Carotenoids are C40 isoprenoid polyene compounds, a group of secondary plant metabolites that can be divided into two groups: the hydrocarbon carotenes such as β-carotene, α-carotene, lycopene and the oxygenated carotenoids, xanthophylls, such as lutein, zeaxanthin and astaxanthin (Figure 1). They are one of the major food bioactives that not only provide humans with essential vitamins but contribute to the maintenance of human health. Although the physicochemical properties of carotenoids have been studied extensively, their bioavailability, metabolism and biological functions and further applications in foods or dietary supplements are still hot topics of research. Particularly, in recent years, attention has been drawn to the geometric isomers of different carotenoids because of their higher bioavailability in human body (Yang et al., 2017). Xanthophylls are of more interest because of their stronger antioxidant activity and potential role in lowing risk of chronic diseases. Xanthophylls are often found in ester forms in plants, algae and marine life.
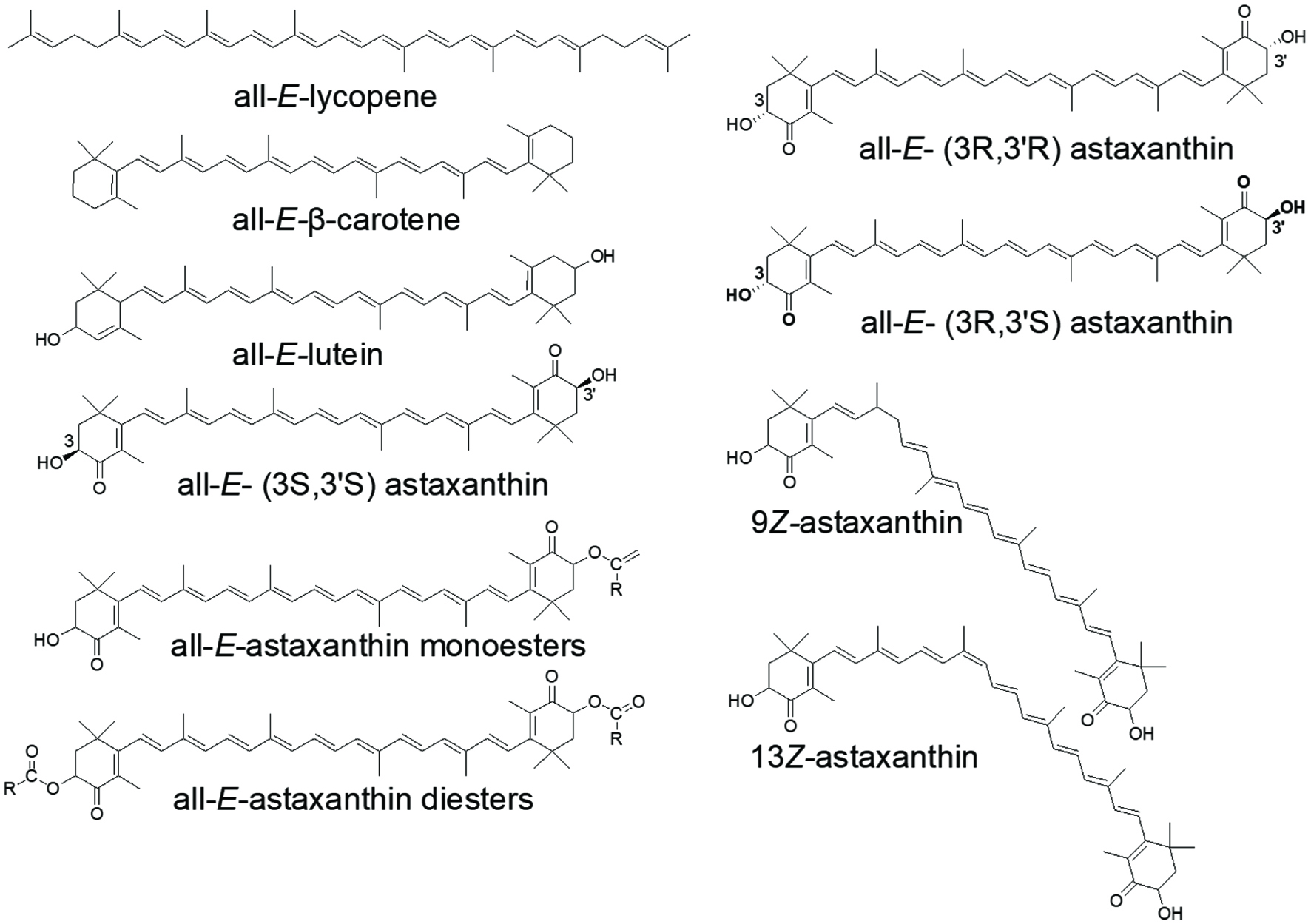 Click for large image | Figure 1. Common dietary carotenoids and different isomeric structures in free and ester forms of carotenoids as represented by astaxanthin. R represents saturated or unsaturated alkyl chains. |
In this contribution, the authors intend to review the new knowledge reported in recent literature in the areas of carotenoid chemistry and biochemistry. The main topics cover the geometric and stereoisomers, ester forms and protein complex, effect of processing and extraction, analytical methods, and updated information for carotenoid bioaccessibility, bioavailability, intestinal uptake mechanism and biological functions related to health, particularly inflammation and intestinal health.
| 2. Dietary carotenoids | ▴Top |
2.1. Biosynthesis and occurrence
The biosynthetic pathway of carotenoids in plants is depicted in Figure 2. It starts with the condensation of two C20 geranylgeranyl pyrophosphate (GGPP) molecules which are synthesized from isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by GGPP synthase in the methylerythritol 4-phosphate (MEP) pathway to form the first C40 tetraterpene, the colorless phytoene via phytoene synthase. Then, phytoene desaturase, ζ-carotene desaturase and carotenoid isomerase (CRTISO) separately convert phytoene to ζ-carotene and further the red colored lycopene. The pathway is then branched into two paths from lycopene including different cyclization reactions catalyzed by lycopene ε-cyclase (LCYE) and lycopene β-cyclase (LCYB), respectively. The cyclization of both ends of the linear lycopene by LCYB generates carotenoids with two β-rings such as β-carotene, while the coordinated action of LCYE and LCYB produces carotenoids with one β-ring and one ε-ring such as α-carotene (Rodriguez-Concepcion et al., 2018). The xanthophylls are then derived from oxygenated α-carotene and β-carotene by the ring-specific hydroxylation. Hydroxylation of α-carotene produces zeinoxanthin or α-cryptoxanthin and further to produce lutein by ring hydroxylase (enzyme CYP97C or/and CYP97A). Hydroxylation of β-carotene produces β-cryptoxanthin and then zeaxanthin by carotene β-hydroxylase. Zeaxanthin can be transformed to antheraxanthin and violaxanthin by epoxidation reactions, and the de-epoxidation reaction could also convert violaxanthin to zeaxanthin with antheraxanthin as the intermediate with the help from the enzymes called violaxanthin de-epoxidase (VDE) and zeaxanthin epoxidase (ZEP). Violaxanthin could also be transformed to neoxanthin by neoxanthin synthase and further to abscisic acid (ABA).
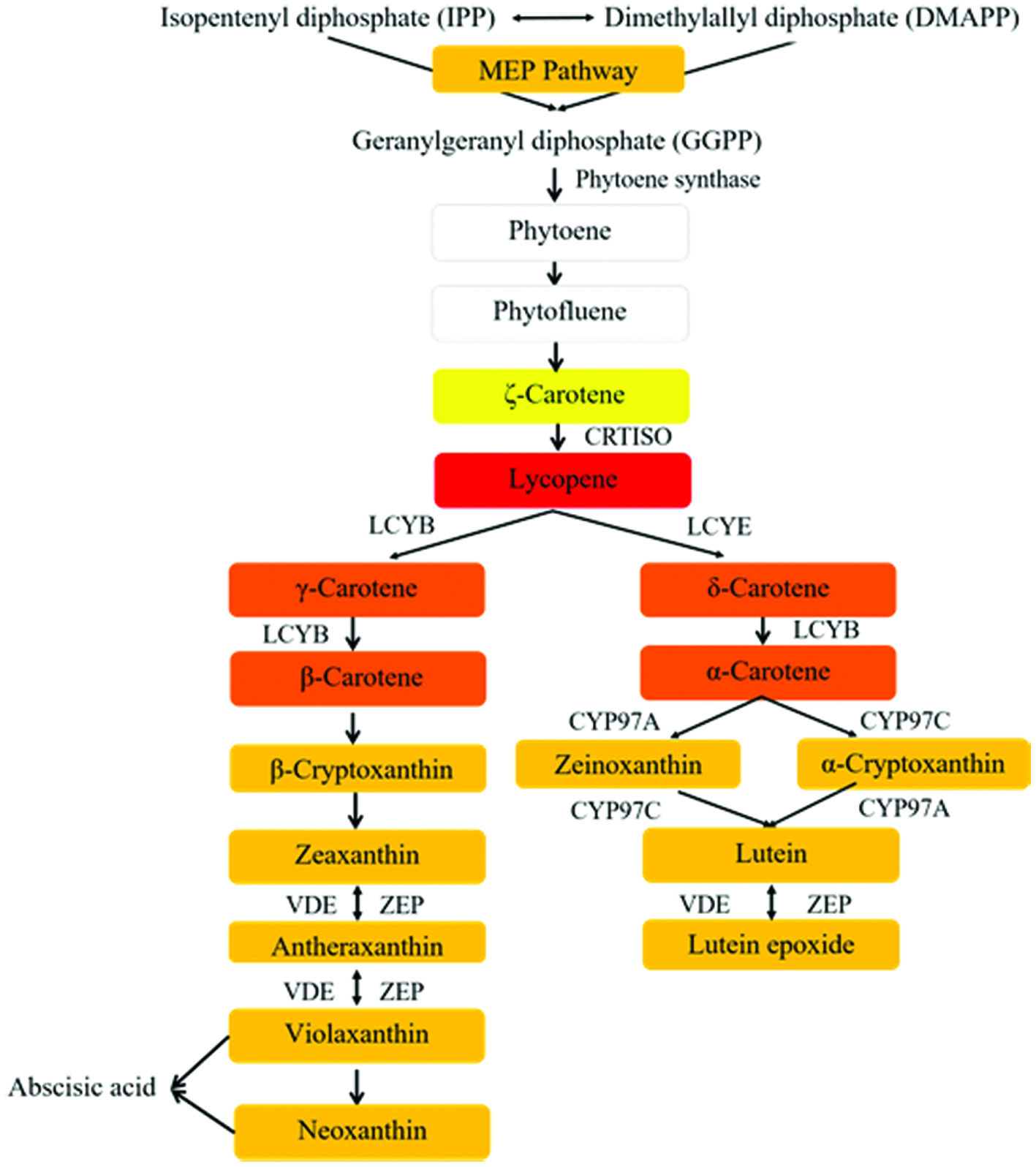 Click for large image | Figure 2. The biosynthetic pathway of carotenoids in plants. The colors of the frames represent the appearance of different compounds. GAP represents glyceraldehyde 3-phosphate; MEP, methylerythritol 4-phosphate; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate. CRTISO, carotenoid isomerase; LCYB, lycopene β-cyclase; LCYE, lycopene ε-cyclase; CYP97C, carotene ε-hydroxylase; CYP97A, cytochrome P450 carotene β-hydroxylase; ZEP, zeaxanthin epoxidase; VDE, violaxanthin de-epoxidase. |
The biosynthesis of carotenoids in bacteria and archaea is different from that in plants. While both pathways start from GGPP to give lycopene, bacteria employ only a single enzyme, the bacterial phytoene desaturase for the catalysis (Rodriguez-Concepcion et al., 2018). Bacterioruberin, a C50 carotenoid derivative is synthesized in archaea using GGPP, so are other carotenoids such as spheroidenone, okenone, isoreniaratene. The geometric isomers of bacterioruberin, namely, 5-cis (Z-), 9Z-, 13Z-, and all-trans (E)-bacterioruberin isomers are produced in Halobacterium salinarum and accounted for 13, 4, 11, and 68%, respectively of the total carotenoids (Mandelli et al., 2012). Additionally, carotenoids from cyanobacteria, a photosynthetic bacteria, are mainly β-carotene, zeaxanthin, canthaxanthin, β-cryptoxanthin instead of lutein, violaxanthin or neoxanthin that are abundant in eukaryotic photosynthetic species.
The carotenoid biosynthesis pathway in microalgae is more diverse and produces downstream products such as β-carotene, lutein, zeaxanthin, violaxanthin, diatoxanthin, fucoxanthin and neoxanthin from lycopene (Henríquez et al., 2016). Different microalgae can produce unique carotenoids or higher amount of a specific carotenoid over others. Cultural conditions can also affect the biosynthesis of certain carotenoids in alga. For instance, the stress conditions including nitrogen deprivation or strong solar irradiation or hyper salinity can cause the accumulation of astaxanthin in Haematococcus pluvialis, β-carotene in Dunaliella salina, and lutein in Muriellopsis sp. and Scenedesmus almeriensis (Del Campo et al., 2007). Research also found that accumulation of carotenoids under stress is often co-occurring with massive production of lipids (Solovchenko, 2012).
Carotenoids are also synthesized by certain fungi, for example neurosporaxanthin, torularhodin, astaxanthin and β-carotene with β-carotene and lycopene are predominant products found in Mucoromycotina such as Blakeslea trispora. β-Carotene is also found in Ustilago maydis and Cercospora nicotianae. A dietary xanthophyll astaxanthin is produced by yeast species such as Xanthophyllomyces dendrorhous and Rhodotorula sp. The biosynthesis of astaxanthin in fungi is considered to be in the branch of the β-carotene side (Rodriguez-Concepcion et al., 2018).
In recent years, genetic engineering techniques have been applied to increase the production or target specific carotenoids in crop plants, microbes and even in arthropods such as hemipteran, dipteran insects and mites (Rodriguez-Concepcion et al., 2018). Astaxanthin for example is a red pigment carotenoid with higher antioxidant activity thus economic value than most other dietary carotenoids such as β-carotene and lycopene. For this reason, astaxanthin has been frequently set as target end-product for metabolic engineering of plants with the endogenous carotenoid pathway. Huang et al. reported that a transgenic tomato plant can produce free (3.12 mg/g in leaves) and esterified astaxanthin (16.1 mg/g in fruits), a 16-fold increase of total carotenoid content (Huang et al., 2013). Tobacco (Nicotiana tabacum) expressing both genes encoding CrtW (β-carotene ketolase) and CrtZ (β-carotene hydroxylase) from a marine bacterium Brevundimonas sp., strain SD212 was able to produce astaxanthin at 0.5% on a dry weight (DW) basis (Hasunuma et al., 2008). Using the same technique, a transgenic potato (Solanum tuberosum) expressing the 4, 4′ β-oxygenase (crtW) and 3, 3′ β-hydroxylase (crtZ) genes from Brevundimonas spp. produced ketocarotenoids such as astaxanthin with significantly higher yield, from the 13% in the wild-type to 45% in the transgenic tubers (Mortimer et al., 2016). Other crops such as sweet potato and oil seeds, could also produce non-native carotenoids or higher amount of its endogenous carotenoids through genetic engineering (Park et al., 2015).
Since humans cannot synthesize carotenoids, we can only rely on dietary sources to meet the nutritional needs for carotenoids, beyond that of the provitamin A carotenoids. Fruit, vegetables and cereals as plants, and certain fungi and marine microalgae are among the most consumed dietary sources rich in carotenoids. Distribution of common carotenoids and their predominant isomers in selected foods are listed in Table 1.
 Click to view | Table 1. Quantitative distribution of common carotenoids and their predominant isomers in selected dietary foods |
2.2. Carotenoids in plants
The color (chromophore) of carotenoids depends on the conjugated carbon–carbon double bonds in the chemical structure. Usually, the existence of carotenoids in fruit or vegetables can be assumed from the distinctive yellow, orange or red color. For example, we now know the red pigment in tomato is from lycopene; the yellow, orange-red color of carrots or pumpkin are from the α-carotene and β-carotene, or lutein. Green leafy vegetables such as kale and spinach can contain very high lutein, at 12.3 mg/100 g FW and 3.7 mg/100 g FW, respectively (Tsao and Yang, 2006). Carotenoids are found in free or esterified forms in plants, and are mostly in all-trans configuration (Figure 1). All-E-lycopene and β-carotene are the most predominant isomers that naturally present in fruits and vegetables. All-E-β-carotene, and its 9Z-, and 13Z-isomers are found in yellow sweet cassava (Carvalho et al., 2012) (Table 1). Study showed that the all-E-lycopene was the predominant carotenoid in 20 tomato cultivars in Canada, ranging from 6.17 to 218 μg/g DW. In addition, all-E-lutein, 9Z-lutein, 13Z-lutein, 5Z-lycopene, 9Z-lycopene, 13Z-lycopene, 15Z-lycopene, di-Z-lycopene, all-E-β-carotene, 9Z-β-carotene, 13Z-β-carotene, 15Z-β-carotenes and di-Z-β-carotene were also found in these tomato cultivars (Li et al., 2012). Lutein used in most dietary supplements is prepared from marigold flowers, which naturally contain high levels of lutein mono- and diesters (Tsao, 2006; Tsao et al., 2004).
Carotenoids may not be distributed evenly in a fruit or vegetable and their content and composition may also change during fruit ripening (Lado et al., 2016). Tsao and Yang, (2006) found that lutein and β-carotene in the flesh of squashes were significantly lower than that in the peel (Tsao and Yang, 2006). Study shows that the formation of xanthophyll esters is closely related to ripening. The transformation of chloroplast (in green fruits) into chromoplast (in ripe fruit) occurs during the ripening and the acylation of xanthophylls is beneficial to its incorporation into chromoplasts through increased liposolubility (Minguez-Mosquera and Hornero-Mendez, 1994).
The greater attention given to the well-known major carotenoid pigments may have missed some opportunities for some minor intermediates of the carotenoid pathway. For instance, in raw and processed tomato products, in addition to lycopene, there are significant quantities of other carotenoids including β-carotene, phytoene, and phytofluene. The colorless phytoene and phytofluene are often ignored, however their bioaccessibility, bioavailability and nutritional/physiological effects have been gaining increased attention in the past decade (Mapelli-Brahm et al., 2017).
The biosynthesis and occurrence of carotenoids in plants have been extensively studied due to its importance in human diet and nutrition. However, many challenges still remain. Questions on the health impact of esterification pattern of xanthophylls, how to direct profile changes and distribution during carotenoid biosynthesis, and rational usage of genetic engineering for improved carotenoid composition and quantity still need to be answered. These new knowledge will help improve the nutritional quality and nutrient density in foods and dietary supplements.
2.3. Carotenoids in algae
Microalgae are ubiquitous in nature as the world largest group of photosynthetic organisms capable of survival using CO2 as the sole carbon source and light as energy. There are significant amount of undescribed microalgae species, however among the already known approximately 60,000 species, only a countable number of microalgae are currently harnessed for commercial use (Henríquez et al., 2016).
Carotenoids are generally accounted for 8–14% of the biomass in microalgae (Henríquez et al., 2016). Insofar Dunaliella sp. and H. pluvialis have been used for commercial scale production of β-carotene and astaxanthin, respectively (Rodriguez-Amaya, 2016). The species D. salina is a unicellular, bi-flagellate and halotolerant green alga with no cell wall. It is the richest natural source of β-carotene, and when exposed to high solar irradiation or nutrient starvation, its production of β-carotene can be over 10% DW (Lamers et al., 2008). Similarly, H. pluvialis is the richest natural source of astaxanthin. It can accumulate lutein and β-carotene when growing under favorable conditions, however, once exposed to stressed conditions such as nitrogen deprivation and intense solar irradiation, the cells stop to divide and start to be transformed to aplanospores, which can produce the red pigment astaxanthin up to 1.5–4% DW (Tanaka et al., 2012) and lipids up to 35% DW (Saha et al., 2013). It was also found that all-E-astaxanthin and its cis- and optical isomers also existed in ester forms in the dark red aplanospores (Shah et al., 2016; Yuan and Chen, 1997). Another alga, Chlorella zofingiensis can also synthesize keto-carotenoids including astaxanthin under photoautotrophic, heterotrophic, and mixotrophic conditions (Liu et al., 2014).
Microalgae such as Muriellopsis sp., C. zofingiensis, Scenedesmus sp. and Chlorella protothecoides have been explored for producing lutein (Sun et al., 2018). Some brown algae or diatoms have been studied for producing unique xanthophylls such as diatoxanthin, diadinoxanthin and fucoxanthin (Bertrand, 2010; Mulders et al., 2014). Moreover, genes such as phytoene synthase from D. salina (Couso et al., 2011) and C. zofingiensis (Fernández Cordero et al., 2011) have been expressed in other species to improve the yield of lutein through genetic engineering. Details on content of bioactive carotenoids in microalgae species can be found in recent literature (Matos et al., 2017). Microalgae such as Chlorella, Dunaliella, Haematococcus, Spirulina and Schizochytrium species containing high concentrations of bioactives such as carotenoids, fatty acids and protein are recognized as GRAS (Generally Regarded as Safe) by the U.S. Food and Drug Administration (García et al., 2017).
As new species of carotenoids continue to be discovered, and more microalgae are being explored for their potential health benefits to humans, the next challenge is to focus on the optimization of cultural conditions of algae and adoption of novel approaches such as by genetic engineering for developing high carotenoid yielding microalgae and commercially viable functional foods and nutraceuticals.
2.4. Carotenoids in marine life
In addition to the algal species, carotenoids and/or carotenoproteins exist in crustaceans (shrimp, lobster, crab), crayfish, trout, salmon, redfish, red snapper, tuna, mollusks (mussel, clam), squid, octopus, sea cucumber, sea bream, Antarctic krill, Pacific herring, pink salmon, sponges, star fish, sea urchin, sea anemones, corals endowed the marine life, with distinctive colors (Cong et al., 2019; Shahidi and Brown, 1998). The distribution and content of carotenoids can vary at different growth stages of marine lives (Persia Jothy et al., 2019), and in the gonads of scallops of different genders (Tan et al., 2020).
Although astaxanthin is not commonly found in plants, it is the major and most valued xanthophyll in marine lives. For instance, crustaceans contain large amounts of astaxanthin in their shells and eggs, significantly higher than that in the flesh (muscle) (Hornero-Méndez, 2019). In some cases, astaxanthin is in a form of carotenoprotein complex and is only released by cooking (Shahidi and Brown, 1998). Astaxanthin is also found in esterified forms with EPA (eicosapentaenoic acid) or DHA (docosahexaenoic acid) in crustaceans (Hornero-Méndez, 2019). In addition, optical (3R, 3′R and 3R, 3′S isomers) and geometric (9Z-, 13Z-, 15Z-, di-Z) isomers of astaxanthin are also found in crustaceans (Yu and Liu, 2020) (Figure 1).
Research has also been carried out to recover carotenoids from the wastes such as shells, shrimp wastes and wastewaters generated from the seafood processing industry. This has been considered a potential way to acquire carotenoids or other highly bioactive molecules for commercialization (Amado et al., 2016; Cahú et al., 2012).
| 3. Characterization of carotenoids | ▴Top |
3.1. Extraction, separation and detection
Different methods have been applied to efficiently extract carotenoids, including oil extraction, organic solvents, ionic liquids, supercritical CO2, and assistance of high pressure, microbial fermentation, enzyme- (Catalkaya and Kahveci, 2019), microwave- or ultrasound-assisted extractions (Routray et al., 2019).
Some extraction techniques include enrichment or purification as well. Liquid-liquid extraction, solid-phase extraction (SPE) with silica or reversed-phase materials such as C18 alkyl-chains can be used to extract and purify carotenoids (Bohn, 2019). Hollow fiber liquid-phase microextraction with a mixed solvent (1-octanol:1-undecanol = 6:4, v:v) was employed to extract lutein from egg yolk; a method that is low-cost, sensitive and easy-to-operate, although not suitable for the industrial extraction (Wang et al., 2016). Recently, pulsed electric field (PEF) and moderate electric field (MEF) assisted extractions have been reported for high extraction yield of carotenoids albeit currently they are only limited to laboratory-scale (Saini and Keum, 2018).
In terms of solvent extraction, organic solvents including dimethylsulfoxide (DMSO), tetrahydrofuran (THF), ethanol, methanol, acetone, isopropanol, hexane and their mixtures have been mostly used. The selection of extraction solvent can depend on sample properties such as the matrixes, the state of the samples (solid or liquid), and the polarity of carotenoids to be extracted. In some cases, treatment with acids or alkalis is applied to digest the exoskeleton or saponify carotenoid esters for carotenoid extraction from crustacean shells, and ultrafiltration for the astaxanthin shrimp cooking wastewater (Amado et al., 2016).
More eco-friendly (green) and efficient extraction methods are being developed for the extraction of bioactive carotenoids. Vieira et al. (2017) reported a single-step method using non-ionic surfactant Tomadol 25-7 that had higher selectivity and less contaminants in extracting carotenoids from brown macroalgae than using ethanol (Vieira et al., 2017). Most recently, naturally occurring sulphides such as isothiocyanates, allyl isothiocyanate and polysulfides have been used as additive to improve the extraction efficiency of carotenoid (astaxanthin, adonirubin, adonixanthin) from Paracoccus carotinifaciens (Honda et al., 2020). The study showed that isothiocyanates and polysulfides can further act as a trans-cis-transformation catalyst favoring the solubility of Z-carotenoid isomers over the all-E-isomers.
The majority of carotenoids absorb lights in the visible (Vis) region of the spectrum with characteristic three-headed absorption maximum between 400–500 nm. cis-Isomers of carotenoids normally have an additional absorption peak in the ultraviolet (UV) region (330–380 nm) depending on the position of the cis double bonds and the type of carotenoids. The UV-vis spectrum and absorption maximum are thus most frequently used to detect and even identify different carotenoids, and the absorption coefficients for quantification. Q-ratio (the absorbance ratio of the cis peak to the middle maximum absorption peak) is used for identifying the cis-isomers (Tsao et al., 2004). UV-Vis absorption has been the conventional detection method for carotenoids in an extract, however, UV-vis spectrum alone is not sufficient to discriminate carotenoids from other compounds with similar chromophores, nor is able to detect those in samples with different geometric or other isomeric configurations.
Carotenoids in an extract must be separated and purified before they can be accurately identified, and this is achieved by applying the various chromatographic techniques. While in-depth review of all separation and detection techniques is beyond the scope of this review, combinations of high performance liquid chromatography (HPLC) or ultra-HPLC (UHPLC) with a diode array detector (DAD) and other hyphenated techniques such as LC coupled with mass spectrometry (MS) and nuclear magnetic resonance (NMR) are worth mentioning as they are particularly important for identification and quantification of the low concentrations of carotenoids and metabolites in biological samples (Yang et al., 2018; Yang et al., 2017). Both C18 and C30 columns have been applied to HPLC separation (Melendez-Martinez et al., 2013; Sun et al., 2016; Yang et al., 2019).
UHPLC systems operate under very high pressure using columns packed with sub-2 μm particles thus offer faster separation of compounds with high resolution (Eriksen et al., 2017). A ultra-high performance supercritical fluid chromatography-MS (UHPSFC-MS) method was reported to separate carotenoids within less than 6 min using a 1-aminoanthracene (1-AA) column (Jumaah et al., 2016). An on-line method coupling the supercritical fluid extraction and supercritical fluid chromatography with triple quadrupole mass spectrometry detection (SFE-SFC-QqQ/MS) has shown to separate and detect carotenoids and apocarotenoids in yellow tamarillo that contained free carotenoids, carotenoid esters and apocarotenoids (Giuffrida et al., 2018). This method is also considered highly efficient for analyzing different carotenoids in human blood samples without preliminary treatment (Zoccali et al., 2018).
3.2. Stability and effect of processing
Different food processing technologies such as thermal, non-thermal, chemical and physical treatments may significantly affect the stability of carotenoids in foods or food products. Thermal treatments include steaming, roasting, boiling, frying and microwave, and non-thermal treatments include high pressure, high-intensity pulsed electric fields and ultrasound processing, may have positive or negative impact on the stability and bioavailability of carotenoids depending on the methods (Cilla et al., 2018). Thermal processing generally lower the carotenoid contents but in different degrees. Boiling was found to decrease the total carotenoids in potato by 92% compared to baking (88%), and lutein was relatively more stable (decreased by 24–43%) than β-carotene (decreased by 78–83%) during thermal processing (Kotikova et al., 2016). Heating treatment not only causes degradation, but leads to isomerization of all-E-carotenoids to cis-isomers (Le Bourvellec et al., 2018). Other food ingredients may contribute to the isomerization of carotenoids during cooking. A recent study showed that cooking with onion, which contains disulfide compounds could facilitate the isomerization of lycopene; increased cooking time and onion content resulted in higher production of 5Z-, 9Z- and 13Z-lycopene in sofrito (de Alvarenga et al., 2017). Similar results have been reported for other tomato-based food products (Honda et al., 2018; Yu et al., 2019). However, all-E-carotenoids are structurally more stable than their cis-isomers. Reverse isomerization from 15Z-carotenoid isomers to all-trans configuration was found to occur in light-harvesting complexes of photosynthetic organisms upon light-induced excitation (Koyama and Fujii, 1999). These findings suggest the mechanisms of the trans-cis and cis-trans isomerization need to be further studied.
In addition to isomerization, conventional thermal pasteurization and ultrasound treatment also caused de-esterification and further degradation of carotenoids profiles in golden berry (Physalis peruviana L.) puree (Etzbach et al., 2019). High-pressure processing on whole-peeled orange fruits before juicing could increase the concentration of certain carotenoids in the juice (De Ancos et al., 2020). Acidic conditions and metal ions in carotenoid-containing foods or beverages could also affect their stability (Yang et al., 2017).
The instability of carotenoids under light or heat is due to the lack of protection by cell walls or embedding system in plant foods (Soukoulis and Bohn, 2018). A study has shown that built-in biomass could greatly maintain the stability of astaxanthin by the thick cell wall of H. pluvialis but not that in its acetone extract (Gouveia and Empis, 2003). Besides, the lipid-core nanocapsules loaded with β-carotene, α-carotene and lutein offered a greater stability of the carotenoids than ethanol extract upon exposure to heat and UV-vis light (da Silva et al., 2017). The presence of lipid or unsaturated fatty acids could also affect the stability of carotenoids. Lycopene and β-carotene in tomato puree and α-carotene and β-carotene in carrot puree containing 5% olive oil were found to be stable with ≥ 97% retention after 6 months of storage in the dark at 20, 30 and 40 °C (Mutsokoti et al., 2017). These indicate that content and composition of lipid and food matrix can affect the stability and degradation of carotenoids.
Nonenzymatic oxidation and isomerization often co-occur to both Z- and E-isomers of carotenoids (Yang et al., 2017). The isomerization of carotenoids can slightly reduce the color saturation, while the oxidation lead to complete color loss of carotenoids. A recent study showed that when exposing to gaseous ozone (80 mg/min) for 5 min, the β-carotene content was significantly reduced because of the oxidation (Mohammadi et al., 2017).
Overall, the effects of food processing and different factors involved in the various processing technologies on the stability of carotenoids are complicated and multifaceted. Further research into the effects of food processing on the bioaccessibility and bioavailability of carotenoids are important as they determine the health and nutritional values.
3.3. Bioaccessibility and bioavailability
The absorption of the lipophilic carotenoids generally include three key steps: (1) release from the food matrix and transfer into the emulsion and mixed micelles during digestion; (2) uptake by the enterocyte; (3) enterocyte transport and packaging into the chylomicrons for secretion into the lymph. Bioaccessibility is defined as fraction of ingested component released from food matrix and available for intestinal absorption, and bioavailability is termed as fraction of ingested component available for utilization in normal physiological functions (Guerra et al., 2012). Therefore, food composition and matrix before ingestion, during digestion and the final metabolism can all impose significant impact on the bioaccessibility and bioavailability of carotenoids in humans.
The bioaccessibility and bioavailability of carotenoids can depend on the molecular structures, such as the different geometric isomers like Z- and all-E forms, optical isomers and patterns of esterification. Studies have shown that Z-astaxanthins and Z-luteins exhibit higher bioaccessibility than their all-trans counterparts during the in vitro gastrointestinal digestion and 9Z-astaxanthin exhibits higher transport efficiency than all-E- and 13Z-astaxanthins in a Caco-2 cell monolayer model, implying a higher bioavailability. The higher solubility of Z-carotenoids is one important factor that lead to better micellization of these isomers with bile salts and pancreatin in the intestinal phase, resulting in improved bioaccessibility (Yang et al., 2018; Yang et al., 2017). Similar results were found for Z-lycopenes in different test models (Failla et al., 2008; Sun et al., 2016). In general, bioaccessibility of different carotenoids are in a decreasing order as follows: phytoene and phytofluene > lutein > β-carotene > lycopene. However, the bioaccessibility and bioavailability of different carotenoids depend on the mechanisms of cellular uptake and transport and models used as well (Mapelli-Brahm et al., 2019). Carotenoid esters generally need to be hydrolyzed prior to absorption because the esters are seldom detected in biological samples, but the effect of esterification pattern on carotenoid bioaccessibility and bioavailability is presently not clear. Contradictory results have been reported for the different bioaccessibilities reported for free carotenoids and their esters such as between lutein and its diester (Mercadante et al., 2017). As different forms of carotenoids are found in nature and used in various functional foods or dietary supplements, ways to increase the bioaccessibility and bioavailability of carotenoids by proper esterification may present as an opportunity for enhanced bioactivity. Studies have also shown that competition exists between certain carotenoids for bioavailability. Lutein and β-carotene when co-ingested through one meal were found to mutually influence the bioavailability (Reboul et al., 2005). Difference in diffusion process between these two compounds was cited as the reason, however, further validation studies are necessary.
Cellular transport and uptake mechanisms of carotenoids are also one of the key factors for the difference in carotenoid bioavailability. It is generally understood that the intestinal absorption of carotenoids occurs by passive diffusion (Reboul, 2013). However, the scavenger receptor class B type I (SR-BI), an enterocyte apical membrane transporter for cholesterol, and cluster determinant 36 (CD36), a fatty acid transporter have been found to be involved in active cellular uptake of β-carotene and α-carotene (Borel et al., 2013; During et al., 2005). SR-BI is also found to be involved in the cross-membrane transport of lutein and lycopene (During et al., 2005; Reboul et al., 2005). More recently, facilitated diffusion process mediated by SR-BI instead of CD36 has been reported for the transport of astaxanthin isomers, phytoene and phytofluene (Mapelli-Brahm et al., 2018; Yang et al., 2019). These indicate that certain transport proteins in intestinal epithelial cells are also important factors that influence the absorption and bioavailability of dietary carotenoids. More studies are necessary to further elucidate the involvement of these and other transport proteins in the absorption of different forms of carotenoid (isomers and esterified forms).
Fats and oils have been shown to aid the solubilization of carotenoids into mixed-micelles and enhance their bioavailability (Nagao, 2014). Furthermore, dietary fats rich in saturated fatty acids such as butter lead to a higher bioavailability of lutein and zeaxanthin than olive or fish oils that are rich in monounsaturated and polyunsaturated fatty acids. The higher bioaccessibility of these xanthophylls in the presence of saturated fatty acids are partially due to the smaller mixed micelles in which the carotenoids are incorporated (Gleize et al., 2013). The bioaccessibility of carotenoids from different food sources can be different. The transfer efficiency of astaxanthin into mixed micelles during digestion of uncooked wild salmon was 43%, but only 12% for uncooked acquacultured salmon (Chitchumroonchokchai and Failla, 2017). Lycopene is markedly more bioavailable from tangerine tomatoes (Solanum lycopersicum) with 94% Z-lycopenes than from red tomato juice with 10% Z-lycopenes (Cooperstone et al., 2015). The presence of dietary fiber was found to interfere with the bioaccessibility of carotenoids, possibly due to its entrapment in the lipids and bile salt molecules in place of carotenoids (Cilla et al., 2018).
The food processing technology not only impacts on the stability of carotenoids but also on their bioaccessibility and bioavailability. Study shows that processing increased the bioaccessibility of carotenoids in persimmon to 54% treated by high hydrostatic pressure compared to 25% in those by thermal pasteurization (Cano et al., 2019). High pressure homogenization (HPH) could increase both the release and micellar incorporation of α- and β-carotene in carrot emulsions to 1.5- to 1.6-fold higher, however, the bioaccessibility of lycopene from tomato was not affected (Svelander et al., 2011). Sonication would increase the bioaccessibility of carotenoids from Chlorella vulgaris to a level comparable to that of Chlamydomonas reinhardtii (β-carotene≥10%; lutein≥15%) (Gille et al., 2016). The bioaccessibility of entrapped astaxanthin in potato protein-astaxanthin nanoparticles is 11 times higher than that of unencapsulated astaxanthin (Edelman et al., 2019). This suggests that entrapping systems such as nanoemulsion and nanostructured lipid carriers can work as efficient delivery system to improve the bioaccessibility and even bioavailability of dietary carotenoids in foods, beverages and nutraceuticals (Sotomayor-Gerding et al., 2016; Yang et al., 2019).
| 4. Roles of dietary carotenoids in human health | ▴Top |
It is generally confirmed that there is a positive relationship between higher consumption of fruits, vegetables and whole grains and reduced risk of chronic diseases such as cardiovascular disease (CVD), cancers, osteoporosis and diabetes. Some of these chronic diseases are often related to metabolic syndrome, which is a collective term for medical conditions that involve risk factors such as obesity, high blood pressure, high blood sugar, and unhealthy cholesterol levels. These conditions are particularly closely associated with the risk of developing CVD and type 2 diabetes. Healthy diets high in fruit, vegetables and whole grains have been known for their ability to improve the cholesterol, insulin resistance, and blood pressure, thus help lower risk of diabetes and cardiovascular disease. These healthy diets are known for their high carotenoid content and have been studied in the context of obesity and diabetes and CVD, and covered in several recent reviews (Le Goff et al., 2019; Mejia et al., 2020).
Most importantly, recent studies have also shown that different chronic diseases are closely related to one another and have overlapping aspects, and the key overlapping aspect is the low grade systemic inflammation. Low grade systemic inflammation is also closely related to immune system and gut health (Cani et al., 2012; Das, 2010; Emanuela et al., 2012). For these reasons, instead of discussing the link between carotenoids and metabolic syndrome, the present review focuses more on the fundamental roles of carotenoids, i.e. their mediating effect on oxidative stress, inflammation and gut health, and molecular mechanisms.
4.1. Antioxidant activities
Oxidative stress (OS) is caused by the imbalance between reactive oxygen or nitrogen species (ROS/RNS) production and the antioxidant defense. Excessive and long term exposure to OS results in damage to vital molecules in human cells such as proteins, membrane lipids, sugars, and nucleic acids, affecting their normal functions and leading to reduced cell viability or cell death. Dietary phytochemicals such as carotenoids are antioxidants that help restore redox homeostasis in the body. Since in situ analysis of the antioxidant activity in vivo is currently unattainable, the antioxidant properties of phytochemicals in plants are mostly evaluated by chemical-based assays (Zhang et al., 2018). Chemical-based antioxidant activity assays such as 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical-scavenging activity, 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation (ABTS+), oxygen radical absorbing capacity (ORAC), photochemiluminescence (PLC), and ferric reducing antioxidant power (FRAP) assays are widely used to determine the antioxidant capacity of different phytochemicals including carotenoids. However, such methods neither provide information on the actual cellular antioxidant mechanisms nor take into consideration the bioavailability (cellular uptake) and metabolism of the antioxidant molecules (Yang et al., 2018). Recently, a cellular antioxidant activity (CAA) assay was developed using cell-permeable 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) as a fluorescence probe. Till now, several cell lines such as Caco-2, human endothelial cell line (EA.hy926), human macrophage cell line (U937), human breast epithelial cell line (MCF-10A) and human lung fibroblasts (WI38, IMR-90) have been adopted for the antioxidant capacities by CAA assay (Yang et al., 2018). In spite of this, both chemical assays and CAA are still widely used in preliminary assessment of antioxidant capacities.
As summarized in Table 2. The antioxidant activity of carotenoids tested by ABTS+ assay follows the order of: lycopene > β-carotene > lutein > α-tocopherol (Zanfini et al., 2010). Furthermore, the antioxidant activity of astaxanthin is at least 10 times stronger than that of β-carotene and more effective than that of α-tocopherol (Matos et al., 2017). The presence of the hydroxyl and keto moieties on each ionone ring could explain its higher antioxidant activity (Liu and Osawa, 2007). However, the antioxidant activity of an individual carotenoid can largely depend on the assay methods. For example, β-carotene showed highest scavenging percentage in the DPPH assay, whereas violaxanthin was most efficient in ABTS+ and other two assays followed by lutein (Fu et al., 2011; Müller et al., 2011; Rodrigues et al., 2012). These studies also showed that assay outcomes were also determined by the solvent used in the protocol. When in combination, carotenoids can exert synergistic antioxidant activities. Study demonstrated that mixtures of lycopene-lutein, lycopene-β-carotene and lutein-β-carotene had higher antioxidant activity than individual compounds alone (Zanfini et al., 2010). It was proposed that the synergism between xanthophylls such as astaxanthin and carotenes such as lycopene, starts with the more hydrophilic xanthophylls serving as molecular wiring across membranes in the electron transfer networks through anchoring in water/lipid interfaces, resulting in synergism with more lipophilic carotenoids (Skibsted, 2012). Further studies are needed to better understand the role of carotenoid structures in their physiological activities and the mechanisms of synergism.
 Click to view | Table 2. Potential health effects of carotenoids and comparison among carotenoid isomers |
The different antioxidant activities exhibited by carotenoid isomers are important as seen in recent studies. 13Z-Astaxanthin had higher antioxidant activity than all-E- and 9Z-astaxanthins in ORAC assay for lipophilic compounds (ORAC-L), PLC and CAA assays, whereas that of 9Z-astaxanthin was higher in DPPH assay (Liu and Osawa, 2007; Yang et al., 2017). The two cis-isomers 9Z- and 13Z-astaxanthins also showed higher protective effect than all-E-astaxanthin against oxidative stress by lowering the secretion and gene expression of the pro-inflammatory cytokine IL-8 in Caco-2 cells treated by H2O2 (Yang et al., 2017). A recent study also showed that 9Z-, 13Z- and all-E-astaxanthin were all able to extend the median lifespan of Caenorhabditis elegans by 59.39, 24.99, and 30.43%, respectively. The ability of lifespan extension was found to be consistent with the degree of decreased intracellular ROS accumulation by astaxanthin isomers, particularly by the 9Z-astaxanthin (Liu et al., 2018).
The 13′Z-lutein also exhibits higher antioxidant activity than 9Z- and all-E-luteins in FRAP, DPPH and ORAC-L assays, but no significant difference was found among the three isomers in CAA assay (Yang et al., 2018). The antioxidant activity of different xanthophyll isomers by chemical-based assays are as follows: 9Z-astaxanthin ≈ 13Z-astaxanthin > all-E-astaxanthin ≈ 13′Z-lutein > 9Z-lutein ≈ all-E-lutein (Yang et al., 2018; Yang et al., 2017). Similar results are found for lycopene isomers in their scavenging activity against peroxyl radicals generated by thermal degradation of 2, 2′-azobis (2-amidinopropane) (AAPH). The AAPH activity of Z-lycopenes was higher than the all-E-isomer, and among cis-isomers 5Z-, 9Z- and 13Z-lycopenes, 5Z-lycopene was the strongest antioxidant. However, significant differences were not found among the isomers in FRAP and ABTS+ assays (Muller et al., 2011), and conflicting results have been reported for the antioxidant activities of geometric isomers of β-carotene and zeaxanthin (Böhm et al., 2002; Lavy et al., 1993; Levin et al., 1997; Mueller and Boehm, 2011).
In addition to the geometric isomers of carotenoids, optical or stereoisomers such as those of astaxanthin are also widely found naturally in some marine lives, and the antioxidant activity of the stereoisomers have not been studied in-depth. One study however showed that the 3S,3′S-astaxanthin exhibited higher antioxidant activity than the 3R,3′R-astaxanthin and a mixture of 3S,3′S-astaxanthin, meso-astaxanthin and 3R,3′R-astaxanthin at 1:2:1 ratio, in both chemical assays and CAA (Liu et al., 2016).
Esterification does not appear to have significant effect on the antioxidant activity of free xanthophylls. The antioxidant activities among lutein, lutein monomyristate and lutein dimyristate, and esters of β-cryptoxanthin and capsanthin were not influenced by esterification (Fu et al., 2010; Matsufuji et al., 1998). However, free astaxanthin showed significantly higher antioxidant effects than its esters in certain assay systems but not in others, again, suggesting the effect of methods used in the assessment (Sowmya and Sachindra, 2012). Further research is required to better understand the effects of fatty acids, especially the polyunsaturated fatty acids, as some of them are known to possess antioxidant activities.
Available research data suggest that although chemical based antioxidant assays lack biological relevance, they are still valid tools for preliminary assessment and quick comparison of the potential antioxidant capacity of carotenoids. The CAA assay offers a good alternative and can serve as a starting point for further in vivo investigations. Considering the various isomers that exist in nature, future studies are warranted to compare and elucidate the mechanisms of antioxidant actions of the different carotenoid geometric and stereoisomers and esters, their synergistic effects, and how food matrix (e.g. protein complex) and formulation affect the total antioxidant activity in foods or supplements.
4.2. Anti-inflammatory activity, immune response, metabolic syndrome and gut health
As mentioned earlier, low grade systemic inflammation is a key overlapping aspect among different conditions of metabolic syndrome, the immune system response and gut health. OS triggers inflammation, and is also implicated in these and other degenerative diseases such neurological disorders (e.g. Parkinson’s and Alzheimer’s diseases) and aging (Yang et al., 2018). The oxidative damage to cells caused by long-term exposure to OS in intestinal epithelial cells can trigger a series of immune system responses resulting in low-grade and sustained inflammation, which is the root cause of chronic diseases such as inflammatory bowel disease (IBD) (Yang et al., 2019).
Results from studies using cellular and animal models have demonstrated that carotenoids such as lycopene and β-carotene are potent anti-inflammatory agents that act by suppressing various inflammation pathways (Cho et al., 2018) (Figure 3). All-E-astaxanthin is reported to relieve retinal OS and exhibit anti-inflammatory effects in cell line and mouse models (Cho et al., 2018). It does not only down-regulate the nuclear factor-κB (NF-κB) signaling pathway, but also improves the insulin signaling cascade by regulating the insulin receptor-beta (IR-β), IRS-1-associated PI3K, phosphorylated Akt/Akt ratio, and translocation of the GLUT-4 (Mohammadzadeh Honarvar et al., 2017). Recently, the cis-isomers of astaxanthin have also been found to exhibit anti-inflammatory effects. Z-Astaxanthins, especially 9Z-astaxanthin exhibited greater anti-inflammatory effect than all-E-astaxanthin by down-regulating pro-inflammatory cytokines COX-2 and TNF-α gene expression in Caco-2 monolayers treated with TNF-α (Table 2). The anti-inflammatory effects of Z-astaxanthin isomers were also found to be via modulating the NF-κB signaling pathway as they down-regulated TNF-α-induced phosphorylation of IκBα (Yang et al., 2019).
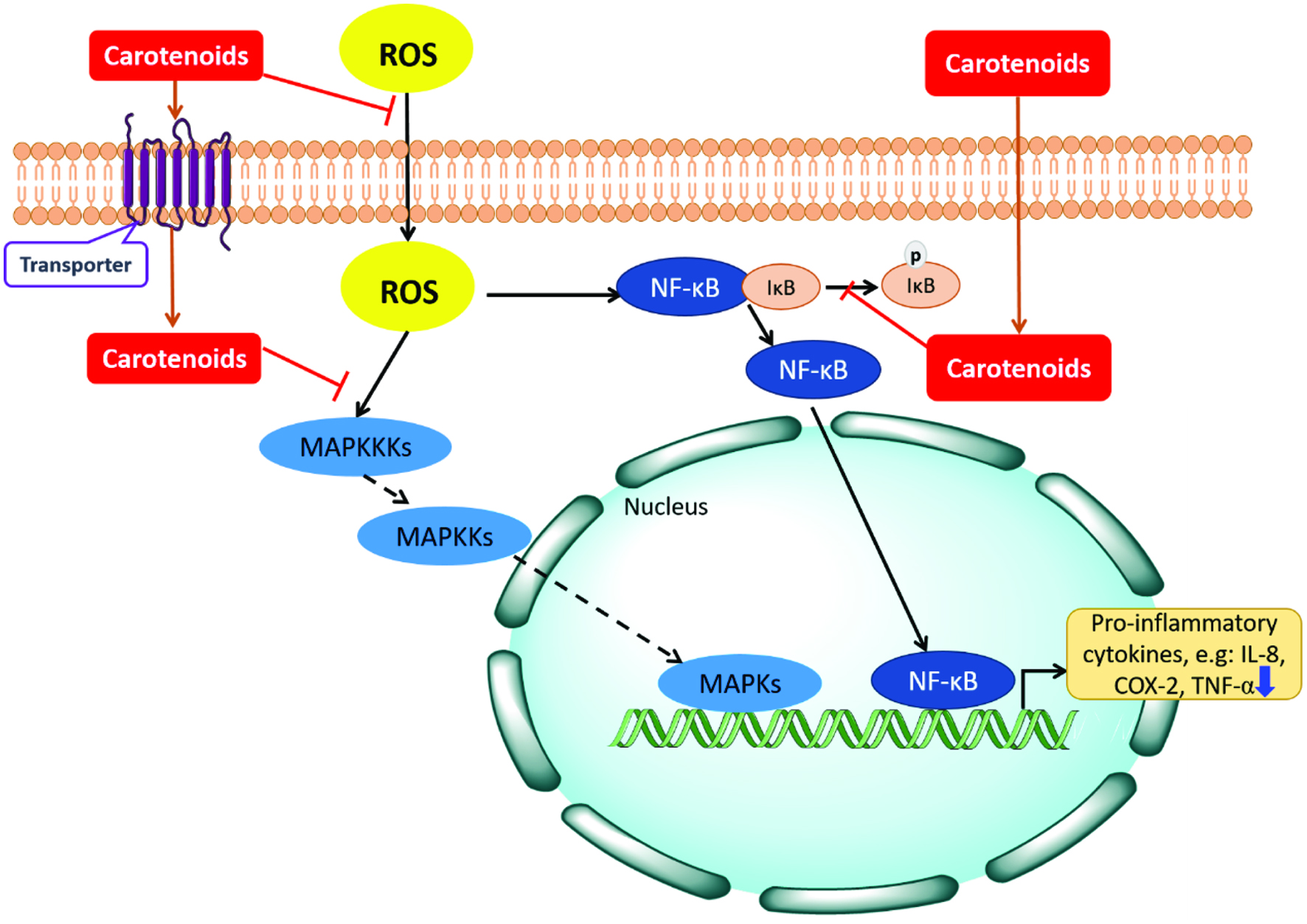 Click for large image | Figure 3. Schematic depiction of inflammatory signaling pathways and actions of carotenoids. Increased ROS causes the oxidative stress inside cells. During exposure to oxidants, IκB (inhibitors of NF-κB) proteins which are bound with NF-κB are rapidly degraded and release NF-κB protein to the nucleus. NF-κB could then bind to DNA sequences, and activate the expression of pro-inflammatory cytokines. The mitogen-activated protein kinase (MAPK) pathway activation begins with the activation of MAPKK-kinases (MAPKKKs). MAPKKKs phosphorylates MAPK-kinases (MAPKKs) subsequently, and the MAPKK further activates and phosphorylates MAPKs. Activated MAPKs then mediate the gene expression in nucleus. Certain carotenoids can inactivate the NF-κB pathway and MAPK signaling pathways to exhibit the anti-inflammatory effects. |
Another xanthophyll, lutein, is the pigment in the retina especially the macula of human eyes to filter the damaging blue light and reduce OS, thus contributes to eye health. It reduces lipid peroxidation and pro-inflammatory cytokine release through suppressing the activation of the NF-κB pathway in the presence of OS (Cho et al., 2018). Moreover, Z-luteins are found disproportionally high in human plasma and retina, however, the physiological roles of Z-luteins in humans, including the various protective effects such as anti-inflammatory and anti-macular degeneration activities, remain unknown (Yang et al., 2018).
Carotenoid extract of D. salina containing all-E-β-carotene and 9Z- or 9′Z-β-carotene was demonstrated to exert its anti-inflammatory effects by inhibiting the nuclear NF-κB p50 subunit translocation and down-regulating the c-Jun NH2-terminal kinase (JNK) activation (Yang et al., 2013). meso-Zeaxanthin [(3R, 3′S)-β, β-carotene-3, 3′-diol] exists in the Henle fibers of retina of primates is found to inhibit paw edema induced by carrageenan, dextran, and formalin in mice and exhibit anti-inflammatory effect against LPS-induced inflammation in macrophages through down-regulating the gene expression of inflammatory mediators like COX-2, TNF-a, and iNOS (Firdous et al., 2015). Colorless carotenoids such as phytoene and phytofluene could also lower the production of inflammatory mediators in UV-irradiated or IL-1-treated fibroblasts by acting either additively or synergistically with CoQ10 (Fuller et al., 2006). In addition to the NF-κB pathway, the anti-inflammatory effects of carotenoids such as fucoxanthin are also found through regulating other pathways including Akt and mitogen-activated protein kinase (MAPK) pathways (Cho et al., 2018). Thus far, little information can be found in the literature about the anti-inflammatory effects of carotenoid esters, largely owing to the fact that only free forms are found in human tissues and organs.
Emerging evidence on the interrelationships between diet, nutritional status, the immune system and gut microbiota in humans has brought explosion of new knowledge in the last decade. Past research has been focused mainly on dietary fibers and their prebiotic effects, however, prebiotic-like effect by phytochemicals especially by carotenoids has not been widely examined. Only a few reports have been published on the role of carotenoids in immune regulation and gut health. Fucoxanthin is a marine carotenoid known for its antioxidant, anti-inflammatory and various other effects, most notably in reducing body fat and obesity. Most recent research showed that in a high-fat diet (HFD) mouse model, fucoxanthin supplementation for 4 weeks significantly changed the composition of both cecal and fecal microbiota, especially the Firmicutes/Bacteroidetes ratio and the abundance of S24-7 and Akkermansia that are best known gut microbes modulating events associated with anti-obesity (Guo et al., 2019). Similar results were also found for astaxanthin. Supplementation of astaxanthin or astaxanthin-producing red yeast (Xanthophyllomyces dendrorhous) for 8 weeks not only reduced the HFD-induced body weight gain, improved the plasma and liver lipid profile, but significantly altered the gut microbiota. Again, the ratio of Bacteroides/Firmicutes was improved, especially in Akkermansia (Wang et al., 2019). These studies demonstrate that carotenoids, especially xanthophylls, are capable of modulating immune responses and gut microbiota composition and ameliorating metabolic syndrome, therefore present a great opportunity for improving gut health and reducing risk of chronic diseases. Further research is needed for other carotenoids, and collective effects by carotenoids and other food components. It is also important to closely examine the effect of carotenoids on the pathogenic bacteria in the gut, as dysbiosis in the gut microbiome increases the number of harmful bacteria in the gut, which may release enterotoxins. Endotoxins can increase the permeability of the intestine, trigger the production of pro-inflammatory cytokines and result in immune dysfunction, damage in intestinal epithelial cells, alteration in energy metabolism, which lead to intestinal inflammation, the ultimate culprit of various chronic diseases.
4.3. Cancer
Increased intake of carotenoid-rich diet has been linked to reduced risk of various cancer types. However, the roles of extracts or food supplements in cancer prevention and treatment are not as clear. The mechanisms of the anti-cancer effects by carotenoids include modulation of antioxidant activity, carcinogen metabolism, and regulation of cell growth, cell cycle progression and cell-to-cell gap junction communication, prevention of cell proliferation, immune modulation and apoptosis induction. Lycopene was found to inhibit disease progression in patients with benign prostate hyperplasia (BPH), and astaxanthin was effective against BPH and prostatic cancer through inhibition of the enzyme 5α-reductase which is involved in abnormal prostate growth (Vílchez et al., 2011). Z-Lycopenes were found to be equivalent and better inhibitors than all-E-lycopene in benign BPH treatment in mice (Zou et al., 2014). The inhibitory effects of 3S,3′S-astaxanthin from H. pluvialis, 3R, 3′R-astaxanthin from Phaffia rhodozyma, and a mixture of different stereoisomers of astaxanthin (S: meso: R = 1:2:1) all inhibited the colon cancer cell growth with statistically same potency (Liu et al., 2016). Despite these positive effects of carotenoids on cancer, cautions have also been made for their potential negative effects. Controversial results on the anti-cancer effects of lycopene on prostate cancer and the protective effects of β-carotene on lung cancer have been reported (Woodside et al., 2015). The source of the carotenoids including dietary foods and supplements, dosage and time intervals for the intake of carotenoids and the life style (e.g. smoking) of the subjects could all impact the results. Tumor growth stage is also important for treating by carotenoids. Astaxanthin given prior to tumor initiation could suppress mammary tumor growth, and increase the natural killer cell populations and plasma interferon-g concentration in BALB/c mice injected with a mammary tumor cell line. However, the astaxanthin supplementation after tumor initiation resulted in more rapid tumor growth and elevated plasma inflammatory cytokines IL-6 and TNF-α (Nakao et al., 2010). All this suggests the importance of checking the redox homeostasis prior to disease initiation and the timing for the supplementation of carotenoids.
4.4. Macular degeneration
Carotenoids also show protective effects against degenerative diseases such as macular degeneration. Two of the leading causes of visual impairment and blindness are age-related macular degeneration (AMD) and age-related cataracts. Both diseases are related to light-induced oxidative damage to the eyes. Lutein and zeaxanthin are concentrated in the macula of the eye. These carotenoids act as a filter of the UV and visible blue light to reduce the light injury and free radical damage in the eyes. As aforementioned, only the cis-isomers have significant UV absorption. This is especially intriguing because even though dietary lutein and zeaxanthin are predominantly all-trans, the cis-isomers are disproportionally higher in the macular. Study also shows that astaxanthin as well as lutein are capable of crossing the blood–brain barrier and deposit in the retina of mammals (Guerin et al., 2003). β-Carotene, in addition to being a pro-vitamin A carotenoid, was also found to reduce the premature aging action of UV rays on the skin and play an important role in retinal synthesis (Matos et al., 2017).
| 5. Conclusions and perspectives | ▴Top |
Research on dietary carotenoids has clearly demonstrated the significant roles that these food bioactives play in promoting health and preventing chronic disease, particularly the OS-associated diseases. Their unique chemical structures allow for extraordinary ability to quench ROS and reduce OS, and the strong antioxidant activities help maintain the redox homeostasis in our body. Carotenoids are bioavailable, however the inherent physicochemical properties of carotenoids such as structure, geometric and stereoisomers and esterification pattern, and food processing, food matrix and formulation, can all affect the bioaccessibility and bioavailability of carotenoids, leading to varied bioactivities. Meanwhile, the OS is also a causative factor for inflammation, especially chronic inflammation. Review of the latest literature in the present paper shows that dietary carotenoids can inhibit production of pro-inflammatory cytokines through modulating cell signally pathways, thereby having strong anti-inflammatory and immune modulatory effects (Figure 3). Existing evidence in vivo also strongly suggests that intake of carotenoids-rich diet or supplements can lower risk of chronic diseases, particularly in alleviating metabolic syndrome. Recent studies on the ability of carotenoids in ameliorating intestinal dysbiosis suggest that these food bioactives may play a significant role in maintaining immune balance and good gut health, an emerging area of research that warrants further exploration. In addition, carotenoids have also been shown to inhibit cancer growth and slowed down progression of degenerative diseases, particularly age-related macular degeneration, although caution should be taken when treating cancer. Overall, carotenoids are a healthy component of diet, and significant evidence does exist for the link between high carotenoid intake and lower risk of various chronic diseases. The present review also led to the following perspectives for future research:
- Means to increase carotenoid content and profile in food crops and microbes. Conventional breeding and rational usage of genetic engineering can lead to better composition in terms of specific carotenoid molecules, geometric and stereoisomers, more bioavailable free or ester forms.
- Means to increase the stability and bioaccessibility of carotenoids during food processing, formulation or large-scale extraction.
- Further studies on the mechanisms of synergistic effects between different carotenoids, different isomers, and with other food bioactives; also, on the interactions with food matrices in terms of their antioxidant and anti-inflammatory activities and potential health benefits.
- The role of carotenoids in modulating gut microbiome, intestinal inflammation and immune response.
These future studies will not only generate new knowledge on how carotenoids contribute to good health beyond their regular nutritional function i.e. provitamin A for some compounds, but development of novel and more efficient functional foods and health products.
Acknowledgments
This project is supported by the A-Base Project (#J-002252.001.04) of Agriculture & Agri-Food Canada and grants from the Fundamental Research Funds for the Central Universities (JUSRP11907), the Natural Science Foundation of Jiangsu Province (BK20190592) and the Natural Sciences Foundation of China (31901654).
| References | ▴Top |